Back to Journals » OncoTargets and Therapy » Volume 12
IL-37 promotes cell apoptosis in cervical cancer involving Bim upregulation
Authors Ouyang P, An W, Chen R, Zhang H, Chen D, Jiang E, Zhu W, Li P, Guo H, Chen Z, Wang S
Received 15 January 2019
Accepted for publication 21 March 2019
Published 10 April 2019 Volume 2019:12 Pages 2703—2712
DOI https://doi.org/10.2147/OTT.S201664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Ping Ouyang,1,* Weifang An,1,2,* Renhuai Chen,1,3,* He Zhang,1 Danrui Chen,1 Enping Jiang,1,4 Wei Zhu,1,4 Peng Li,4 Hongsheng Guo,4 Zhangquan Chen,1 Sen Wang1,4
1Guangdong Provincial Key Laboratory of Medical Molecular Diagnostics, Dongguan Scientific Research Center, Guangdong Medical University, Dongguan, Guangdong Province 523808, People’s Republic of China; 2Pathology Department, Shenzhen Longhua District Central Hospital, Shenzhen, Guangdong Province, 518110, People’s Republic of China; 3Pathology Department, Dongguan Tungwah Hospital, Dongguan, Guangdong Province, 523110, People’s Republic of China; 4Basic Medicine Department, Guangdong Medical University, Dongguan, Guangdong Province 523808, People’s Republic of China
*These authors contributed equally to this work
Background: Growing evidence has indicated that interleukin-37 (IL-37) is a potential anticancer molecule that mainly plays an inhibiting role in different kinds of cancers, but data for the role of IL-37 on cell apoptosis in cancers remains rare. The present study aimed to explore the role of IL-37 in cell apoptosis in cervical cancer, and the involved apoptosis-related molecules.
Methods: IL-37 was overexpressed by transfecting the pIRES2-EGFP-IL-37 plasmid in HeLa and C33A cells. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed to detect the mRNA expression of IL-37, Bcl-2, Bax and Bim. Western blotting was performed to detect the protein expression of IL-37 and Bim. Cell apoptosis was detected by flow cytometry.
Results: IL-37 upregulated the mRNA expression levels of Bim by 138.40% for HeLa (P<0.05) and 58.95% for C33A (P<0.05), and increased the protein expression levels of BimL by 69.10% (P<0.05) and 56.66% (P<0.05) in HeLa and C33A, respectively. Overexpression of IL-37 increased the apoptosis rates by 152.86% for HeLa (P<0.01) and 25.4% for C33A (P<0.05). Knockdown of Bim by specific siRNA interference fragments (SiBim) reduced the apoptosis rates by 36.00% for HeLa (P<0.05) and 14.66% for C33A (P<0.05). Compared with the IL-37 overexpression group, the apoptosis rate in cotransfecting the IL-37 overexpression plasmid and SiBim group decreased by approximately 31% (P<0.05) and 24.35% (P<0.05) in HeLa and C33A, respectively.
Conclusion: IL-37 upregulated Bim in cervical cancer cells. Furthermore, IL-37 can promote cervical cancer cell apoptosis, but Bim knockdown decreased this promotion through IL-37. Thus, IL-37 can promote cervical cancer cell apoptosis, which involve the upregulation of Bim.
Keywords: IL-37, Bim, cervical cancer, apoptosis
Introduction
Cervical cancer (CC) is the fourth most common cancer in both incidence and mortality for women, with approximately 570,000 cases and 311,000 deaths in 2018 worldwide.1 Furthermore, approximately 95% of CC cases are caused by persistent human papillomavirus (HPV) infection and related long-term chronic inflammation.2 This fact indicates the important role of inflammation in the development of CC. Hence, the anti-inflammatory approach might be a possible way to treat CC.
