Back to Archived Journals » Reports in Medical Imaging » Volume 8
Imaging techniques for the diagnosis of soft tissue tumors
Authors Afonso D, Mascarenhas V
Received 15 September 2014
Accepted for publication 20 December 2014
Published 30 April 2015 Volume 2015:8 Pages 63—70
DOI https://doi.org/10.2147/RMI.S54490
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Tarik Massoud
P Diana Afonso,1,2 VV Mascarenhas2
1Department of Radiology, Hospital Beatriz Angelo, Loures, 2Department of Radiology, Hospital da Luz, Lisbon, Portugal
Abstract: The primary aim in soft tissue tumor imaging should be to reach a specific diagnosis or to narrow the differential diagnosis, and to help to decide whether biopsy, surgical intervention, or simple observation is required for further management. In addition to contributing toward diagnosis, imaging has an important role in the staging of soft tissue malignancies and potentially in response assessment. This general review article highlights a rational diagnostic imaging approach to patients presenting with soft tissue tumors, emphasizing the fundamental principles inherent to soft tissue tumor imaging and diagnosis.
Keywords: soft tissue tumors, ultrasound, CT, PET, MRI
Introduction
Soft tissue malignancies are an uncommon heterogeneous group of mesenchymal lesions. They account for 1% of adult malignant tumors1–3 and are estimated to represent about 1% of all malignant tumors with a lifetime risk of development estimated at 0.33%.4
Long-term local and systemic disease-free survival depends on patient age and tumor type, accurate initial staging, surgical excision (often with neoadjuvant or adjuvant radiation and chemotherapy), and early detection of disease recurrence.2,4
The past years have witnessed remarkable advancements in diagnostic imaging techniques, which in turn have resulted in significant improvements in musculoskeletal tumor imaging. Imaging can accurately delineate the morphology of lesions including size, location, and extent. It can also provide useful information related to the underlying biology of lesions, often able to depict the underlying physical composition of tumors. As a result, diagnostic imaging can effectively limit the differential diagnosis for skeletal lesions, and it often can accurately arrive at the diagnosis.5
Imaging of soft tissue tumors requires a multimodality approach, with no single imaging modality being ideal for every tumor.6 This review article highlights the general imaging approach to patients presenting with soft tissue tumors. It is not intended as a comprehensive review, but rather as an overview, emphasizing the fundamental principles inherent to tumor imaging.5,6
Conventional radiography
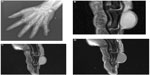
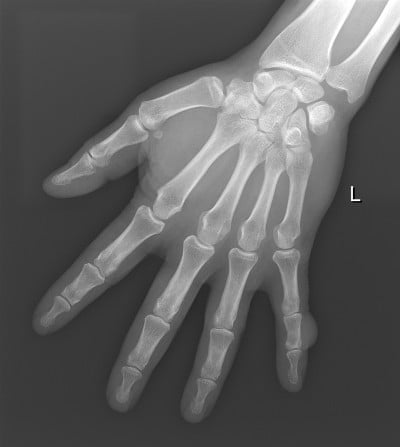
The imaging evaluation of a suspected soft tissue mass begins with conventional radiography, particularly for extremity and other superficial masses.4–8 Although radiographs are frequently unrewarding, they can provide valuable information when positive (Figure 1).
Radiographs evaluate whether the soft tissue tumor is actually originating from the bone and is in fact an osseous lesion, and similarly provide an excellent method for assessment of osseous involvement by a truly soft tissue tumor (such as remodeling, periosteal reaction, or overt cortical destruction).
Radiographs also evaluate for the presence of mineralization that may be suggestive, and at times characteristic, of a certain diagnosis. For example, they may reveal the phleboliths within a hemangioma, or the peripherally more mature ossification of myositis ossificans.6,7,9
Ultrasound
Ultrasound (US) is a readily available and cost-effective imaging technique. However, it is highly dependent on the skill of the radiologist/sonographer and the quality of the equipment. High-resolution US requires linear-array, high-frequency transducers (>9–18 MHz).
In patients with a suspected soft tissue “lump and bump”, US is ideally suited as initial triage imaging modality, given that the suspected tumor is accessible by sonography (deeply seated tumors pose obvious problems). Furthermore, it is a simple first-line study for children.
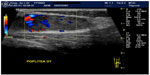
Following the confirmation of a soft tissue mass, sonographic assessment of its nature (ie, solid versus cystic), size, shape, number, vascularity (color or power Doppler), location, and anatomical relationships to adjoining structures aids in characterization and determining whether further imaging or biopsy is required (Figure 2).
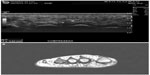
For some benign soft tissue masses (eg, homogenous small lipomas, bursas, cysts and ganglion, Morton neuromas [Figure 3], and foreign bodies), the US findings may be sufficient to obviate the need for further imaging.
