Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 5
IL-15 as a potential target in leukemia
Authors Xiong Y, Bensoussan D, Decot V
Received 29 November 2014
Accepted for publication 28 January 2015
Published 9 April 2015 Volume 2015:5 Pages 55—63
DOI https://doi.org/10.2147/BLCTT.S78347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor David Dingli
Video abstract presented by Yu Xiong
Views: 525
Yu Xiong,1,2 Danièle Bensoussan,1,3 Véronique Decot1,3
1Cell Therapy Department, University Hospital of Nancy, Vandoeuvre les Nancy, France; 2Institute of Hepatobiliary Diseases, Transplant Center, Wuhan University, Hubei Key Laboratory of Medical Technology on Transplantation, Zhongnan Hospital of Wuhan University, Wuhan, People's Republic of China; 3UMR CNRS UL 7365, Lorraine University, Vandoeuvre les Nancy, France
Abstract: Leukemia, one of the most aggressive hematopoietic malignancies, is characterized by excessive proliferation, survival, and impaired differentiation of hematopoietic stem cells. Interleukin 15, a proinflammatory cytokine, induces proliferation and promotes cell survival of human T and B lymphocytes, as well as natural killer cells. However, it may also play a detrimental role in the onset of leukemia. This review provided an overview of the aberrant expression of Interleukin 15 and its role in the development and progression of this hematological malignancy. Also, we critically explored the potential therapeutic opportunities involved in targeting the disruption of interleukin-15 signaling as well as in interleukin-15-mediated enhancement of antitumor immunity.
Keywords: leukemia, interleukin-15, immunotherapy
Leukemia
Leukemia is a complex and heterogeneous group of diseases and is characterized as the abnormal proliferation of immature cells of the hematopoietic system. The most widely accepted classification system is the World Health Organization system, which encompasses etiologic, morphologic, immunophenotypic, clinical, genetic, and molecular features and incorporates scientific and clinical information to refine diagnostic criteria. Among the most common forms of leukemia are two acute variants, acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), and two chronic variants, chronic myeloid leukemia (CML) and chronic lymphoblastic leukemia (CLL).1
Current treatment options and patient outcomes
AML is characterized by the inhibition of differentiation and the subsequent accumulation of hematopoietic stem cells at various stages of incomplete maturation, and by reduced production of healthy hematopoietic elements.2 AML is a heterogeneous disease that can be classified in as many as seven subtypes.3 These subtypes are characterized by a variety of cytogenetic and cell surface markers. The standard treatment for AML is induction chemotherapy with an anthracycline/cytarabine combination, followed by either consolidation chemotherapy (commonly high-dose cytarabine) or allogeneic stem cell transplantation, depending on the patient’s ability to tolerate intensive treatment and the likelihood of cure with chemotherapy alone.4 The goal of induction chemotherapy is to achieve complete remission (CR). Once this is achieved, consolidation is given to maintain the remission.5 The duration of initial CR,6 and cytogenetics and molecular studies in AML,7 together with antecedent of myelodysplasic syndrome, multidrug resistance, or other medical comorbidities8 provide important prognostic information.7
ALL, the most common form of cancer in children, is a clonal stem cell malignancy of excessive B- or T-lymphoblast proliferation.9 The genetics of ALL are quite complex and are comprised of a variety of chromosome fusions, which can be used to distinguish different subtypes of the disease. Treatment for ALL is categorized broadly as induction phase, consolidation therapy, maintenance therapy, central nervous system (CNS) prophylaxis, and allogeneic stem cell transplantation. The goal of induction therapy is to achieve CR. Standard therapy will achieve CR in 80%–90% of patients.5
CML is characterized by a balanced genetic translocation, t (9; 22) (q34; q11.2), involving a fusion of the ABL oncogene from chromosome 9q34 with the BCR gene on chromosome 22q11.2. This rearrangement is known as the Philadelphia chromosome. This translocation results in expression of the constitutively active protein tyrosine kinase BCR-ABL.10 The current standard of care for CML is tyrosine kinase inhibitor (TKI) therapy, a very specific inhibitor of the BCR-ABL fusion protein. Imatinib, a TKI, has revolutionized the treatment of CML by transforming it from an invariably fatal disease to a chronic but manageable condition. Treatment with imatinib results in a 5-year progression-free survival rate of approximately 89%.11 Resistance to imatinib occurs in certain cases, usually through mutations in the imatinib binding site on BCR-ABL, hence, second generation TKIs (dasatinib and nilotinib) were introduced to the pharmacological armamentarium, successfully rescuing about 50% of imatinib-resistant patients.12
