Back to Journals » OncoTargets and Therapy » Volume 12
Ilex hainanensis Merr targets ITGAV to suppress the proliferation and metastasis of osteosarcoma cells
Authors Pei Y , Zhang YY, Zheng K, Shang GN , Wang YM, Wang W, Qiu ED, Li SL, Liu F, Zhang XJ
Received 16 February 2019
Accepted for publication 27 April 2019
Published 7 June 2019 Volume 2019:12 Pages 4499—4507
DOI https://doi.org/10.2147/OTT.S205688
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Yi Pei,1 YueYan Zhang,2 Ke Zheng,1 GuanNing Shang,1 YuMing Wang,1 Wei Wang,1 EnDuo Qiu,1 ShengLong Li,1 Fei Liu,1 XiaoJing Zhang1
1Department of Bone and Soft Tissue Surgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, 110042, People’s Republic of China; 2Clinical Pathology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, 110042, People’s Republic of China
Background: Osteosarcoma (OS) is the most common primary malignant bone tumor. Hence, there is an urgent need to identify effective and safe therapeutic agents against OS. It has been reported that Ilex hainanensis Merr (IME) possesses antitumor properties. Integrin subunit alpha V (ITGAV) is important for the diagnosis, treatment, and prognosis of tumors.
Purpose: The objective of this study was to whether IME can play a role in the treatment of osteosarcoma by regulating ITGAV.
Methods: Western blot and real-time PCR were used to detect the expression of ITGAV in non-tumorous tissues, osteosarcoma tissues, and metastatic tumors. The expression of ITGAV in MG63, U2OS, and hFOB1. A total of 19 cells was determined through Western blotting and real-time PCR. The expression of ITGAV in OS cells treated with different concentrations of DDP was determined through Western blotting. Agter transfecting with control or si-ITGAV, and subsequently treated with control or 5 μmol/L DDP, MTT assay and transwell assay were used to detect the proliferaion and migration of cells. Western blot was used to detect the expression of ITGAV in cells treated with different concentrations of IME and MTT assay and transwell assay were used to detect the proliferaion and migration of cells. MG63 and U2OS cells were treated with control, 5 μmol/L DDP, 25 μmol/L IME, or 5 μmol/L DDP combined with 25 μmol/L IME, the expression of ITGAV was determined through Western blotting and real-time PCR. MTT assay and transwell assay were used to detect the proliferation and migration of cells. Inhibitory effect of IME on lung metastasis of osteosarcoma in vivo.
Results: ITGAV was highly expressed in tumors, with the highest expression found in metastatic tumors and higher in OS cells. A low concentration of DDP (5 μmol/L) inhibited the expression of ITGAV. However, ITGAV may be related to the development of resistance to DDP. Silencing of ITGAV downregulates the proliferation and migration of OS cells as the effect of low-concentration DDP (5 μmol/L). IME inhibited the proliferation and migration of MG63 and U2OS cells in a concentration-dependent manner and decreased the expression of ITGAV. MTT and Transwell assays showed that 25 μmol/L IME and 5 μmol/L DDP exhibited similar inhibitory effects on the proliferation and migration of OS cells. The combination of IME with DDP resulted in the amplification of these inhibitory effects. Both DDP and IME downregulated the expression of ITGAV, and the inhibition of ITGAV was amplified by the combination of IME with DDP. In-vivo studies have shown that IME and DDP, independently or in combination, may significantly inhibit the metastasis of OS to the lungs.
Conclusion: IME may reduce the resistance of OS cells to DDP to some extent.