Large size at presentation (>5 cm), rapid growth, deep location, and hyperemic chaotic-type vasculature on Doppler imaging are all more common in malignant tumors. However, other solid benign and malignant soft tissue masses demonstrate considerable overlap in their sonographic appearances, and further evaluation is needed.7,10,11
Image-guided procedures such as biopsy or aspiration can also be easily performed under US guidance.6,10
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is the imaging method with the best soft tissue contrast, provides multiplanar capability, and lacks ionizing radiation; thus, MRI has emerged as the preferred modality for evaluating soft tissue masses. Vascular structures can also be more easily recognized, even without the need of intravenous contrast agents.
MRI should be considered instead of (or in addition to) US whenever there is clinical suspicion of malignancy and/or painful, deep-seated, or (fast)-growing masses.6,8,12,13
It serves to further characterize the tumor, to perform local staging reliably and reproducibly for therapy planning, and to help selecting appropriate biopsy regions. Also, it is the modality of choice for local surveillance after malignant soft tissue tumor resection/assessment of postsurgical site.2,3
MRI protocols for the evaluation of soft tissue masses must be performed in at least two orthogonal planes and include T1-weighted and fluid-sensitive weighted sequences, with or without fat suppression. Additional sequences to consider include gradient-echo imaging for the detection of hemorrhage, T1-weighted fat-suppressed images to differentiate fat from hemorrhage, and static-enhanced imaging after contrast administration.6,7 Gadolinium-enhanced imaging identifies viable solid tumor (versus necrosis) for biopsy, and demonstrates enhancement in solid lesions with a cystic-like appearance (like myxoid tumors).
MRI lesion characterization includes assessment of signal intensity (often nonspecific, but can detect fat, blood products, and fluid), size, morphology, location, and relationship to adjacent structures, and multiplicity (including other lesions on the field of view, eg, lymph nodes, skip metastases).
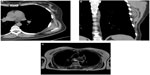
While MRI accurately elucidates the anatomic location of a tumor (Figure 4) and the lesion’s relationship to the neurovascular bundle and bone, it remains partially limited in its ability to accurately detect patterns of soft tissue calcification, and to reliably differentiate between some benign and malignant soft tissue tumors (ability to predict histology by MRI is limited). Highest confidence in characterization occurs with benign masses – many benign tumors such as lipomas, hemangioma/arteriovenous malformations, neurogenic tumors, fibromatosis, cysts, hematomas, and abscesses may be diagnosed based on their MR appearance alone.6
Features favoring malignancy are large size (>5 cm), deep location (regardless of size), heterogeneous signal/enhancement (necrosis), and bone/neurovascular bundle involvement. However, pitfalls should also be considered: both benign and malignant soft tissue masses often have well-defined margins; superficial sarcomas often appear less “aggressive” than deep sarcomas (smaller), and gadolinium enhancement may be seen in both benign and malignant lesions.
Additional sequences may include dynamic contrast enhancement and diffusion-weighted sequences, which can be helpful in characterizing the tumor.14,15
Dynamic contrast-enhanced MRI
Dynamic contrast-enhanced MRI differentiates benign and malignant tumors by evaluating the difference in rates of enhancement over time as a measure of lesion vascularity and perfusion.
In general, malignant lesions have a greater degree and rate of enhancement. Yet, overlap exists secondary to highly vascularized benign lesions and poorly vascularized (or necrotic) malignant tumors. In a study by van Rijswijk et al, more than 100 patients were prospectively evaluated, and it was determined that dynamic contrast-enhanced imaging was significantly superior to both unenhanced imaging and static gadolinium-enhanced imaging in the predication of malignancy. However, controversy persists, and in another study by Mirowitz et al, they showed no advantage in the use of dynamic imaging due to significant overlap in the enhancement rate of benign and malignant soft tissue tumors. As such, additional research is required.4,14–16
Diffusion-weighted MRI
Diffusion-weighted MRI (DWI) analyzes tissue cellularity and cell membrane integrity by measuring the random motion of water molecules in biological tissues. The diffusion of water in highly cellular tissues is restricted and as such will have higher signal intensity on DWI and lower signal intensity on apparent diffusion coefficient (ADC) maps.