Interleukin-37 (IL-37; formerly IL-1 family member 7) is recently identified as an anti-inflammatory cytokine.3 IL-37 contains six exons that encode five splicing subtypes, which are named as IL-37a to IL-37e. Among these, IL-37b has exons 1, 2, 4 and 6, which encodes a protein of approximately 25 kDa.4 Furthermore, IL-37b possesses the most complex biological functions, and is the mostly researched isoform.5,6 IL-37 can be detected in many human tissues, such as lung, thymus, bone marrow and uterus tissues, but the expression of different isoforms is tissue-specific.4 The level of IL-37 is normally low, but this significantly increases under severe inflammatory conditions. Thus, IL-37 plays an important regulatory role in the development of a variety of inflammatory and autoimmune diseases.7–12 Increasing evidence suggests that IL-37 may be an anti-cancer molecule due to its inhibiting role in different kinds of cancers.3,13–24 For example, IL-37 can inhibit cell invasion and metastasis through the IL-6/STAT3 signaling pathway in non-small cell lung cancer.15 Furthermore, IL-37 can also suppress the proliferation, migration and invasion of human lung adenocarcinoma A549 cells.16 In addition, IL-37 can inhibit colon cancer development through beta-catenin suppression, and function as a novel prognostic indicator and potential therapeutic target.25 In lung cancer, Li et al reported that IL-37 suppressed tumor metastasis by inhibiting Rac1 activation.26 In hepatocellular carcinoma cells, Li et al reported that IL-37 induced autophagy by inhibiting the PI3K/AKT/mTOR pathway.17 In our previous study, it was found that IL-37 inhibited STAT3 expression, and suppressed cell proliferation and invasion in CC cells.3 Cell apoptosis is regarded as a tumor inhibitory mechanism, however, to our knowledge, the effect of IL-37 on tumor cell apoptosis has rarely been researched in recent studies. Hence, the present study discussed the role of IL-37 in the cell apoptosis of CC.
B-cell lymphoma 2 family (Bcl-2 family) proteins contain at least one of the four Bcl-2 homology domains, and contribute to programmed cell death or apoptosis. Some of the members (Bcl-2, Bcl-XL and Mcl-1) are anti-apoptotic, while the others (Bim, Bax, Bak, and Bok) are pro-apoptotic.27 Recently, Bcl-2 family proteins have been identified to play roles in many apoptotic-related diseases, including cancer. Moreover, there are some clues showed the relationship between IL-37 and the Bcl-2 family members. In SMMC-7721 cells, IL-37 induced cell apoptosis and increased Bax, LC3 and beclin 1 levels, but decreased Bcl-2 levels.28 In renal cell carcinoma, IL-37 promoted cancer cell apoptosis and decreased the expression of IL-6, pSTAT3, Bcl-2, and cyclin D1.23 IL-37 has also been found to reduce cell death and increase Bcl-2 levels in hepatocytes.29 Among the Bcl-2 family members, Bim is a well-known pro-apoptotic molecule of 198 amino acids. Previous studies have shown that loss of Bim renders lymphocytes more resistant to diverse apoptotic stimuli.30 Bim takes part in the apoptosis of many tissues, including cardiomyocytes,31 as well as neurons and lymphocytes.32 Importantly, Bim has been reported to be a crucial tumor suppressor gene in various cancer types, such as lung cancer,33,34 breast cancer,35 neck squamous cell carcinomas,36 and hematopoietic tumor cells.37 In our preliminary experiment, it was found that Bim could promote the cell apoptosis of CC cell lines HeLa and C33A. Thus, the present study aimed at exploring the apoptosis role of IL-37 in HeLa and C33A cells, and the relationship between IL-37 and Bim.
Materials and methods
Cell lines, plasmids and transfection
Two CC cell lines, HPV(+) HeLa and HPV(-) C33A used in present study were purchased from Cell bank of typical culture preservation Committee of Chinese Academy of Sciences (Shanghai, China). Cells were maintained in DMEM medium containing 10% fetal bovine serum (FBS, Invitrogen), supplemented with 2.0 mmol/L of L-glutamine, 50 U of penicillin and 50 µg/ml of streptomycin.3 All cells were routinely cultured in a 5% CO2 atmosphere at 37 °C. The empty vector pIRES2-EGFP was purchased from Shanghai Geneary Biotech Co., Ltd (Shanghai, China). The expression plasmid pIRES2-EGFP/IL-37 was constructed based on IL-37 (NM_014439), which could express IL-37 protein in eukaryotic cells. This plasmid was stored in our laboratory as described before.3 Plasmids pcDNA3.1 and pcDNA3.1/IL-37 used for flowcytometry experiment was a gift from Professor Zhangquan Chen (Guangdong Medical University, Guangdong, China).