Keywords: Ilex hainanensis Merr, osteosarcoma, integrin subunit alpha V, cisplatin
Introduction
Osteosarcoma (OS) is the most common primary malignant bone tumor, mainly occurring in children and adolescents. The combination of surgical resection with intensive chemotherapy has improved the 5-year overall survival rate. However, due to its low efficacy, chemotherapy is associated with the development of adverse effects and multidrug resistance.1,2 Therefore, there is an urgent need to identify new effective and safe therapeutic agents against OS.3
The ethanol extract of Ilex hainanensis Merr (IME) is obtained from the leaves of IME, which belongs to the family Aquifoliaceae. Recently, studies demonstrated that IME possesses excellent lipid modification and anti-inflammatory properties.4 It has also been reported that IME can inhibit the occurrence and development of melanoma.5
Integrin subunit alpha V (ITGAV) belongs to the integrin subunit alpha family. Studies showed that ITGAV plays an important role in angiogenesis and metastasis of tumors.6 It binds to extracellular ligands, promoting the secretion and activation of matrix metalloproteinases. This results in the activation and migration of vascular endothelial cells through a series of cascade reactions. ITGAV inhibits the apoptosis of endothelial cells and induces angiogenesis by synergistically interacting with the basic fibroblast growth factor and vascular endothelial growth factor. A study has implied that the level of ITGAV expression is important for the diagnosis, treatment, and prognosis of tumors.7 The expression of ITGAV is closely related to the progression of colon cancer and the degree of malignancy of glioma. In addition, it is associated with the differentiation of squamous cell carcinomas of the larynx and hypopharynx and their metastases to the lymph nodes.8 Overexpression of ITGAV in patients with cancer often indicates poor prognosis. Researchers showed that the expression of ITGAV is closely associated with the degree of malignancy and clinical stage of OS.9
This study analyzed the effect of ITGAV on DDP-resistant osteosarcoma cells, hoping to find a better treatment for osteosarcoma.
Materials and methods
Materials
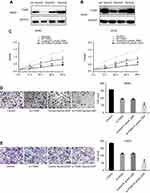
IME (Figure 1A), DDP, MTT, and Trypan Blue were purchased from Bioss Biotechnology (Beijing, China). FBS and RPMI-1640 medium were obtained from Gibco (Thermo Fisher Scientific Inc., Waltham, MA, USA). Lipofectamine 2000 and TRIzol were obtained from Invitrogen (Invitrogen, Carlsbad, CA, USA). The RT reaction kit and SYBR Premix Ex Taq were obtained from TaKaRa (Kusatsu, Japan). The anti-ITGAV and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) antibodies were purchased from Santa Cruz (Santa Cruz, CA, USA). The horseradish peroxidase-conjugated secondary antibody, bicinchoninic acid protein assay kit, and enhanced chemiluminescence solution were purchased from Beyotime Biotechnology (Shanghai, China).
Samples
Tissue samples were obtained from patients undergoing surgery at Liaoning Cancer Hospital and Institute (Shenyang, China). The original histopathologic reports were obtained for all patients, and the diagnosis of OS was confirmed. Part of the excised tissue was embedded in paraffin, while the remaining sample was snap-frozen at −80°C. All patients provided written informed consent, and the study was approved by the Ethics Committee of the Academic Medical Center (Shenyang, China).
Cell culture
MG63, U2OS, and hFOB1.19 cells were obtained from the Cell Bank of Chinese Academy of Sciences (Beijing, China) and cultured in RPMI-1640 medium with 10% FBS in a humidified atmosphere at 37°C under an atmosphere of 5% CO2.
Cell viability assay
Cells (1×104/well) were plated in 96-well plates; 12 hrs later, the medium was removed and the cells were treated with IME for 12, 24, 36, and 48 hrs. At the end of the treatment, the capability of cellular proliferation was measured using the MTT assay. For this purpose, 0.01 mL of MTT solution (5 mg/mL in PBS) was added to each well. After 4 hrs of incubation at 37°C, the medium was replaced by 0.15 mL dimethyl sulfoxide. After 15 mins of incubation at 37°C, the optical density was measured at 490 nm using a Microplate Reader (BIO-RAD, Beijing, China).
Transwell assay
Transwell assays were performed using modified Boyden chambers with polycarbonate nucleopore membranes. Following treatment with IME for 24 hrs, 1×105 cells in 100 μL FBS-free DMEMwere placed in the upper part of each chamber, whereas the lower compartment was filled with 600 μL of RPMI-1640 containing 10% FBS. After incubation for 8 hrs at 37°C, the invading cells on the lower surface of the filter were fixed, stained using Trypan Blue, and counted under high-power magnification.
Transfection
Cells were transfected with vector/ITGAV or pRS-si-NC (negtive control)/pRS-siITGAV (Shanghai GeneChem Company, Shanghai, China) to stably overexpress or silence ITGAV and were subsequently selected using puromycin (1.5 μg/mL). Lipofectamine 2000 was used for cell transfection according to the protocol provided by the manufacturer.