DWI has been applied to soft tissue tumors with variable results. van Rijswijk et al reported a significant difference in true diffusion coefficients between malignant and benign soft tissue tumors but noted considerable variation within the liposarcoma and myxofibrosarcoma groups. Besides, the true diffusion coefficient in fibromatosis was indistinguishable from malignancy.14,15
Again, Einarsdóttir et al reported significant overlap of the ADC values between benign and malignant soft tissue tumors, and thus determined the sequence to be of no diagnostic usefulness. In a targeted study, Oka et al reported a significant difference in the ADC value of chronic-expanding hematomas when compared to soft tissue tumors, and thus, DWI may have a role in the distinction of hematomas versus hemorrhagic malignant soft tissue tumors.17,18
MR spectroscopy can characterize lesions based on metabolic constituents, including choline, a marker for membrane turnover. Again, a degree of controversy persists because some published works have shown significantly different choline peaks and choline signal-to-noise ratios in benign and malignant lesions, thus allowing for distinction. However, overlap with metabolically active benign lesions and abscesses has been reported.6,19
Computed tomography
MR has largely replaced computed tomography (CT) for the evaluation of soft tissue tumors. However, image quality has markedly improved due to the introduction of multidetector scanners and high-quality multiplanar-reformatted images, providing faster scanning times, which decreases motion artifacts and allows larger volumes of coverage.
As such, there is still a role for CT in the evaluation of soft tissue masses, since CT is the most effective modality for detailed evaluation of osseous architecture, particularly in areas with complex osseous anatomy (eg, chest wall); also, CT is able to assess osseous remodeling, periosteal reaction, and matrix when these are not adequately delineated on initial radiographs or US.
CT is also useful in identifying extrinsic osseous erosions, subtle areas of mineralization, or soft tissue gas that may not be apparent on MRI or US. Fat is also well identified, and lipomas are easily characterized on both CT and MRI.
Lastly, CT is useful in patients with contraindications to MRI.6,8
Metastatic spread of soft tissue sarcomas mainly is hematogenous, and pulmonary metastases are most common, accounting for 75%–80% of metastases. A CT scan of the chest (without contrast) is thus recommended for the identification of pulmonary metastases, particularly in large sarcomas (>5 cm) and sarcomas with moderate-to-poor differentiation.6,8,12
Positron emission tomography
Positron emission tomography (PET) utilizes radioisotopes that undergo positron emission decay. A detector surrounding the patient detects the paired gamma photons released as a consequence of decay and registers the interaction in the form of an image. The radionuclide most commonly used for PET is [18F]-fluoro-2-deoxy-d-glucose (FDG). In vivo, FDG behaves like glucose and provides a means of quantifying glucose metabolism. Unlike glucose, the metabolite of FDG is not a substrate for glycolytic enzymes. Therefore, the radioactive tracer is trapped in the cell, allowing subsequent imaging. The amount of tracer accumulation reflects the tissue’s glucose metabolism.
Theoretically, high-grade malignancies would have higher rates of glycolysis and therefore FDG uptake (and thus a higher standardized uptake value [SUV]).6 Also, intensity of uptake and identification of necrosis on pretreatment staging studies could be used as prognostic markers; also, they would help to direct biopsy to metabolically active area of tumor.
While PET–CT has become well established in the staging and long-term management of malignancies (like non-small-cell lung, head and neck, gastrointestinal (Figure 5), and lymphoma), for soft tissue neoplasms, the role of PET–CT is still under debate because overlap with benign, inflammatory, and/or aggressive lesions persists as it is well known that FDG is not specific for malignant cells, and it also accumulates in the infectious/inflammatory processes; it may lead to upstaging in only a minority of patients and is, to date, therefore not recommended for routine use for soft tissue tumors.20
For instance, synovial sarcoma and liposarcoma did not have average SUVs significantly higher than benign lesions; also, sarcoidosis and giant cell tumor of tendon sheath could not be differentiated from high-grade sarcomas based on the SUV.6,21 In another study, similar findings were reported in the evaluation of both soft tissue and bone tumors showing no significant difference in the uptake of aggressive lesions (as fibromatosis) from malignant lesions.22
Although benign aggressive soft tissue masses may be misinterpreted as malignant, the converse is also possible with well-differentiated slow-growing malignant tumors misdiagnosed as benign lesions.6,22 As such, for initial staging, therapy control, and follow-up of soft tissue tumors, PET role is still developing.
However, information from PET may be used for other purposes; for example, even though SUVs acquired from a PET study unlikely could be relied upon to obviate biopsy, PET can be used to determine which area of a mass to biopsy if there is heterogeneity in SUV, targeting areas with more metabolic activity.
More is yet to come, especially with the availability of hybrid PET/MR scanner.
Conclusion
Imaging of soft tissue tumors requires a multimodality approach, with no single imaging modality being ideal for every tumor.
The diagnostic evaluation should ideally begin with radiographs of the mass (or region) in question. Primary US or MRI is chosen according to the clinical characteristics, location of the soft tissue tumor, and patient “concern”. Soft tissue masses that should raise suspicion are those located deep to the deep fascia, larger than 5 cm, rapidly growing, and painful. MRI is the modality of choice for diagnostic and local staging of soft tissue tumors, but US may be enough for simple, superficial, nongrowing benign lesions. CT is indicated to better define the osseous and matrix architecture and in patients with contraindications to MRI.