CLL, the most common type of adult leukemia,13 is characterized by the accumulation of mature CD5+ B cells in the peripheral blood and lymphoid organs. As in the leukemias described above, chromosomal aberrations and gene mutations are common in CLL. Over the past decades, there has been a transition from single-agent alkylator-based therapies to nucleoside analogues, combinations of both alkylators and nucleoside analogues, and most recently, chemoimmunotherapy. CR rates have improved from 7% to a maximum of 70%.14,15
IL-15
IL-15 was codiscovered in 1994 by two independent groups while studying the human T cell lymphotrophic virus-1 (HTLV-1)-infected T-cell line, HUT102, in the absence of IL-216 and its ability to stimulate proliferation of the IL-2-dependent CTLL-2 T cell line in the presence of neutralizing anti-IL-2 antibodies.17 IL-15 is a member of the four α-helix bundle and shares receptor signaling components (IL-2/15 receptor [R]βγ) with IL-2; as a result, the two cytokines have similar biologic properties in vitro. However, specificity for IL-15 versus IL-2 is provided by unique private α-chain receptors that complete the IL-15Rαβγ and IL-2Rαβγ heterotrimeric high-affinity receptor complexes.18
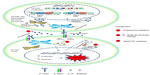
IL-15 is a 14–15 kDa glycoprotein encoded by a 34 kb region on human chromosome 4q31 and the central region of mouse chromosome 8.19 There are two isoforms of IL-15 messenger(m)RNA that differ in their signal peptides lengths, containing either a short signal peptide or long signal peptide (Figure 1). Both isoforms produce the same mature protein; however, they each have distinct intracellular trafficking, localization, and secretion patterns. The IL-15 long signal peptide is located to the Golgi apparatus, early endosomes, and the endoplasmic reticulum and is often secreted from the cell as a soluble protein. The IL-15 short signal peptide is restricted to the cytoplasm and nucleus and may play a role in its transcriptional regulation.20
Although IL-15 mRNA can be found in many tissues and cells, including fibroblasts, muscle cells, keratinocytes, kidney cells, lymphocytes, mast cells, and tumor cells,21 it is produced as a mature protein mainly by dendritic cells (DCs), monocytes, macrophages, and stromal cells.22 Translationally inactive IL-15 mRNA is stored in the cell, ready to be rapidly translated upon specific signals.22 The prevailing mechanism of IL-15 action seems to be transpresentation (juxtacrine signaling) (Figure 1), although it also includes intracrine, autocrine, paracrine, and endocrine signaling.20 IL-15 is also capable of efficiently signaling in cis through IL-15 Rα and IL2Rβ/γc expressed on the surface of a single cell.
Once IL-15 is secreted out of the cell, it binds to IL-15Rα and is presented to IL-2Rβγ complexes expressed on nearby effector cells, leading to a series of signaling events. These include activation of the JAK/STAT proteins for cellular activation.24 Binding of IL-15 to the IL-2/15Rβγ heterodimer induces JAK1 activation that subsequently phosphorylates STAT3 via the β-chain and JAK3/STAT5 activation via its γ-chain (Figure 1).24 Additional signaling pathways involve the recruitment of Shc to a phosphorylated site on the IL-2/15Rβ chain, followed by activation of Grb2. From there, Grb2 can proceed down the PI3K-Akt signaling pathway or can bind the guanine nucleotide exchange factor SOS to activate the RAS-RAF-MAPK signaling pathway.25,26 Collectively, these signaling mechanisms induce expression and activation of downstream effector molecules, such as proto-oncogenes c-Myc, c-Fos, and c-Jun, and antiapoptotic protein Bcl-2 for cellular proliferation.27
IL-15 plays an important role in the development, homeostasis, and function of memory CD8+ T cells, natural killer (NK) cells, NKT cells, and intestinal intraepithelial T cells.28,29 This cytokine induces proliferation of naïve CD8+ and memory CD4+ and CD8+ T cells, promoting development of the primary response of effector CD8+ T cells,30 and makes both CD4+ and CD8+ T cells resistant to the action of regulatory T cells.31 IL-15, which is itself produced by DCs, participates in their activation, proliferation, and differentiation,32 promoting expression of major histocompatibility complex (MHC) class II, CD40, and CD80/86 molecules,33 and making them potent inducers of CD4+ and CD8+ T cell responses.34 More importantly, it inhibits IL-2-induced activation-induced cell death.35 The antiapoptotic effects of IL-15 in granulocytes and lymphocytes are caused by its regulatory actions on the expression of proteins of the Bcl-2 family and on the activity of NF-κB and caspases.36 IL-15 induces proliferation and promotes cell survival of human T and B lymphocytes, and NK cells. However, it may also play a role in certain leukemias: human lymphotropic virus type-1 (HTLV-1)-associated adult T cell leukemia (ATL), large granular lymphocyte (LGL) leukemia, ALL, AML, B-cell CLL, and CML (Figure 1).