For transfection, cells were plated onto 6-well plates or 24-well plates (Corning, NPY) at a density of 2.5×105 cells per 6-well or 5×104 cells per 24-well, respectively. When the cells were grown to a confluence of 50–70%, it was ready for transfection. The cells were transfected with HilyMax reagent (Dojindo, Shanghai, China) in serum- and antibiotic-free Opti-MEM (Gibco-BRL, USA), according to manufacturer’s instructions. The HilyMax (μl)-to-DNA (μg) rate was 15: 3.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was performed to detect the mRNA expression levels of IL-37, Bim, Bcl-2, Bax and GAPDH. Total RNAs were extracted from cells using TRIzol (Invitrogen, Carlsbad, CA, USA), according to manufacturer’s instructions. Then, the RNA was reversely transcripted to DNA through the following reactions: 2 μl of 5×PrimeScript RT Master Mix, 500 ng of total RNA, and RNase-free dH2O was added to reach a volume of 10 μl. The real-time PCR was performed on Prism 7500 (ABI, Foster City, CA, USA) using a standard SYBR green assay protocol. The primers were as follows: IL-37-F: 5ʹ-GATCACAAAGTACTGGTCCTGG-3ʹ, IL-37-R: 5ʹ-TCCTTTATCCTTGTCACAGTAG-3ʹ; Bim-F: 5ʹ-CAGACAGGAGCCCAGCACC-3ʹ, Bim-R: 5ʹ-TCCAATACGCCGCAACTCTT-3ʹ; Bcl-2-F: 5ʹ-ATGTGTGTGGAGAGCGTCAA-3ʹ, Bcl-2-R: 5ʹ-ACAGTTCCACAAAGGCATCC-3ʹ; Bax-F: 5ʹ-ACAGACAGGAGCCCAGCACC-3ʹ, Bax-R: 5ʹ-CAGTTTGCTGGCAAAGTAGAAAAG-3ʹ. GAPDH was used as the normal control, and the primers were 5ʹ-TGACTTCAACAGCGACACCCA-3ʹ (forward) and 5ʹ-CACCCTGTTGCTGTAGCCAAA-3ʹ (reverse). Quantitive PCR (qPCR) was performed on the 7500 Real-Time PCR ABI system (ABI, USA) using a 96-well plate format. PCR was performed in triplicate in a 20 μl reaction volume containing 10 μl of 2×FastStart Universal SYBR Green Master (ROX; Invitrogen, Guangzhou, China), 0.8 μl of forward primer (10 μM), 0.8 μl of reverse primer (10 μM), 1 μl of cDNA and 7.4 μl of RNase-free dH2O. The reaction conditions were 95 °C for 10 mins, 40 cycles of 95 °C for 15 seconds, and 60 °C for one minute. Data were analyzed using the sequence detection software version 1.6.3 supplied by Applied Biosystems (ABI, USA). The relative mRNA expression levels of IL-37 or Bim were calculated and normalized using the 2−ΔΔCt method relative to GAPDH.38
Western blotting
Cells were lysed in RIPA buffer (Beyotime, Shanghai, China), and the lysates were collected into tubes and centrifuged at 12,000 g for 20 mins at 4 °C. Then, the supernatants were collected, and the protein concentration was quantified using a BCA Protein Quantitative Kit (Beyotime, Shanghai, China). Western blotting was performed according to the manual. An equal amount of protein (20 μg) was separated on 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, MA, USA), which were blocked with 5% non-fat milk for one hour and incubated overnight at 4 °C with rabbit anti-human antibodies against IL-37 (Abcam, Cambridge, MA, USA, ab116282; diluted at 1:1,000), Bim (CST; diluted at 1:1,000), and GAPDH (Goodhere Technology, Hangzhou, China; AB-P-R001; diluted at 1:500), respectively. Then, this was followed by incubation with goat anti-rabbit HRP-conjugated secondary antibody (Abcam, Cambridge, MA, USA, ab116282; diluted at 1:1,000) for one hour, and detection by ECL. The reactive bands were revealed using an Immobilon Western Chemiluminescent kit (WBKLS0100; Millipore, USA) through the Roche Cobas e601 automated chemiluminescence image analysis system (Roche, USA).
Cell apoptosis assay
The combination of Annexin-V-FITC and propidium iodide (PI) staining methods was applied on cells for the following groups: (a) control group, transfection of control palsmid; (b) IL-37 group, transfection IL-37; (c) SiBim group, transfection of siRNA fragments against Bim (SiBim); (d) IL-37+SiBim group, co-transfection IL-37 and SiBim. Cells (5×104/500 μl of medium) were seeded onto 24-well culture plates. After transfecting for 48 hrs, these cells were trypsinized with EDTA-free typsine, washed, and resuspended in 500 μl of binding buffer with 5 μl of Annexin-VFITC and 5 μl of PI (Sigma, USA). Then, these cells were incubated for 5–15 mins at room temperature in the dark. Afterwards, the number of cells was immediately read by flow cytometry (Beckman Coulter). The proportion of apoptotic cells (Annexin-V positive cells) were presented as mean ± standard deviation (SD). There were four controls: empty control, Annexin V-FITC staining control, PI staining control, and Annexin V-FITC/PI double staining control. Each group contained three parallels, and the experiments were performed in triplicate.
