Back to Journals » Cancer Management and Research » Volume 12
IGFBP-3 Is the Key Target of Sanguinarine in Promoting Apoptosis in Hepatocellular Carcinoma
Authors Wang H, Wang H, Li K, Li S, Sun B
Received 11 October 2019
Accepted for publication 11 January 2020
Published 11 February 2020 Volume 2020:12 Pages 1007—1015
DOI https://doi.org/10.2147/CMAR.S234291
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Huiwen Wang,1,* He Wang,1,* Kai Li,1 Shijie Li,1 Bingyi Sun2
1Department of Interventional, Harbin Medical University Cancer Hospital, Harbin 150081, Heilongjiang Province, People’s Republic of China; 2Department of General Surgery, The First Hospital of Qiqihar, Qiqihar 161005, Heilongjiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bingyi Sun
Department of General Surgery, The First Hospital of Qiqihar (Equals to: Affiliated Qiqihar Hospital, Southern Medical University), Qiqihar 161005, Heilongjiang Province, People’s Republic of China
Email [email protected]
Introduction: Chemotherapeutic treatment of hepatocellular carcinoma (HCC) has always been plagued by nonspecific and side effects. Plant extracts have potential anticancer capabilities with low cytotoxicity and few side effects, but their detailed mechanisms are still unclear, thus limiting their clinical applications.
Methods: In this study, five plant extracts were chosen, their inhibition on HCC cell viability was compared by CCK-8 assay and sanguinarine (SAN) was selected. Then, wound healing assay, transwell assay, and apoptosis assay were carried out in Hep3B cells. Bioinformatics methods were performed and IGFBP-3 was predicted the targets of SAN in HCC. The mechanism of SAN regulating IGFBP-3 was explored using qRT-PCR, Western blotting, cell viability assay and apoptosis assay. Meanwhile, knockdown of IGFBP-3 were used by small interfering RNA (siRNA).
Results: In five plant extracts, SAN inhibited the proliferation of HCC cell lines most considerably. In addition, apoptosis was promoted, and invasion and migration were inhibited in the Hep3B cell line by treatment with SAN at 2 μM. Bioinformatics indicated that SAN could affect HCC apoptosis through the TP53/IGFBP-3 pathway, and further verification experiments showed that SAN upregulated the expression of insulin-like growth factor binding protein-3 (IGFBP-3) in the Hep3B cell line; SAN also inhibited the expression of Bcl-2 and promoted the expression of BAX and caspase-3. After using siRNA to inhibit the expression of IGFBP-3, the effect of SAN was blocked.
Conclusion: Our study further reveals a novel mechanism that IGFBP-3 is an important target of SAN, by upregulating expression of IGFBP-3, SAN promotes apoptosis in HCC.
Keywords: sanguinarine, IGFBP-3, apoptosis, hepatocellular carcinoma
Plain Language Summary
Chemotherapy for hepatocellular carcinoma has many side effects, while plant extracts have better advantages; thus, further clarification of the mechanism of plant extracts in HCC is urgently needed. Previous studies have suggested that sanguinarine elicits potential antitumor effects by promoting the apoptosis of cancer cells, but the specific mechanism is still unclear. This study found that sanguinarine can effectively promote the apoptosis of hepatocellular carcinoma cells, and IGFBP-3 is the key target for this effect. Further study on SAN could provide a new model for treating HCC.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy and the second most common cause of cancer-related death worldwide.1 At present, according to the Barcelona Clinic Liver Cancer (BCLC) staging classification, the treatment of HCC mainly consists of surgery, chemoembolization and systemic therapy.2 However, the existing treatment is not adequate and has numerous side effects, and the mortality rate of HCC remains high.3 The identification of more effective therapeutic approaches to treat HCC and other malignancies is necessary. Although new methods are constantly being proposed, the side effects are often overlooked. Recently, the use of plant materials as anticancer agents has gained a great deal of attention for their possible therapeutic efficacy with minimal toxicity.4–8
By comparing the antitumor effects of 5 known plant extracts: sanguinarine (CID: 5154), betulinic acid (CID: 64971), amygdalin (CID: 656516), solanesol (CID: 5477212), and (-)-sparteine (CID: 644020), we found that sanguinarine (SAN) could significantly reduce the viability of HCC cell lines. As a benzophenanthridine alkaloid, it is derived from the root of Sanguinaria canadensis and has been proven to induce cell apoptosis in several human carcinoma cell lines.8–10 Nevertheless, research on the related mechanism of SAN in HCC is rare.