Role of IL-15 in leukemias
HTLV-1-associated ATL
Both IL-15 and IL-15Rα levels are elevated in ATL patients.37,38 Using an antibody towards IL-2/IL-15R, Azimi et al37 demonstrated a role of this cytokine in the proliferation of peripheral mononuclear cells of infected patients. In these patients, an elevation of IL-15 mRNA was also detected. This phenomenon was also confirmed in another study, where an increase of IL-15Rα, IL-15 mRNA and protein level was detected in HTLV-1-associated diseases. Furthermore, researchers observed that the viral protein Tax was involved in the activation of IL-15Rα. These findings suggest a role for IL-15Rα in the aberrant T cell proliferation observed in HTLV-I-associated diseases. Other studies have observed that the mechanism of HLTV-1 oncogenesis relies on a constitutive activation of the JAK/STAT pathway, especially on an association between JAK1 and STAT3, two molecules that are downstream to the IL-2/IL-15 receptor.39 However, Chung et al observed that IL-13 mRNA was highly expressed in HTLV-1-infected, IL-2-dependent T-cell lines, but IL-4, IL-10, IL-2, or IL-15 mRNAs were either below detection limits or did not correlate with HTLV-1 infection.40 These conflicting results may rely on the fact that they were performed on different biological samples. Further studies on the role of IL-15 in ATL patients are warranted to determine definitively whether IL-15 may participate in the initiation, maintenance, or progression of the disease.
LGL leukemia
LGL leukemia can arise from a CD3+ T-cell lineage or from a CD3− NK-cell lineage, which can be classified into two categories: NK LGL leukemia and chronic NK lymphocytosis.41 In a study where they deciphered the role of IL-15 in LGL, Zambello et al demonstrated the presence of IL-15 on leukemic LGL blasts, suggested a role of IL-15 in the pathogenesis of this disease, and showed that IL-15 may induce proliferation and cytotoxicity of LGL through the β and γ chains shared with IL-2.42 The study of Chen et al showed increased expression of IL-15Rα in T-cell LGL (T-LGL) leukemia and suggested that higher IL-15Rα expression may lower the IL-15 response threshold in vivo and therefore, may contribute to the pathogenesis of the disease.43 Chronic IL-15-mediated activation via the JAK/STAT pathway, especially STAT3 and STAT5, can be leukaemogenic.44 Somatic mutations in the SH2 domain of STAT3 have been discovered at the frequency of 40% in T-LGL leukemia and 30% in NK-LGL leukemia patients.45,46 Mishra et al showed that excessive IL-15 initiates cancer through the induction of Myc, Hdac-1, and NF-κBp65, which mediate downregulation of microRNA (miR)-29b and consequent overexpression of Dnmt3b, thereby hastening the onset of LGL leukemia.47 Hodge et al demonstrated that IL-15 alters expression of Bcl-2 family members and control of Bid that potentially links this cytokine to leukemogenesis through targeted proteasome degradation of Bid and offers the possibility that proteasome inhibitors may aid in the treatment of LGL leukemia.36 Yu et al demonstrated T-LGL leukemias in IL-15 transgenic mice that expressed NKp46; NKp46-expression was the hallmark of a minute population of wild-type NKT cells with higher activity and potency to become leukemic.48 Taken together, all these studies showed a role of IL-15 receptor and its signaling pathway in the pathogenesis of LGL leukemia and suggest that these may be useful targets in the treatment of patients with T-LGL or NKT leukemia.