Real-time polymerase chain reaction (PCR)
Following treatment for 24 hrs, total RNA was extracted using TRIzol according to the protocol provided by the manufacturer. Total RNA (1 µg) was reverse transcribed to cDNA in a total volume of 20 μL using an RT reaction kit. Real-time PCR was performed using an Mx 3000P real-time PCR system (Applied Biosystems, Foster City, CA, USA) and SYBR Premix Ex Taq. PCR was carried out for 40 cycles at 95°C for 10 s and 60°C for 30 s. The primer sequences used for the detection of mRNA expression are shown in Table 1. All reactions were performed at least in triplicate. The levels of gene expression, relative to GAPDH, were calculated using the Stratagene Mx 3000P software.
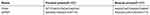 | Table 1 The primers used for the detection of mRNA expression |
Western blotting analysis
Following treatment, cells and tissues were lysed using lysis solution at 4°C for 30 mins. An equal amount of supernatant obtained from each sample was resolved in 10% SDS-PAGE, and the proteins were transferred onto polyvinylidene difluoride membranes. The membrane (Amersham) was blocked with 5% non-fat dry milk in Tris-buffered saline and Tween 20 for 1 hr at room temperature, and the proteins were probed with specific antibodies against ITGAV and GAPDH. The gels were stripped and reprobed with antibodies against GAPDH to ensure equal loading. The proteins on polyvinylidene difluoride membranes were detected through enhanced chemiluminescence.
Effect of IME in vivo
Tumors were established via intravenous injection of MG63 cells (1×105 cells/animal) into the tail of 3- to 4-week-old female BALB/c (nu/nu) mice (Beijing, China). The mice were housed under standard conditions with a 12-hr light/dark cycle and had ad libitum access to water and food. Each mouse in the IME and DDP groups received 100 μL of IME (5 mg/kg, diluted with PBS) or DDP (8 mg/kg, diluted with PBS), respectively, every 2 days through tail vein injection. Each mouse in the IME+DDP group received 100 μL of IME+DDP (5 mg/kg IME+8 mg/kg DDP, diluted with PBS) in the same manner. Finally, the control mice were injected with 100 μL of PBS. After 21 days, the mice were sacrificed, and all lungs were dissected and fixed in formalin. The investigation conformed to the Guide for the Care and Use of Laboratory Animals established by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). The Animal Research Committee at Liaoning Cancer Hospital approved all animal studies, and animal care was provided in accordance with institutional guidelines.
H&E stain
Tissues were blocked in 10% formalin and embedded in paraffin. The paraffin-embedded tissues were cut into sections (thickness: 3 μm), and these sections were de-waxed in xylene and rehydrated using a graded alcohol series. Finally, the sections were counterstained with H&E.
Statistical analysis
All data are presented as the mean ± SEof the mean. Statistical significance between the two groups was evaluated via the Student’s t-test (two-tailed) using the GraphPad Prism (GraphPad, Inc., La Jolla, CA, USA) software. A P<0.05 denoted statistical significance.
Results
Expression of ITGAV in OS
We examined the expression of ITGAV in nontumorous tissues, OS tissues, and metastatic tumors obtained from patients with OS. Western blotting and real-time PCR analyses showed that ITGAV was highly expressed in tumors, with the highest expression found in metastatic tumors (Figure 1B, C). The results showed that the expression of ITGAV was markedly higher in MG63 and U2OS cells versus hFOB1.19 cells (Figure 1D, E).
ITGAV is involved in the development of resistance to DDP
We used different concentrations of DDP to treat MG63 and U2OS cells. The results of the Western blotting and real-time PCR analyses showed that a low concentration of DDP pi re 3,j (5 μmol/L) inhibited the expression of ITGAV. However, an increase in the concentration of DDP (ie, 10 μmol/L and 15 μmol/L) was associated with an increase in the level of ITGAV. This finding indicates that ITGAV may be related to the development of resistance to DDP (Figure 2A, B). In addition, we found that silencing of ITGAV downregulates the proliferation of OS cells. Notably, low-concentration DDP (5 μmol/L) inhibits the proliferation of OS cells. Following the silencing of ITGAV, the inhibitory effect of DDP on the proliferation of OS cells was amplified (Figure 2C). The Transwell experiment demonstrated that low-concentration DDP (5 μmol/L) amplifies the decrease in cell migration induced by silencing of ITGAV (Figure 2D, E).