Statistical analysis
The statistical analysis was performed using SPSS 15.0 (
Results
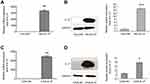
IL-37 was successfully overexpressed in HeLa and C33A cells
In the present study, the eukaryotic plasmid pIRES2-EGFP-IL-37 was used to overexpress IL-37 in HeLa and C33A cells, respectively. As a result, in HeLa cells, the mRNA expression level of IL-37 increased by 3,328.35% (P<0.01, Figure 1A), while the protein expression level of IL-37 increased by 1,043.9% (P<0.001, Figure 1B), when compared to the control group. In C33A cells, the mRNA expression level of IL-37 increased by 2,424.88% (P<0.01, Figure 1C), while the protein expression level of IL-37 increased by 210.1% (P<0.05, Figure 1D), when compared to the control group. These results suggest that transfection of pIRES2-EGFP-IL-37 in HeLa and C33A cells can successfully express IL-37 protein.
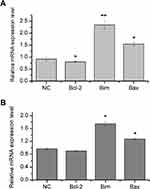
Expression changes of apoptosis-related genes induced by IL-37
In order to detect the influence of IL-37 on apoptosis-related genes, the mRNA expression levels of Bcl-2, Bim and Bax were analyzed by RT-qPCR (Figure 2). These results revealed that the overexpression of IL-37 exhibited no obvious regulation on the Bcl-2 gene (22.2% for Hela cells and 7.9% for C33A cells). However, the mRNA expression levels of Bim and Bax were significantly upregulated by IL-37 in HeLa and C33A cells. Bim level increased to be 239.4% in Hela cells and 169.3% in C33A cells, while Bax level increased to be 151.2% in Hela cells and 125.6% in C33A cells. Among these three genes, the mRNA expression level change of Bim was the most obvious. Thus, Bim was chosen for the subsequent experiments.
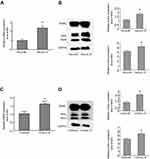
IL-37 upregulated the expression of Bim in HeLa and C33A cells
In order to test the relationship between IL-37 and Bim, IL-37 was first overexpressed using pIRES2-EGFP-IL-37 in HeLa and C33A. Then, the mRNA and protein expression levels of Bim were detected. Compared with the control, the mRNA expression levels of Bim increased by 138.4% (P<0.05) and 58.95% (P<0.05) in HeLa and C33A, respectively (Figure 3A and C), while the protein expression levels of BimL increased by 69.10% (P<0.05) and 56.66% (P<0.05) in HeLa and C33A, respectively. Furthermore, the total protein of Bim increased by 25.52% (P<0.05) and 31.55% (P<0.05) in HeLa and C33A, respectively (Figure 3B and D).
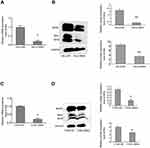
Bim expression was knocked down by siRNA in HeLa and C33A cells
In order to investiage the effects of Bim on the apoptosis of HeLa and C33A cells, the RNA interference method was applied using specific siRNA fragments against Bim (SiBim). Bim was knocked down with SiBim in HeLa and C33A cells. Then, the mRNA and protein expression levels of Bim were counted by RT-qPCR and western blotting, respectively.
In HeLa cells, the mRNA expression level of Bim in the SiBim group decreased by 80.3% (P<0.05, Figure 4A), protein expression level of BimL decreased by 84.00% (P<0.01), and the total protein of Bim decreased by 60.57% (P<0.01, Figure 4B), when compared to the control group. In C33A cells, the mRNA expression level of Bim decreased by 78.00% (P<0.05, Figure 4C), the protein expression level of BimL decreased by 66.55% (P<0.05), and the total protein of Bim decreased by 37.11% (P<0.05, Figure 4D), when compared to the control group. These results suggest that the siRNA fragments against Bim were efficient in both cervical cancer cell lines.