AML
Very few studies have been published about the putative role of IL-15 in the development of the disease. Physiologically low amounts of IL-15 can be secreted by bone marrow stromal cells.49 This suggests that in the pathological condition, IL-15 could play a juxtacrine role, together with other factors, in promoting the survival and proliferation of IL-2Rβ/γ+ neoplastic clones in the bone marrow or lymph node microenvironment. This is likely to occur more frequently in IL-2Rβ/γ+ lymphoproliferative disorders;50,51 however, it seems that only a fraction of AML express this receptor.52 Drexler et al showed that only a fraction (3/19) of a panel of AML cell lines examined displayed responsiveness to IL-15, although the possible involvement of IL-2Rβ/γ was not studied.53 These results suggested that IL-15, produced by bone marrow stromal cells, could be an antiapoptotic growth factor for a particular subset of AML cells equipped with a functional IL-2Rβ/γ complex.54
ALL
A high expression of IL-15 correlates with CNS disease in childhood ALL55,56 and neurocognitive impairment.57 Moreover, several IL-15 single nucleotide polymorphisms (SNPs) have been associated with minimal residual disease;58 five SNPs (rs10519612, rs10519613, rs35964658, rs17007695, and rs17015014) located in the IL-15 locus were associated with childhood ALL treatment response.59 In adult ALL, the expression of IL-15 has also been correlated with the immunophenotypes of ALL, therefore Lin et al genotyped the five SNPs of the IL-15 gene by polymerase chain reaction – restriction fragment length polymorphism assays and observed a excess risk of developing ALL for the rs10519612 CC and rs17007695 TC genotype carriers in the adult Chinese population.60 Aly et al also observed a higher risk of developing T-cell ALL for rs10519612 CC and an increased risk of developing B-cell ALL for rs17007695 TC and rs17007695 CC genotype carriers in Egypt.61 These results suggested that several IL-15 SNPs, which were associated with minimal residual disease and correlated with the immunophenotypes of ALL, may provide important prognostic information in ALL patients.
CML
Autologous transplantation of stem cells could represent an alternative treatment to allogeneic bone marrow transplantation for CML patients, in whom NK cells may constitute the main cytolytic immune cells effect of mediating the graft-versus-leukemia effect.62 Therefore, Carayol et al investigated NK cell differentiation of CD34+ progenitors from CML patients and found that CD34+ cells from CML patients did not proliferate nor differentiate in the presence of stem cell factor and IL-15. Furthermore, anti-IL-15 monoclonal antibody (mAb) failed to induce the downregulation of the γc chain or the disruption of its interaction with JAK3 in leukemic cells. Therefore, in leukemic cells, the constitutive expression of the γc chain, as well as the continuous production of IL-15, would explain the sustained proliferation of leukemic precursors in vivo and their survival. As all the patients included in this study were positive for BCR/ABL, the alteration of the IL-15 differentiation pathway could be an indirect consequence of the BCR/ABL protein expression and its subsequent enhanced tyrosine kinase activity.63 In this respect, BCR/ABL expression through NF-κB activation64 may be involved in IL-15 transcription,65 thus the IL-15 signaling pathway may play a role in the development and progression of CML.
CLL
Purified CLL cells do not proliferate and tend to undergo spontaneous apoptosis in vitro, suggesting that factors inducing CLL cell survival and proliferation might be present in the microenvironment of lymphoid organs or of the bone marrow.66 Several cytokines, such as the TNF family members BAFF and APRIL,67 the chemokine SDF-1,68 IL-2,69 and IL-15,70 stimulate CLL cell proliferation and/or survival in vitro. Bernasconi et al demonstrated that IL-15 and CpG oligonucleotides promote memory B-cell activation and proliferation,71 and Park et al suggested that IL-15 plays an important role in supporting germinal center B cell proliferation, proposing a new target for treatment of B cell tumors.72 Importantly, IL-15 has also been described as a growth factor in non–germinal center–derived CLL mature B-cell survival and proliferation, through a strong activation of both STAT5 and ERK1/2 pathways.73 Interestingly, the mitogenic and antiapoptotic effects of IL-15 were enhanced when CLL B cells were preactivated by CD40. Epron et al found that in the presence of CD40L signaling, IL-15 essentially increased B-cell proliferation.74 These studies demonstrated that the IL-15 signaling pathway plays a role in CLL cell survival and proliferation and that the cooperation between IL-15 and CD40L increased CLL B cell growth.