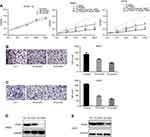
IME inhibits the proliferation and migration of OS cells
The results of the MTT assay showed that IME inhibited the proliferation of MG63 and U2OS cells in a concentration-dependent manner. However, the inhibitory effect on the proliferation of hFOB1.19 cells was limited (Figure 3A). The IC50 of IME in MG63 and U2OS cells after 24 hrs was approximately 48.96 μmol/L and 53.69 μmol/L, respectively (Table 2).
 | Table 2 The IC50 of IME in MG63 and U2OS cells |
The Transwell assay showed that IME significantly decreased the migration of MG63 and U2OS cells in a dose-dependent manner (Figure 3B, C). Western blotting and real-time PCR showed that, after exposure to IME for 24 hrs, the expression of ITGAV was drastically decreased (Figure 3D, E).
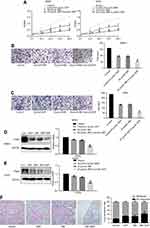
IME enhances the inhibitory effect of DDP on OS cells
The results of the MTT and Transwell assays showed that 25 μmol/L IME and 5 μmol/L DDP exhibited similar inhibitory effects on the proliferation and migration of OS cells. Moreover, the combination of IME with DDP resulted in the amplification of these inhibitory effects (Figure 4A–C). Western blotting and real-time PCR showed that both DDP and IME downregulated the expression of ITGAV, and the inhibition of ITGAV was amplified by the combination of IME with DDP (Figure 4D, E). In vivo studies have shown that IME and DDP, independently or in combination, may significantly inhibit the metastasis of OS to the lungs (Figure 4F).
Discussion
The overall treatment effects and long-term disease-free survival rate in patients with OS remain unsatisfactory. Therefore, further study of the genes involved in the development of OS is warranted to determine the occurrence, evolution, prognosis, and appropriate treatment of this disease.10,11 Active ingredients extracted from natural plants and Chinese medicines have attracted considerable attention as potential anticancer agents, owing to the following advantages: multicomponent, multiboot effect, high biological activity, favorable safety profile, and less drug resistance.12 Paclitaxel, camptothecin, vincristine, and their derivatives from plants have been widely used in clinical practice as first-line antineoplastic drugs.13 In addition, curcumin, bark hormone, oridonin, matrine, and oleanolic acid have shown strong antitumor activity in preclinical studies.14 Moreover, studies have demonstrated that IME exhibited significant antimelanoma activity in vitro and in vivo.15
The expression of ITGAV decreased in OS tissues sensitive to chemotherapy. Conversely, the expression of ITGAV did not decrease in tissues which were not sensitive to chemotherapy.16 These data indicate that the expression of ITGAV may serve as a predictor of the degree of malignancy and an indicator of the effect of chemotherapy in OS.16 This study indicates that ITGAV is highly expressed in OS and metastatic OS tissues. The expression of ITGAV in metastatic OS tissues was higher than that reported in primary OS tissues. The results showed that the expression of ITGAV was correlated with the occurrence and development of OS. Researchers found that the expression of ITGAV in OS cells was upregulated after treatment with a high concentration of DDP, suggesting the involvement of ITGAV in the development of resistance to DDP. Subsequent data indicated that the inhibition of OS cell proliferation and migration by DPP was significantly enhanced after silencing ITGAV.
Following the treatment of OS cells with different concentrations of IME, we found that IME inhibited the proliferation and migration of OS cells in a dose-dependent manner. In addition, IME downregulated the expression of ITGAV in OS cells. Subsequently, we combined IME and DDP to examine the effect on the proliferation and migration of OS cells. The results showed that the combination of IME with DDP significantly inhibited the proliferation and migration of OS cells. This finding indicates that IME may promote the therapeutic effect of DDP on OS and treat DDP-resistant OS to a certain extent.