Effects of IL-37 and Bim on cell apoptosis
IL-37 can regulate the expression of Bim, which is a crucial pro-apoptotic factor in both HeLa and C33A cells, but their combination roles on the apoptosis of these two cells remain unclear. In the present study, cells were assigned into four groups: (a) normal control (NC) group, transfection control plasmid; (b) IL-37 group, transfection IL-37; (c) SiBim group, transfection of SiBim; (d) IL-37+SiBim group, co-transfection IL-37 and SiBim. The following were the four controls: empty control, Annexin V-FITC staining control, PI staining control, and Annexin V-FITC/PI double staining control.
In HeLa cells, the cell apoptosis rates were 16.56%, 42.00% and 10.56% in the HeLa-NC, HeLa-IL-37 and HeLa-SiBim groups, respectively (Figure 5A–C), when compared to controls. Compared with the HeLa-NC group, the apoptosis rate increased by 152.86% (P<0.01) in the HeLa-IL-37 group, while the apoptosis rate decreased by 36% (P<0.05) in the HeLa-SiBim group. Compared with the HeLa-IL-37 group, the apoptosis rate of HeLa-IL-37+SiBim decreased by approximately 31% (P<0.05). In C33A cells, the cell apoptosis rates were 30.35%, 38.06% and 25.9% in the C33A-NC, C33A-IL-37 and C33A-SiBim groups, respectively (Figure 5F–H), when compared to controls. Compared with the C33A-NC group, the apoptosis rate increased by 25.4% (P<0.05) in C33A-IL-37 group, while the apoptosis rate decreased by 14.66% (P<0.05) in the C33A-SiBim group. Compared to the C33A-IL-37 group, the apoptosis rate decreased by approximately 24.35% (P<0.05) in C33A-IL-37+SiBim group. The results in both cell lines were consistent with each other and indicated IL-37 could efficiently promote apoptosis in CC cells. Furthermore, Bim inhibition could antagonize the apoptotic role of IL-37, which suggests that Bim is involved in the IL-37-induced apoptosis in cervical cancer cells.
Discussion
IL-37 is concerned for its role in inflammation, and innate and adaptive immunity science, when it was renamed in 2010. Recent studies have begun to report its role in cancer. It has been previously found that IL-37 could suppress CC cell proliferation through STAT3. However, the potential role of IL-37 in the cell apoptosis of CC is rarely known. In the present study, it was found that IL-37 promoted cell apoptosis in two CC cell lines. IL-37 could upregulate the expression of pro-apoptotic factor Bim, while the inhibition of Bim can attenuate the apoptosis induced by IL-37.
In the present study, two CC cell lines, HPV(+) HeLa and HPV(-) C33A, were used. The normal expression of IL-37 was very low in both cell lines. The transfection of the IL-37 plasmid led to the 10-fold upregulation of IL-37 mRNA expression in HeLa cells and 2-fold upregulation for C33A cells. This expression difference may be due to the HPV infection, which might suggest that IL-37 may exert a higher anti-cancer efficiency in HPV (+), when compared to that in HPV(-) cervical cancer cells. This result is consistent with previous studies. For example, it has been reported that Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid) has more anti-tumor activity in different HPV cervical cancer cell types, when compared to HPV(-) cells.39
IL-37 can efficiently promote cell apoptosis in HeLa cells, as well as in C33A cells. The anti-tumor role of IL-37 in different cancer types has been reported by inhibiting tumor cell proliferation, migration and invasion.14,15 However, the effect of IL-37 on tumor cell apoptosis is limited. Jiang et al reported that IL-37 markedly inhibited the migration and proliferation, and promoted the apoptosis of renal cell carcinoma cells.23 The investigators previously reported that IL-37 could suppress cell proliferation and invasion in CC cells.3 In the present, it was further revealed that IL-37 can promote cell apoptosis in cervical cancer cells, which was consistent with the results reported by Jiang.23 Compared with the control group, the apoptosis rate increased by 152.86% and 25.4% in HeLa and C33A, respectively, in the IL-37 group. Hence, it appears that HeLa is more sensitive to IL-37 than C33A.