Although not yet part of routine clinical practice, FDG PET and new, dedicated MR sequences show promise in the diagnosis and treatment of soft tissue tumors in the near future.
Disclosure
The authors report no conflicts of interest in this work.
References
Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2002:9–175. | |
Diana Afonso P, Kosinski AS, Spritzer CE. Following unenhanced MRI assessment for local recurrence after surgical resection of mesenchymal soft tissue tumors, do additional gadolinium-enhanced images change reader confidence or diagnosis? Eur J Radiol. 2013;82(5):806–813. | |
Godland JR, Fletcher CDM, Smith MA. Surgical resection of primary soft-tissue sarcoma. Incidence of residual tumour in 95 patients needing re-excision after local resection. J Bone Joint Surg Br. 1996;78-B:658–661. | |
Beaman F, Jelinek J, Priebat D. Current imaging and therapy of malignant soft tissue tumors and tumor-like lesions. Semin Musculoskelet Radiol. 2013;17(02):168–176. | |
Peterson J. Current developments and recent advances in musculoskeletal tumor imaging. Semin Musculoskelet Radiol. 2013;17(02):099–100. | |
Knapp EL, Kransdorf MJ, Letson GD. Diagnostic imaging update: soft tissue sarcomas. Cancer Control. 2005;12(1):22–26. | |
Bancroft L, Pettis C, Wasyliw C. Imaging of benign soft tissue tumors. Semin Musculoskelet Radiol. 2013;17(02):156–167. | |
National Guideline Clearinghouse. National Guideline Clearinghouse | Print: ACR Appropriateness Criteria® soft-tissue masses; August 8, 2014. Retrieved August 8, 2014. | |
Sherman C, O’Connor M. Musculoskeletal tumor imaging: an orthopedic oncologist perspective. Semin Musculoskelet Radiol. 2013;17(02):221–226. | |
Smith S, Salanitri J, Lisle D. Ultrasound evaluation of soft tissue masses and fluid collections. Semin Musculoskelet Radiol. 2007;11(2):174–191. | |
Hung E, Griffith J. Pitfalls in ultrasonography of soft tissue tumors. Semin Musculoskelet Radiol. 2014;18(01):079–085. | |
National Cancer Institute: General Information About Adult Soft Tissue Sarcoma. [Webpage on the Internet]. http://www.cancer.gov/cancertopics/pdq/treatment/adult-soft-tissue-sarcoma/HealthProfessional/ page1. Accessed January 9, 2015. | |
Verstraete KL, Lang P. Bone and soft tissue tumors: the role of contrast agents for MR imaging. Eur J Radiol. 2000;34(3):229–246. | |
van Rijswijk CS, Geirnaerdt MJ, Hogendoorn PC, et al. Soft-tissue tumors: value of static and dynamic gadopentetate dimeglumine-enhanced MR imaging in prediction of malignancy. Radiology. 2004;233(2):493–502. | |
van Rijswijk CS, Kunz P, Hogendoorn PC, Taminiau AH, Doornbos J, Bloem JL. Diffusion-weighted MRI in the characterization of soft-tissue tumors. J Magn Reson Imaging. 2002;15(3):302–307. | |
Mirowitz SA, Totty WG, Lee JK. Characterization of musculoskeletal masses using dynamic Gd-DTPA enhanced spin-echo MRI. J Comput Assist Tomogr. 1992;16(1):120–125. | |
Einarsdóttir H, Karlsson M, Wejde J, Bauer HCF. Diffusion-weighted MRI of soft tissue tumors. Eur Radiol. 2004;14(6):959–963. | |
Oka K, Yakushiji T, Sato H, et al. Ability of diffusion-weighted imaging for the differential diagnosis between chronic expanding hematomas and malignant soft tissue tumors. J Magn Reson Imaging. 2008;28(5):1195–1200. | |
Doganay S, Altinok T, Alkan A, Kahraman B, Karakas HM. The role of MRS in the differentiation of benign and malignant soft tissue and bone tumors. Eur J Radiol. 2011;79(2):e33–e37. | |
Roberge D, Vakilian S, Alabed YZ, Turcotte RE, Freeman CR, Hickeson M. FDG PET/CT in initial staging of adult soft-tissue sarcoma. Sarcoma. 2012;2012:960194. | |
Aoki J, Watanabe H, Shinozaki T, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol. 2003;32(3):133–138. | |
Feldman F, van Heertum R, Manos C. 18FDG PET scanning of benign and malignant musculoskeletal lesions. Skeletal Radiol. 2003;32(4):201–208. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.