In this study, we investigated the effect of SAN on the cell growth, migration, and invasion of HCC. Subsequent experiments found that IGFBP-3 was the key target for SAN in promoting apoptosis in HCC.
Materials and Methods
Chemicals and Reagents
Five natural extracts, sanguinarine, betulinic acid, amygdalin, solanesol, and (-)-sparteine, of analytical grade (HPLC purity ≥98%) were purchased from Sigma. Each extract was prepared with DMSO to form a stock solution with a final concentration of 20 mM.
Cell Culture and Treatment with Reagents
The HCC cell lines HepG2 and Hep3B were obtained from ATCC and cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin under standard conditions (37°C, 5% CO2). The culture medium was changed every day. Cells for assays were detached by a solution of 0.25% trypsin and 0.02% EDTA. For the cell viability study, all extract stock solutions were used at a final concentration of 2 μM. For the study of SAN dose-dependent experiments, SAN stock solution was set at different final concentrations of 0.5, 1, 2, and 4 μM.
Cell Viability Assays
In the cell viability assay, cells were seeded into each well of a 96-well plate with 5 replicates for each group at each time point. After 24 hrs of incubation, 100 μL of medium containing 10% CCK-8 (Dojindo, Japan) was added to each well. After incubation for 2 hrs, OD values were measured at 450 nm using a Sunrise Microplate Reader (Tecan, Groedig, Austria).
qRT-PCR
For qRT-PCR, cDNA synthesis was performed by utilizing the PrimeScript qRT-PCR Kit (TaKaRa, Dalian, China), and cDNA samples were amplified by using specific primers (GenePharma Shanghai, China) (Supplementary Table 1). The mean Ct values of the target genes were normalized to the average Ct values of the endogenous control GAPDH. The ratio of mRNA expression of the target gene to GAPDH was defined as 2–ΔΔC(t).
Western Blotting
Cells were collected and lysed in RIPA lysis buffer, and the protein concentrations were determined using a BCA protein assay kit (Thermo Scientific, USA). Equal amounts of protein extracts (20 μg) were loaded in the SDS-PAGE gel and transferred to HyBond ECL nitrocellulose membranes. Western blot analyses were performed using the following primary antibodies: anti-IGFPB3, anti-Bcl-2, anti-caspase-3, anti-BAX, and anti-β-actin, diluted according to the operation manual (Abcam, USA). After incubation with the IgG-HRP secondary antibody, immunoreactivity was detected using an ECL detection instrument, and the protein bands were quantified using ImageJ software and normalized to the signal intensity of β-actin.
Wound Healing Assays
Hep3B cells were resuspended and adjusted to 1×106/mL and then seeded on ibidi inserts in μ-dishes (ibidi, Germany) at 70 μL/well. After 12 hrs, the insert was removed carefully, and 500 μL serum-free DMEM with/without SAN was added. After 24 hrs, the cells were observed and photographed under an inverted phase contrast microscope. The area of the cell gap in each visual field was measured by ImageJ software, and then the average migration distance was calculated.
Transwell Invasion Assays
The migration of Hep3B cells was measured by Transwell filters (8-μm pores; BD Labware, USA). Cells were seeded in 200 μL serum-free DMEM in the upper chamber with Matrigel (BD Labware, USA) coated on the membrane of the filters, with/without SAN treatment. DMEM containing 10% fetal bovine serum (500 μL) was placed in the lower chamber. After 24 hrs, the cells on the outer side of the membrane were fixed with 90% alcohol and stained with crystal violet. After decolorization of citric acid, the OD value at 570 nm was measured by a spectrophotometer (Shimadzu, Japan).