The influence of IL-37 was first analyzed on three Bcl-2 family members, Bim, Bax and Bcl-2. Among these, Bim and Bax are pro-apoptotic, while Bcl-2 is anti-apoptotic. The results revealed that IL-37 can significantly upregulate the mRNA expression levels of Bim and Bax in both cell lines, but significantly inhibit Bcl-2 in HeLa (Figure 2). This result was consistent with previous studies. It has been reported that in SMMC-7721 cells, renal cell carcinoma cells and hepatocytes, IL-37 could promote cell apoptosis through either increasing Bax or decreasing Bcl-2.28 In addition, the Bcl-2 family members are involved in many other uterus diseases. For example, Bcl-2 is involved in the apoptosis regulation of endometriosis, which is characterized by insufficient apoptosis.40,41 Since IL-37 can regulate some Bcl-2 family members, it seemed that IL-37 might also play a role in those pathological processes through Bim or other Bcl-2 protein, which shows IL-37’s broad application prospect in gynecological diseases.
In this study, we found the apoptotic role of IL-37 in CC cell lines may be at least partially through Bim. The results in this study showed overexpression of IL-37 can upregulate Bim in CC cells. Since the normal expression levels of IL-37 in both CC cells were low, we didn’t check the expression change of Bim under the condition of IL-37 knock down. IL-37 increased the apoptosis rate, while SiBim attenuated this phenotype in both CC cell lines. Moreover, compared with the overexpression IL-37 group, the apoptosis rates of the IL-37+SiBim group reduced 31% and 24.35% in HeLa and C33A, respectively. This result suggests that other factors may have taken part in the IL-37-induced apoptosis, but it was confirmed that Bim was one of these. Thus, it was speculated that IL-37 could increase the expression of Bim and other factors, and trigger the apoptosis process. To our knowledge, the relationship between IL-37 and Bim has been less reported. However, it is not surprise that many proteins carry out their apoptotic effects through Bim. For example, the 26S proteasome-associated PAD1 homolog 1 (POH1)-induced tumor cell apoptosis via Bim and the ability of TMEM16A/ANO1 to inhibit apoptosis by downregulating the expression of Bim.40 In the present study, it was revealed that Bim was also involved in the IL-37 induced apoptosis process.
In addition, there are many markers have been reported for early CC dignosis or differentiating carcinoma-in-situ (CIS) from microinvasive cancer of CC.41,42 Some markers such as p16ink4a, p16, E-cadherin, Ki67, pRb and p53 were showed to be able to detect early cervical lesions have more chance to evolve to invasive forms. Other markers such as CEA, SCC-Ag and CD44 have been developed to detect invasive forms.43 Moreover, PDL1 was the newly reported marker and would be useful in differentiating CIS from microinvasive cancer in CC.44 A study also suggest IL-6 and IL-37 may serve as biomarkers for the diagnosis of endometriosis.45 However, there are no reports suggesting IL-37 might be a biomarker in CC. In future studies, the relationships among IL-37 and these markers should be investigated to help offer better diagnosis and treatment for CC patients.
In summary, the present study revealed that IL-37 could induce apoptosis in CC cells, which involved the upregulation of Bim (Figure 6). The results expanded our knowledge on the role of IL-37 in cell apoptosis, and also suggested that IL-37 may be a potential drug for CC treatment in the future.
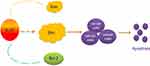 | Figure 6 IL-37 promoted cell apoptosis of cervical cancer which involved Bim. |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Koh W-J, Greer BE, Abu-Rustum NR, et al. Cervical cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13(4):395–404.
3. Wang S, An W, Yao Y, et al. Interleukin 37 expression inhibits STAT3 to suppress the proliferation and invasion of human cervical cancer cells. J Cancer. 2015;6(10):962–969. doi:10.7150/jca.12266
4. Jia H, Liu J, Han B. Reviews of Interleukin-37: functions, receptors, and roles in diseases. Biomed Res Int. 2018;2018:3058640. doi:10.1155/2018/3058640
5. Pu XY, Zheng DF, Shen A, et al. IL-37b suppresses epithelial mesenchymal transition in hepatocellular carcinoma by inhibiting IL-6/STAT3 signaling. Hepatobiliary Pancreat Dis Int. 2018;17(5):408–415. doi:10.1016/j.hbpd.2018.08.009