Critical analysis of the potential for targeting IL-15 in cancer
Therapeutic interventions of targeting IL-15 have been found to include: proteasomal inhibitors,45 anti-IL-15R antibodies and pharmacologic inhibitors (Figure 1).75
IL-15 can lower the proapoptotic Bid protein in LGL leukemia, which was shown to result from increased proteasomal degradation, whereas the induction of Bid by the proteasome inhibitor bortezomib increased leukemic cell death, suggesting that proteasome inhibitors could be an effective treatment option for this disease.36 Furthermore, a formulation of a proteasomal inhibitors provides long-term disease-free survival in leukemic mice,49 thus offering a new approach to treating patients with aggressive LGL leukemia.
Another therapeutic approach targeting IL-15 in leukemias has been the use of the anti-IL-15R mAb Mikβ1 to block the presentation of IL-15 to the IL-2/IL-15Rβ, thereby inhibiting proliferation of IL-15-dependent leukemia cells (Table 1).76 A Phase I clinical trial of IL-15 blockade in T-cell LGL leukemia was conducted by Waldmann et al using the Hu-Mikβ1 mAb that blocks IL-15 transpresentation to cells expressing IL-2/IL-15Rβ and γc (Table 1).76 However, Hu-Mikβ1 did not block IL-15 action on the cells that express the heterotrimeric receptor in a cis orientation. In this trial, Hu-Mikβ1 therapy was not effective in the treatment of patients with monoclonal T-LGL.
Finally, Epling-Burnette et al demonstrated that STAT3 activation contributed to accumulation of the leukemic LGL clones, as a variety of STAT3 inhibitors come forth to the clinic, suggested that investigation could focus on STAT3 inhibitors in the treatment of LGL leukemia.77
However, IL-15 can activate many immune antitumor mechanisms and can be considered as a good candidate for application in tumor therapy. The most important cells engaged in IL-15 antitumor activity seem to be T-cells, in particular CD8+ T cytotoxic cells and NK cells.22
IL-15 increases the number of specific CD8 T cells in adoptive T-cell therapy,78 and in vitro assays have shown that treatment of CML monocytes with IFN-α and GM-CSF resulted in the rapid generation of activated DCs expressing IL-15. An additional activation/maturation step of these DCs can render these cells fully capable of presenting CML-specific antigens inducing tumor-specific CD8+ effector cells, which represents an important requisite for achieving an immune-mediated control of tumor progression in CML patients.79–81
It had been proved that IL-15 increases NK cell cytotoxicity in an NKG2D-dependent fashion,82 which could be dependent on the presence of the NKG2D ligand ULBP1 on the tumor cell,83 and that IL-15-expanded NK cells enhanced their expression of KIR and NKG2D.84 Furthermore, IL-15 may be used in NK cell expansion protocols, in combination with SCF, FLT3-L, and IL-21, to generate NK cells from CD34+ cells. This protocol was developed by Yoon et al to expanded NK cells into AML or ALL patients shortly after haploidentical stem cell transplantation. This feasibility study showed no toxicity of the NK cell infusion.85
There are additional ways in which NK cells are important for IL-15 antitumoral therapy. For instance, in vitro assays have indicated that NK cells in the presence of IL-15 become more efficacious mediators of ADCC against cultures of B-lymphoma cells in the presence of the anti-CD20 mAb rituximab.86
The encouraging data of IL-15 immunotherapy in murine models, together with its low toxicity in mice and primates, has led to the design of clinical trials in cancer patients. The first-in-human clinical trial of recombinant human IL-15 (rhIL-15) in patients with cancer, by Conlon et al, demonstrated that IL-15 could be safely administered to patients with metastatic malignancy. IL-15 administration markedly altered homeostasis of NK cells and γδ cells in the blood.87 There were also other clinical trials initiated recently, as shown in Table 1. Six of the trials use the rhIL-15 protein, administered either alone or combined with the administration of patient-derived tumor-infiltrating lymphocyte or combined with NK cells. Three trials used IL-15 as an ex vivo ancillary agent to enhance DC or NK cell expansion and function when used for anticancer vaccination or immunotherapy. The remaining four trials aimed to determine the safety and effectiveness of an IL-15 super agonist complex (ALT-803) administered weekly for 4 or 6 consecutive weeks, alone or mixed together with Calmette-Guerin bacillus.
Conclusion
The recent published data have highlighted the role of IL-15 and the aberrancies in its regulation in the development and progression of leukemias. The better understanding of the IL-15/IL-15R system has led to the identification of new therapeutic targets, which will open the possibility for the development of new drugs. However, IL-15’s ability to stimulate the development and activity of effector and memory CD8+ T cells, and NK and NKT cells, which all contribute significantly to the antitumor immune response, raises the hope that this cytokine could become a valuable adjuvant in antitumor immunotherapy.