Apoptosis Assays
Hep3B apoptosis was detected by flow cytometry (FCM) and caspase activation assays. For FCM assays, the Annexin V-FITC/PI Apoptosis Detection Kit (BD Pharmingen, USA) was used according to the manufacturer protocols. Cells were collected and suspended in annexin-V binding buffer. After incubation with Annexin V-FITC (25°C, 15 mins), Propidium iodide (PI) was added, and apoptotic cells were analyzed with a FACScan instrument (Becton Dickinson). Apoptosis was described as cells with annexin V-positive staining. For the apoptosis assay, the Caspase-3 Activity Assay Kit (Beyotime Biotechnology, China) was used to assess caspase activation according to the manual. Cells were cultured at a density of 1×106/well with/without SAN or si-IGFBP-3. Then, the cells were harvested and lysed (0°C, 30 mins), and the supernatants were collected after centrifugation (0°C, 12,800 g × 10 mins) and incubation with 2 mM Ac-DEVD-pNA (37°C, 1 hr). The OD values at 405 nm were detected and calibrated with the p-NA standard using a spectrophotometer (Shimadzu, Japan).
Cell Transfection
Hep3B cells were transfected with pcDNA3.1-IGFBP-3 and si-IGFBP-3, and the siRNA sequence was designed and synthesized by GenePharma (GenePharma, Shanghai, China) (Supplementary Table 1). Cells were transfected with pcDNA3.1-IGFBP-3 and IGFBP-3 siRNA plus Lipofectamine 2000 (Invitrogen, California, USA). All operations were carried out in accordance with the manual for instructions.
Statistical Analyses
The measurement data are presented as the mean±SD. Using t-test for two independent groups, using ANOVA for multiple comparisons (two-way ANOVA for CCK-8 assays and one-way ANOVA for multiple comparisons) and Tukey’s multiple comparisons for post hoc test. Statistical analyses and graphics presentation were performed using GraphPad Prism 7, P<0.05 was considered statistically significant.
Results
SAN Inhibited the Viability of the HCC Cell Line Hep3B in a Concentration-Dependent Manner and Also Inhibited the Viability of HepG2 Cells
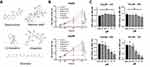
To understand the effects of different plant extracts on hepatocellular carcinoma, based on previous studies, 5 plant extracts with potential antitumor effects, sanguinarine (SAN), betulinic acid, sparteine, amygdalin and solanesol, were selected for this study (Figure 1A). HepG2 and Hep3B cell lines were used to evaluate the cell viability after treatment with the mentioned extracts using working concentrations of 2 μM. After 72 hrs, the results of CCK-8 assays showed that each extract presented different degrees of proliferation inhibition, and SAN treatment resulted in the most significant proliferation inhibition in both HepG2 cells (q=21.66, P<0.01) and Hep3B cells (q=18.55, P<0.01) (Figure 1B). The inhibitory effect of SAN in Hep3B cells was observed with different concentrations, at 0, 0.5, 1, 2 and 4 μM, and showed an approximately linear change with increasing concentration (Figure 1C). These results indicated that SAN elicited the most significant inhibition of proliferation of HCC cell lines among the five plant extracts, and the effects occurred in a typical concentration- and time-dependent manner.
SAN Inhibited the Malignant Properties and Promoted the Apoptosis of the Hep3B Cell Line
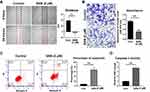
To further understand the phenotype of HCC cell lines with/without SAN, apoptosis, wound healing and Transwell invasion assays were performed using the Hep3B cell line. Wound healing assays showed that the mean migration distance of the SAN group was significantly shorter than that of the control group (t=5.513, P=0.0067) (Figure 2A). In the Transwell assay, the invasion of the SAN group decreased significantly (t=17.46, P<0.01) (Figure 2B). Flow cytometry showed that the percentage of apoptotic cells in the SAN group was significantly higher than that in the control group (t=11.90, P=0.0003) (Figure 2C). Caspase-3 activity assays demonstrated the activation of caspase-3 after SAN treatment (t=14.75, P<0.01) (Figure 2D). These experiments confirmed that SAN could effectively inhibit the malignant phenotype of the Hep3B cell line.