6. Luo C, Shu Y, Luo J, et al. Intracellular IL-37b interacts with Smad3 to suppress multiple signaling pathways and the metastatic phenotype of tumor cells. Oncogene. 2017;36(20):2889–2899. doi:10.1038/onc.2016.444
7. Chen B, Huang K, Ye L, et al. Interleukin-37 is increased in ankylosing spondylitis patients and associated with disease activity. J Transl Med. 2015;13:36. doi:10.1186/s12967-015-0541-x
8. Zhuang X, Wu B, Li J, Shi H, Jin B, Luo X. The emerging role of interleukin-37 in cardiovascular diseases. Immun Inflamm Dis. 2017;5(3):373–379. doi:10.1002/iid3.159
9. Shou X, Lin J, Xie C, Wang Y, Sun C. Plasma IL-37 elevated in patients with chronic heart failure and predicted major adverse cardiac events: a 1-year follow-up study. Dis Markers. 2017;2017:1–6. doi:10.1155/2017/9134079
10. Pfeiler S, Winkels H, Kelm M, Gerdes N. IL-1 family cytokines in cardiovascular disease. Cytokine. 2017. doi:10.1016/j.cyto.2017.11.009
11. Cavalli G, Dinarello CA. Suppression of inflammation and acquired immunity by IL-37. Immunol Rev. 2018;281(1):179–190. doi:10.1111/imr.12605
12. Liu H, Zheng R, Wang P, et al. IL-37 confers protection against mycobacterial infection involving suppressing inflammation and modulating T cell activation. PLoS One. 2017;12(1):e0169922. doi:10.1371/journal.pone.0169922
13. Zhu B, Luo J, Jiang Y, Yu L, Liu M, Fu J. Prognostic significance of nomograms integrating IL-37 expression, neutrophil level, and MMR status in patients with colorectal cancer. Cancer Med. 2018;7(8):3682–3694. doi:10.1002/cam4.1663
14. Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;281(1):57–61. doi:10.1111/imr.12614
15. Jiang M, Wang Y, Zhang H, et al. IL-37 inhibits invasion and metastasis in non-small cell lung cancer by suppressing the IL-6/STAT3 signaling pathway. Thorac Cancer. 2018;9(5):621–629. doi:10.1111/1759-7714.12628
16. Chen YH, Zhou BY, Wu GC, et al. Effects of exogenous IL-37 on the biological characteristics of human lung adenocarcinoma A549 cells and the chemotaxis of regulatory T cells. Cancer Biomark. 2018;21(3):661–673. doi:10.3233/CBM-170732
17. Li TT, Zhu D, Mou T, et al. IL-37 induces autophagy in hepatocellular carcinoma cells by inhibiting the PI3K/AKT/mTOR pathway. Mol Immunol. 2017;87:132–140. doi:10.1016/j.molimm.2017.04.010
18. Huo J, Hu J, Liu G, Cui Y, Ju Y. Elevated serum interleukin-37 level is a predictive biomarker of poor prognosis in epithelial ovarian cancer patients. Arch Gynecol Obstet. 2017;295(2):459–465. doi:10.1007/s00404-016-4258-8
19. Ding VA, Zhu Z, Steele TA, et al. The novel role of IL-37 in prostate cancer: evidence as a promising radiosensitizer. Med Oncol. 2017;35(1):6. doi:10.1007/s12032-017-1070-7
20. Abulkhir A, Samarani S, Amre D, et al. A protective role of IL-37 in cancer: a new hope for cancer patients. J Leukocyte Biol. 2017;101(2):395–406. doi:10.1189/jlb.5RU0816-341R
21. Ding VA, Zhu Z, Xiao H, Wakefield MR, Bai Q, Fang Y. The role of IL-37 in cancer. Med Oncol. 2016;33(7):68. doi:10.1007/s12032-016-0782-4
22. Wang WQ, Zhao D, Zhou YS, et al. Transfer of the IL-37b gene elicits anti-tumor responses in mice bearing 4T1 breast cancer. Acta Pharmacol Sin. 2015;36(4):528–534. doi:10.1038/aps.2015.3
23. Jiang Y, Wang Y, Liang L, et al. IL-37 mediates the antitumor activity in renal cell carcinoma. Med Oncol. 2015;32(11):250. doi:10.1007/s12032-015-0695-7
24. Zhao JJ, Pan QZ, Pan K, et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Sci Rep. 2014;4:5177. doi:10.1038/srep05177
25. Yan X, Zhao J, Zhang R. Interleukin-37 mediates the antitumor activity in colon cancer through beta-catenin suppression. Oncotarget. 2017;8(30):49064–49075. doi:10.18632/oncotarget.17093
26. Li Y, Zhao M, Guo C, et al. Intracellular mature IL-37 suppresses tumor metastasis via inhibiting Rac1 activation. Oncogene. 2017;37(8):1095–1106. doi:10.1038/onc.2017.405
27. Zhao M, Zhang Y, Li J, et al. Histone deacetylation, as opposed to promoter methylation, results in epigenetic BIM silencing and resistance to EGFR TKI in NSCLC. Oncol Lett. 2018;15(1):1089–1096. doi:10.3892/ol.2017.7411
28. Li T, Zhu D, Mou T, et al. [Interleukin-37 induces apoptosis and autophagy of SMMC-7721 cells by inhibiting phosphorylation of mTOR]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;3(4):440–445.