Acknowledgments
This study was supported by an Oversea Study Fellowship from the People’s Republic of China Scholarship Council.
Disclosure
The authors report no conflicts of interest in this work.
References
Ntziachristos P, Mullenders J, Trimarchi T, Aifantis I. Mechanisms of epigenetic regulation of leukemia onset and progression. Adv Immunol. 2013;117:1–38. | |
Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381(9865):484–495. | |
de Jonge HJ, Huls G, de Bont ES. Gene expression profiling in acute myeloid leukaemia. The Netherlands Journal of Medicine. 2011;69:167–176. | |
Roboz GJ. Current treatment of acute myeloid leukemia. Curr Opin Oncol. 2012;24(6):711–719. | |
Dis Mon. 2012;58:219–238. | |
Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–1978. | |
Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. | |
Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. | |
Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. New Engl J Med. 2006;354(2):166–178. | |
Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112(13):4808–4817. | |
Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. | |
Holtz MS, Forman SJ, Bhatia R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19(6):1034–1041. | |
Cramer P, Hallek M. Hematological cancer in 2011: New therapeutic targets and treatment strategies. Nat Rev Clin Oncol. 2012;9(2):72–74. | |
Catovsky D, Richards S, Matutes E, et al; UK National Cancer Research Institute (NCRI) Haematological Oncology Clinical Studies Group; NCRI Chronic Lymphocytic Leukaemia Working Group. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370(9583):230–239. | |
Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. | |
Bamford RN, Grant AJ, Burton JD, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91(11):4940–4944. | |
Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264(5161):965–968. | |
Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. | |
Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33(1):35–41. | |
Tagaya Y, Kurys G, Thies TA, et al. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc Natl Acad Sci U S A. 1997;94(26):14444–14449. | |
Bulfone-Paus S, Bulanova E, Budagian V, Paus R. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. Bioessays. 2006;28(4):362–377. | |
Jakobisiak M, Golab J, Lasek W. Interleukin 15 as a promising candidate for tumor immunotherapy. Cytokine Growth Factor Rev. 2011;22(2):99–108. | |
Olsen SK, Ota N, Kishishita S, et al. Crystal structure of the interleukin-15. interleukin-15 receptor alpha complex: insights into trans and cis presentation. J Biol Chem. 2007;282(51):37191–37204. | |
Johnston JA, Bacon CM, Finbloom DS, et al. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci U S A. 1995;92(19):8705–8709. | |
Gu H, Maeda H, Moon JJ, et al. New role for Shc in activation of the phosphatidylinositol 3-kinase/Akt pathway. Mol Cell Biol. 2000;20(19):7109–7120. | |
Adunyah SE, Wheeler BJ, Cooper RS. Evidence for the involvement of LCK and MAP kinase (ERK-1) in the signal transduction mechanism of interleukin-15. Biochem Biophys Res Commun. 1997;232(3):754–758. | |
Miyazaki T, Liu ZJ, Kawahara A, et al. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81(2):223–231. | |
Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. | |
Imamura M, Shook D, Kamiya T, et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood. 2014;124(7):1081–1088. | |
Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177(9):6072–6080. | |
Ben Ahmed M, Belhadj Hmida N, Moes N, et al. IL-15 renders conventional lymphocytes resistant to suppressive functions of regulatory T cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol. 2009;182(11):6763–6770. | |
Gil M, Park SJ, Chung YS, Park CS. Interleukin-15 enhances proliferation and chemokine secretion of human follicular dendritic cells. Immunology. 2010;130(4):536–544. | |
Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167(3):1179–1187. | |
Pulendran B, Dillon S, Joseph C, Curiel T, Banchereau J, Mohamadzadeh M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur J Immunol. 2004;34(1):66–73. | |
Waldmann T. The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res. 2002;4 Suppl 3:S161–S167. | |