IGFBP-3 Was Predicted as a Potential Target for SAN in HCC by Bioinformatics Analysis
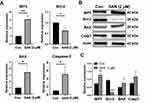
To explore the possible mechanisms of SAN in HCC cells, bioinformatics methods were used. According to the BATMAN-TCM database (http://bionet.ncpsb.org/batman-tcm/), the number of predicted candidate target genes (including known targets) of SAN was 290 (score cutoff ≥20). After clustering with KEGG, the top 20 terms, including 6 significant terms, were listed, including the p53 signaling pathway (Supplementary Table 2). Further analysis revealed that 63 of the 290 candidate genes were highly correlated with clinical disease, and 2 genes were related to HCC, TP53 and IGFBP-3 (Figure 3A), which are involved in the regulation of apoptosis through the p53 signaling pathway (Figure 3B). By means of bioinformatics, we speculate that SAN regulates the apoptosis of HCC cells by affecting IGFBP-3.
IGFBP-3 and Apoptosis-Related Proteins Were Activated by Treatment with SAN
To verify the results of the bioinformatics analysis, qRT-PCR and Western blotting were performed to detect the expression of IGFBP-3 and apoptosis-related genes and proteins treated with/without SAN. The qRT-PCR results showed that with SAN treatment, IGFBP-3, BAX, and caspase-3 increased significantly, while Bcl-2 decreased significantly (t=8.087, 4.051, 3.907, 4.267, P<0.05) (Figure 4A). Western blotting also showed consistent results (Figure 4B and C). Overall, the results of bioinformatics analysis were verified, and with SAN treatment, HCC cells overexpressed IGFBP-3 and initiated changes in apoptosis-related proteins.
IGFBP-3 Was the Key Target by Which Sanguinarine Promoted Hep3B Cell Apoptosis
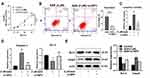
The relationship between the upregulation of IGFBP-3 expression and the activation of apoptosis in HCC still requires further investigation. Using siRNA to inhibit the expression of IGFBP-3 in Hep3B cells, CCK-8 assays showed that the proliferation of HCC cells treated with SAN was markedly inhibited (Control vs SAN, q=16.34, P<0.001), but with si-IGFBP-3, the inhibition of SAN in HCC cells was attenuated (Control vs SAN+si-IGFBP-3, q=2.969, P=0.1112), suggesting that SAN mainly inhibited the proliferation of HCC cells through IGFBP-3 (Figure 5A). Flow cytometry assays were used to detect the above two groups of cells after SAN treatment, and the apoptotic rate of the SAN+si-IGFBP-3 group was decreased (SAN vs SAN+si-IGFBP-3, t=8.029, P=0.0013) (Figure 5B). In the caspase-3 activity assay, caspase-3 was activated after SAN treatment (Control vs SAN, q=24.47, P<0.01), but in the si-IGFBP-3 group, SAN treatment could not cause caspase-3 activation (Control vs SAN+si-IGFBP-3, q=2.654, P=0.2252) (Figure 5C). qRT-PCR assays showed that si-IGFBP-3 could eliminate the regulatory effect of SAN on apoptosis-related genes (Bcl-2: Control vs SAN, q=5.396, P=0.0206; Control vs SAN+si-IGFBP-3, q=0.7734, P=0.8518) (Caspase-3: Control vs SAN, q=19.95, P<0.01; Control vs SAN+si-IGFBP-3, q=1.281, P=0.6568) (Figure 5D), and Western blotting also achieved similar results (Figure 5E). These results indicated that SAN mainly affects the apoptosis of HCC cells by upregulating IGFBP-3, while inhibition of IGFBP-3 expression could reverse this effect.