29. Sakai N, Van Sweringen HL, Belizaire RM, et al. Interleukin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27(10):1609–1616. doi:10.1111/j.1440-1746.2012.07187.x
30. Kelly PN, White MJ, Goschnick MW, et al. Individual and overlapping roles of BH3-only proteins Bim and Bad in apoptosis of lymphocytes and platelets and in suppression of thymic lymphoma development. Cell Death Differ. 2010;17(10):1655–1664. doi:10.1038/cdd.2010.43
31. Xu Y, Xing Y, Xu Y, et al. Pim-2 protects H9c2 cardiomyocytes from hypoxia/reoxygenation-induced apoptosis via downregulation of Bim expression. Environ Toxicol Phar. 2016;48:94–102. doi:10.1016/j.etap.2016.10.011
32. Akhter R, Saleem S, Saha A, Biswas SC. The pro-apoptotic protein Bmf co-operates with Bim and Puma in neuron death induced by beta-amyloid or NGF deprivation. Mol Cell Neurosci. 2018;88:249–257. doi:10.1016/j.mcn.2018.02.011
33. Yuan J, Li B, Zhang N, et al. Clinical implications of the BIM deletion polymorphism in advanced lung adenocarcinoma treated with Gefitinib. Clin Lung Cancer. 2018;19(4):e431–e438. doi:10.1016/j.cllc.2018.02.007
34. Wu D, Chen B, Cui F, et al. Hypoxia-induced microRNA-301b regulates apoptosis by targeting Bim in lung cancer. Cell Proliferat. 2016;49(4):476–483. doi:10.1111/cpr.12264
35. Hoshino Y, Katsuno Y, Ehata S, Miyazono K. Autocrine TGF-beta protects breast cancer cells from apoptosis through reduction of BH3-only protein Bim. J Biochem. 2011;149(1):55–65. doi:10.1093/jb/mvq114
36. Godse NR, Khan N, Yochum ZA, et al. TMEM16A/ANO1 inhibits apoptosis via downregulation of Bim expression. Clin Cancer Res. 2017;23(23):7324–7332. doi:10.1158/1078-0432.CCR-17-1561
37. Fujiwara D, Tsubaki M, Takeda T, et al. Statins induce apoptosis through inhibition of Ras signaling pathways and enhancement of Bim and p27 expression in human hematopoietic tumor cells. Tumor Biol. 2017;39(10):1010428317734947. doi:10.1177/1010428317734947
38. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
39. Souza RP, de Souza Bonfim-Mendonca P, Damke G, et al. Artepillin C induces selective oxidative stress and inhibits migration and invasion in a comprehensive panel of human cervical cancer cell lines. Anticancer Agents Med Chem. 2018;18(12):1750–1760. doi:10.2174/1871520618666180604092930
40. Wang CH, Lu SX, Liu LL, et al. POH1 knockdown induces cancer cell apoptosis via p53 and Bim. Neoplasia. 2018;20(5):411–424. doi:10.1016/j.neo.2018.02.005
41. Vetvicka V, Laganà AS, Salmeri FM, et al. Regulation of apoptotic pathways during endometriosis: from the molecular basis to the future perspectives. Arch Gynecol Obstet. 2016;294(5):897–904. doi:10.1007/s00404-016-4195-6
42. Laganà AS, Vitale SG, Salmeri FM, et al. Unus pro omnibus, omnes pro uno: a novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses. 2017;103:10–20. doi:10.1016/j.mehy.2017.03.032
43. Valenti G, Vitale SG, Tropea A, Biondi A, Laganà AS. Tumor markers of uterine cervical cancer: a new scenario to guide surgical practice? Updates Surg. 2017;69(4):441–449. doi:10.1007/s13304-017-0491-3
44. Nicol AF, de Andrade CV, Gomes SC
45. Jiang J, Jiang Z, Xue M. Serum and peritoneal fluid levels of interleukin-6 and interleukin-37 as biomarkers for endometriosis. Gynecol Endocrinol. 2019;11:1–5. doi:10.1080/09513590.2018.1554034
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.