Hodge DL, Yang J, Buschman MD, et al. Interleukin-15 enhances proteasomal degradation of bid in normal lymphocytes: implications for large granular lymphocyte leukemias. Cancer Res. 2009;69(9):3986–3994. | |
Azimi N, Jacobson S, Leist T, Waldmann TA. Involvement of IL-15 in the pathogenesis of human T lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis: implications for therapy with a monoclonal antibody directed to the IL-2/15R beta receptor. J Immunol. 1999;163(7):4064–4072. | |
Mariner JM, Lantz V, Waldmann TA, Azimi N. Human T cell lymphotropic virus type I Tax activates IL-15R alpha gene expression through an NF-kappa B site. J Immunol. 2001;166(4):2602–2609. | |
Takemoto S, Mulloy JC, Cereseto A, et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci U S A. 1997;94(25):13897–13902. | |
Chung HK, Young HA, Goon PK, et al. Activation of interleukin-13 expression in T cells from HTLV-1-infected individuals and in chronically infected cell lines. Blood. 2003;102(12):4130–4136. | |
Lamy T, Loughran TP. Current concepts: large granular lymphocyte leukemia. Blood Rev. 1999;13(4):230–240. | |
Zambello R, Facco M, Trentin L, et al. Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood. 1997; 89(1):201–211. | |
Chen J, Petrus M, Bamford R, et al. Increased serum soluble IL-15Rα levels in T-cell large granular lymphocyte leukemia. Blood. 2012;119(1):137–143. | |
Mishra A, Sullivan L, Caligiuri MA. Molecular pathways: interleukin-15 signaling in health and in cancer. Clin Cancer Res. 2014;20(8):2044–2050. | |
Koskela HL, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366(20):1905–1913. | |
Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120(15):3048–3057. | |
Mishra A, Liu S, Sams GH, et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell. 2012;22(5):645–655. | |
Yu J, Mitsui T, Wei M, et al. NKp46 identifies an NKT cell subset susceptible to leukemic transformation in mouse and human. J Clin Invest. 2011;121(4):1456–1470. | |
Mrózek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87(7):2632–2640. | |
Trentin L, Cerruti A, Zambello R, et al. Interleukin-15 promotes the growth of leukemic cells of patients with B-cell chronic lymphoproliferative disorders. Blood. 1996;87(8):3327–3336. | |
Zambello R, Facco M, Trentin L, et al. Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood. 1997;89(1):201–211. | |
Rosolen A, Nakanishi M, Poplack DG, et al. Expression of interleukin-2 receptor beta subunit in hematopoietic malignancies. Blood. 1989;73(7):1968–1972. | |
Drexler HG, Zaborski M, Quentmeier H. Cytokine response profiles of human myeloid factor-dependent leukemia cell lines. Leukemia. 1997;11(5):701–708. | |
Meazza R, Basso S, Gaggero A, et al. Interleukin (IL)-15 induces survival and proliferation of the growth factor-dependent acute myeloid leukemia M-07e through the IL-2 receptor beta/gamma. Int J Cancer. 1998;78(2):189–195. | |
Cario G, Izraeli S, Teichert A, et al. High interleukin-15 expression characterizes childhood acute lymphoblastic leukemia with involvement of the CNS. J Clin Oncol. 2007;25(30):4813–4820. | |
Williams MT, Yousafzai Y, Cox C, et al. Interleukin-15 enhances cellular proliferation and upregulates CNS homing molecules in pre-B acute lymphoblastic leukemia. Blood. 2014;123(20):3116–3127. | |
Petranovic D, Pilcic G, Valkovic T, Sotosek Tokmadzic V, Laskarin G. Perforin- and granulysin-mediated cytotoxicity and interleukin 15 play roles in neurocognitive impairment in patients with acute lymphoblastic leukaemia. Med Hypotheses. 2014;83(1):122–126. | |
Zhang XJ, Yan KL, Wang ZM, et al. Polymorphisms in interleukin-15 gene on chromosome 4q31.2 are associated with psoriasis vulgaris in Chinese population. J Invest Dermatol. 2007;127(11):2544–2551. | |
Yang JJ, Cheng C, Yang W, et al. Genome-wide interrogation of germline genetic variation associated with treatment response in childhood acute lymphoblastic leukemia. JAMA. 2009;301(4):393–403. | |
Lin D, Liu C, Xue M, et al. The role of interleukin-15 polymorphisms in adult acute lymphoblastic leukemia. PLoS One. 2010;5(10):e13626. | |
Aly RM, Taalab MM, Ghazy HF. Influence of interleukin-15 polymorphism on the survival of adult patients with acute lymphoblastic leukaemia in Egypt. Leuk Lymphoma. Epub June 17, 2014. | |