Discussion
HCC development and progression are the consequences of multiple factors involving various molecular events. Major factors include telomere maintenance, cell cycle control, WNT/β-catenin signaling, oxidative stress, epigenetic and chromatin remodeling, AKT/mTOR/MAPK signaling, and angiogenesis,1 and HCC can be derived from a variety of cell types, including stem/progenitor cells and mature hepatocytes.11–13 However, research on HCC treatment seems to have not kept pace with research on the occurrence and development of HCC. Although chemotherapy has been widely used, most treatments cannot distinguish cancer cells from normal cells effectively in vivo, thus causing systemic toxicity and side effects. Finding more appropriate drugs or delivery methods with lower cytotoxicity and fewer side effects are important tasks for HCC chemotherapy.14,15
Alkaloids are organic compounds containing nitrogen that occur nature, most of which are basic, some of which are neutral (eg, colchicine) or even acidic (eg, theophylline, theobromine). A wide range of pharmacological properties and effects have been reported, such as antimalarial (eg, quinine), antiasthma (eg, ephedrine), anticancer (eg, homoharringtonine), cholinomimetic (eg, galantamine), vasodilatory (eg, vincristine), antiarrhythmic (eg, quinidine), analgesic (eg, morphine), antibacterial (eg, chelerythrine), and antihyperglycemic activities (eg, vincristine and vinblastine).16 SAN [13-methyl-(1,3) benzodioxolo-(5,6-C)-1,3-dioxolo(4,5)-phenanthrene] is the most widely used benzophenanthridine alkaloid, which originates from the Canadian Haemorrhoa and other Papaver plants,17 and has antimicrobial, antioxidant and anti-inflammatory properties.18 SAN is a low toxicity, safe alkaloid and is relatively specific for cancer cells;19,20 it has been approved with known safety and bioavailability by the Food and Drug Administration (FDA) and included in the Prestwick Chemical Library.21,22
SAN has effects on a variety of human cancers, including breast, lung, prostate, pancreatic, and colon cancer, and erythroleukemia, promyelocytic leukemia, etc.,23–26 exhibiting cytotoxicity and cell inhibition. Some studies suggest that SAN exerts cytotoxic effects by acting on Na+-K+-ATPase, which plays a key role in the regulation of the MAPK pathway, reactive oxygen species and intracellular calcium ions.27 SAN also affects apoptosis of colon cancer cells by regulating Bcl-2/BAX,28 and prostate cancers by increasing nitric oxide synthase (NOS) formation29 or STAT3 activation.30 In breast cancer, SAN induces the accumulation of reactive oxygen species (ROS) in MDA-231 cell lines and inhibits the expression of Bcl-2 and C-Flip.31 Nevertheless, the mechanism by which SAN acts on HCC remains unclear. Potential mechanisms of SAN were assumed by using bioinformatics analysis, and further experiments confirmed that SAN regulated apoptosis-related proteins by upregulating IGFBP-3 and consequently promoted the apoptosis of HCC.
IGFBP-3 is the principal binding protein of insulin-like growth factor-1 (IGF-1). IGFBP-3 recruits IGF-1 so that it can bind with insulin-like growth factor-1 receptor (IGF-1R) and produces a series of cellular biological effects.32 IGFBP-3 can interact with vitamin D receptor (VDR) directly, and upregulation of IGFBP-3 can inhibit the differentiation of osteoblasts.33 In HCC and lung adenocarcinoma, downregulation of IGFBP-3 can trigger the activation of PI3K/Bcl-2 and RAS/JUN signaling and then facilitate tumor progression.34 In patients with advanced HCC who received antiangiogenic therapy, high IGFBP-3 promotes high IGF-1 levels, which are associated with a better disease control rate (DCR), progression-free survival (PFS) rate, and overall survival (OS) rate.35 According to our study, we found that SAN can upregulate IGFBP-3 expression in HCC cell lines, and more details of the relative mechanisms need to be understood.