In summary, our data showed that ITGAV may be involved in the development of resistance to DDP in OS. IME can significantly inhibit the proliferation and migration of OS cells by blocking the expression of ITGAV. The combination of IME with DDP can reduce – to a certain extent – the resistance of OS to DDP, and this effect may be achieved through the inhibition of ITGAV expression.
Ethical approval and informed consent
All patients provided written consent and approval for the use of clinical materials for research purposes, according to the institutional regulations, at the Department of Bone and Soft Tissue Surgery of Liaoning Cancer Hospital.
Consent for publication
We have obtained consent from the patients for the publication of individual patient data.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang N, Meng X, Liu Y, Chen Y, Liang Q. LPS promote osteosarcoma invasion and migration through TLR4/HOTAIR. Gene. 2019;680:1–8. doi:10.1016/j.gene.2018.09.031
2. Shao YW, Wood GA, Lu J, et al. Cross-species genomics identifies DLG2 as a tumor suppressor in osteosarcoma. Oncogene. 2019;38(2):291–298.
3. Fu Y, Zhang L, Hong Z, et al. Methanolic extract of pien tze huang induces apoptosis signaling in human osteosarcoma MG63 cells via multiple pathways. Molecules. 2016;21(3):283. doi:10.3390/molecules21030283
4. Yang X, Yang G, Li W, Zhang Y, Wang J. Therapeutic effect of ilex hainanensis Merr. Extract on essential hypertension: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2018;9:424. doi:10.3389/fphar.2018.00424
5. Zhao WW, Zan K, Wu JY, et al. Antibacterial triterpenoids from the leaves of ilex hainanensis Merr. Nat Prod Res. 2018;19:1–5.
6. Flum M, Kleemann M, Schneider H, et al. miR-217-5p induces apoptosis by directly targeting PRKCI, BAG3, ITGAV and MAPK1 in colorectal cancer cells. J Cell Commun Signal. 2018;12(2):451–466. doi:10.1007/s12079-017-0410-x
7. Linhares MM, Affonso RJ
8. Viana Lde S, Affonso RJ
9. Waisberg J, De Souza Viana L, Affonso Junior RJ, et al. Overexpression of the ITGAV gene is associated with progression and spread of colorectal cancer. Anticancer Res. 2014;34(10):5599–5607.
10. Kun-Peng Z, Chun-Lin Z, Xiao-Long M, Lei Z. Fibronectin-1 modulated by the long noncoding RNA OIP5-AS1/miR-200b-3p axis contributes to doxorubicin resistance of osteosarcoma cells. J Cell Physiol. 2019;234(5):6927–6939. doi:10.1002/jcp.27435
11. Jie Z, Xie Z, Zhao X, et al. Glabridin inhibits osteosarcoma migration and invasion via blocking the p38- and JNK-mediated CREB-AP1 complexes formation. J Cell Physiol. 2019;234(4):4167–4178. doi:10.1002/jcp.27171
12. Wang H, Zhou JW, Fu DH, Zhou Y, Cheng WZ, Liu Z-L. Gynura procumbens ethanolic extract suppresses osteosarcoma cell proliferation and metastasis in vitro. Oncol Lett. 2013;6(1):113–117. doi:10.3892/ol.2013.1315
13. Yang H, Liu C, Zhang YQ, et al. Ilexgenin A induces B16-F10 melanoma cell G1/S arrest in vitro and reduces tumor growth in vivo. Int Immunopharmacol. 2015;24(2):423–431. doi:10.1016/j.intimp.2014.12.040
14. Chang JL, Wang WY, Li YM, et al. Chinese herbal medicine for osteosarcoma in the mouse: a systematic review and meta-analysis. Chin J Integr Med. 2018. doi:10.1007/s11655-018-2565-6
15. Zhang YQ, Yang H, Sun WD, et al. Ethanol extract of ilex hainanensis Merr. Exhibits anti-melanoma activity by induction of G1/S cell-cycle arrest and apoptosis. Chin J Integr Med. 2018;24(1):47–55. doi:10.1007/s11655-017-2544-8
16. Luo Z, Li D, Luo X, et al. Decreased expression of miR-548c-3p in osteosarcoma contributes to cell proliferation via targeting ITGAV. Cancer Biother Radiopharm. 2016;31(5):153–158. doi:10.1089/cbr.2016.1995
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.