Carella AM, Lerma E, Corsetti MT, et al. Autografting with philadelphia chromosome-negative mobilized hematopoietic progenitor cells in chronic myelogenous leukemia. Blood. 1999;93(5):1534–1539. | |
Carayol G, Giron-Michel J, Azzarone B, et al. Altered natural killer cell differentiation in CD34+ progenitors from chronic myeloid leukemia patients. Oncogene. 2000;19(23):2758–2766. | |
Reuther JY, Reuther GW, Cortez D, Pendergast AM, Baldwin AS. A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev. 1998;12(7):968–981. | |
Washizu J, Nishimura H, Nakamura N, Nimura Y, Yoshikai Y. The NF-kappaB binding site is essential for transcriptional activation of the IL-15 gene. Immunogenetics. 1998;48(1):1–7. | |
Ghia P, Circosta P, Scielzo C, et al. Differential effects on CLL cell survival exerted by different microenvironmental elements. Curr Top Microbiol Immunol. 2005;294:135–145. | |
Nishio M, Endo T, Tsukada N, et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood. 2005;106(3):1012–1020. | |
Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96(8):2655–2663. | |
de Totero D, Francia di Celle P, Cignetti A, Foa R. The IL-2 receptor complex: expression and function on normal and leukemic B cells. Leukemia. 1995;9(9):1425–1431. | |
Trentin L, Cerutti A, Zambello R, et al. Interleukin-15 promotes the growth of leukemic cells of patients with B-cell chronic lymphoproliferative disorders. Blood. 1996;87(8):3327–3335. | |
Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298(5601):2199–2202. | |
Park CS, Yoon SO, Armitage RJ, Choi YS. Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form. J Immunol. 2004;173(11):6676–6683. | |
de Totero D, Meazza R, Capaia M, et al. The opposite effects of IL-15 and IL-21 on CLL B cells correlate with differential activation of the JAK/STAT and ERK1/2 pathways. Blood. 2008;111(2):517–524. | |
Epron G, Ame-Thomas P, Le Priol J, et al. Monocytes and T cells cooperate to favor normal and follicular lymphoma B-cell growth: role of IL-15 and CD40L signaling. Leukemia. 2012;26(1):139–148. | |
Caceres-Cortes JR. A potent anti-carcinoma and anti-acute myeloblastic leukemia agent, AG490. Anticancer Agents Med Chem. 2008;8(7):717–722. | |
Waldmann TA, Conlon KC, Stewart DM, et al. Phase 1 trial of IL-15 trans presentation blockade using humanized Mikβ1 mAb in patients with T-cell large granular lymphocytic leukemia. Blood. 2013;121(3):476–484. | |
Epling-Burnette PK, Liu JH, Catlett-Falcone R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107(3):351–362. | |
Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117(6):1888–1898. | |
Gabriele L, Borghi P, Rozera C, et al. IFN-alpha promotes the rapid differentiation of monocytes from patients with chronic myeloid leukemia into activated dendritic cells tuned to undergo full maturation after LPS treatment. Blood. 2004;103(3):980–987. | |
Molldrem JJ, Lee PP, Kant S, et al. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest. 2003;111(5):639–647. | |
Schwartz J, Pinilla-Ibarz J, Yuan RR, Scheinberg DA. Novel targeted and immunotherapeutic strategies in chronic myeloid leukemia. Semin Hematol. 2003;40(1):87–96. | |
Le Maux Chansac B, Missé D, Richon C, et al. Potentiation of NK cell-mediated cytotoxicity in human lung adenocarcinoma: role of NKG2D-dependent pathway. Int Immunol. 2008;20(7):801–810. | |
Sutherland CL, Rabinovich B, Chalupny NJ, Brawand P, Miller R, Cosman D. ULBPs, human ligands of the NKG2D receptor, stimulate tumor immunity with enhancement by IL-15. Blood. 2006;108(4):1313–1319. | |
Decot V, Voillard L, Latger-Cannard V, et al. Natural-killer cell amplification for adoptive leukemia relapse immunotherapy: comparison of three cytokines, IL-2, IL-15, or IL-7 and impact on NKG2D, KIR2DL1, and KIR2DL2 expression. Exp Hematol. 2010;38(5):351–362. | |
Yoon SR, Lee YS, Yang SH, et al. Generation of donor natural killer cells from CD34(+) progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplant. 2010;45(6):1038–1046. | |
Laprevotte E, Voisin G, Ysebaert L, et al. Recombinant human IL-15 trans-presentation by B leukemic cells from chronic lymphocytic leukemia induces autologous NK cell proliferation leading to improved anti-CD20 immunotherapy. J Immunol. 2013;191(7):3634–3640. | |
Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33(1):74–82. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.