Conclusions
In conclusion, among the five extracts with potential antitumor effects, SAN most potently inhibits the HCC malignant phenotype. By upregulating IGFBP-3, SAN effectively promotes the apoptosis of HCC cells, and IGFBP-3 is essential to this effect. Considering the limited cytotoxicity and side effects of SAN, it could have advantages in HCC treatment. Further study on SAN could provide a new model for treating HCC.
Abbreviations
HCC, hepatocellular carcinoma; SAN, sanguinarine; IGFBP-3/IBP3, insulin-like growth factor binding proteins-3; NOS, nitric oxide synthase; ROS, reactive oxygen species; IGF-1, insulin-like growth factor-1; IGF-1R, IGF-1 receptor; PI, Propidium iodide.
Data Sharing Statement
Target gene analysis of Sanguinarine was based on BATMAN-TCM database (http://bionet.ncpsb.org/batman-tcm/). Wynn diagram analysis was done by Bioinformatics & Evolutionary Genomics website (http://bioinformatics.psb.ugent.be/webtools/Venn/). Pathway analysis was based on KEGG (https://www.kegg.jp/).
Acknowledgments
Thanks to Affiliated Qiqihar Hospital, Southern Medical University for providing labs to complete part of the experiments in this study. Thanks to AJE® for providing the professional language service.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declared there are no financial competing interests and non-financial competing interests in this study.
References
1. Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi:10.1038/nrdp.2016.18
2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
3. Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol. 2019;70(6):1262–1277. doi:10.1016/j.jhep.2019.01.028
4. Yao N, Li YJ, Lei YH, et al. A piperazidine derivative of 23-hydroxy betulinic acid induces a mitochondria-derived ROS burst to trigger apoptotic cell death in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2016;35(1):192. doi:10.1186/s13046-016-0457-1
5. Shi J, Chen Q, Xu M, et al. Recent updates and future perspectives about amygdalin as a potential anticancer agent: a review. Cancer Med. 2019;8(6):3004–3011. doi:10.1002/cam4.2197
6. Yao X, Bai Q, Yan D, Li G, Lü C, Xu H. Solanesol protects human hepatic L02 cells from ethanol-induced oxidative injury via upregulation of HO-1 and Hsp70. Toxicol in Vitro. 2015;29(3):600–608. doi:10.1016/j.tiv.2015.01.009
7. Eichelbaum M, Gross AS. The genetic polymorphism of debrisoquine/sparteine metabolism – clinical aspects. Pharmacol Ther. 1990;46(3):377–394. doi:10.1016/0163-7258(90)90025-W
8. Och A, Zalewski D, Ł K, Kołodziej P, Kocki J, Cytotoxic B-KA. Proapoptotic activity of sanguinarine, berberine, and extracts of chelidonium majus L. and berberis thunbergii DC. toward hematopoietic cancer cell lines. Toxins (Basel). 2019;11(9):485. doi:10.3390/toxins11090485
9. Wang Y, Zhang B, Liu W, et al. Noninvasive bioluminescence imaging of the dynamics of sanguinarine induced apoptosis via activation of reactive oxygen species. Oncotarget. 2016;7(16):22355–22367. doi:10.18632/oncotarget.7971
10. Sarkhosh-Inanlou R, Molaparast M, Mohammadzadeh A, Shafiei-Irannejad V. Sanguinarine enhances cisplatin sensitivity via glutathione depletion in cisplatin resistant ovarian cancer (A2780) cells. Chem Biol Drug Des. 2019. doi:10.1111/cbdd.13621
11. Huang Q, Pu M, Zhao G, et al. Tg737 regulates epithelial-mesenchymal transition and cancer stem cell properties via a negative feedback circuit between Snail and HNF4α during liver stem cell malignant transformation. Cancer Lett. 2017;402:52–60. doi:10.1016/j.canlet.2017.05.005
12. Pu M, Wang J, Huang Q, et al. High MRPS23 expression contributes to hepatocellular carcinoma proliferation and indicates poor survival outcomes. Tumour Biol. 2017;39(7):1010428317709127. doi:10.1177/1010428317709127
13. Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer. 2015;15(11):653–667. doi:10.1038/nrc4017
14. Gong X, Zheng Y, He G, Chen K, Zeng X, Chen Z. Multifunctional nanoplatform based on star-shaped copolymer for liver cancer targeting therapy. Drug Deliv. 2019;26(1):595–603. doi:10.1080/10717544.2019.1625467
15. Dong H, Wu G, Xu H, et al. N-acetylaminogalactosyl-decorated biodegradable PLGA-TPGS copolymer nanoparticles containing emodin for the active targeting therapy of liver cancer. Artif Cells Nanomed Biotechnol. 2018;46(sup2):260–272. doi:10.1080/21691401.2018.1455055
16. Singh N, Sharma B. Toxicological effects of berberine and sanguinarine. Front Mol Biosci. 2018;5:21. doi:10.3389/fmolb.2018.00021
17. Laster LL, Lobene RR. New perspectives on sanguinaria clinicals: individual toothpaste and oral rinse testing. J Can Dent Assoc. 1990;56(7 Suppl):19–30.
18. Firatli E, Unal T, Onan U, Sandalli P. Antioxidative activities of some chemotherapeutics. A possible mechanism in reducing gingival inflammation. J Clin Periodontol. 1994;21(10):680–683. doi:10.1111/cpe.1994.21.issue-10
19. Ahmad N, Gupta S, Husain MM, Heiskanen KM, Mukhtar H. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6(4):1524–1528.
20. Malíková J, Zdarilová A, Hlobilková A, Ulrichová J. The effect of chelerythrine on cell growth, apoptosis, and cell cycle in human normal and cancer cells in comparison with sanguinarine. Cell Biol Toxicol. 2006;22(6):439–453. doi:10.1007/s10565-006-0109-x
21. Sun M, Lou W, Chun JY, et al. Sanguinarine suppresses prostate tumor growth and inhibits survivin expression. Genes Cancer. 2010;1(3):283–292. doi:10.1177/1947601910368849
22. Das M, Ansari KM, Dhawan A, Shukla Y, Khanna SK. Correlation of DNA damage in epidemic dropsy patients to carcinogenic potential of argemone oil and isolated sanguinarine alkaloid in mice. Int J Cancer. 2005;117(5):709–717. doi:10.1002/(ISSN)1097-0215
23. Weerasinghe P, Hallock S, Bax LA. Bcl-2, and NF-kappaB expression in sanguinarine induced bimodal cell death. Exp Mol Pathol. 2001;71(1):89–98. doi:10.1006/exmp.2001.2355
24. Matkar SS, Wrischnik LA, Hellmann-Blumberg U. Sanguinarine causes DNA damage and p53-independent cell death in human colon cancer cell lines. Chem Biol Interact. 2008;172(1):63–71. doi:10.1016/j.cbi.2007.12.006
25. Vrba J, Dolezel P, Vicar J, Ulrichová J. Cytotoxic activity of sanguinarine and dihydrosanguinarine in human promyelocytic leukemia HL-60 cells. Toxicol in Vitro. 2009;23(4):580–588. doi:10.1016/j.tiv.2009.01.016
26. Park H, Bergeron E, Senta H, et al. Sanguinarine induces apoptosis of human osteosarcoma cells through the extrinsic and intrinsic pathways. Biochem Biophys Res Commun. 2010;399(3):446–451. doi:10.1016/j.bbrc.2010.07.114
27. Janovská M, Kubala M, Simánek V, Ulrichová J. Interaction of sanguinarine and its dihydroderivative with the Na+/K+-ATPase. Complex view on the old problem. Toxicol Lett. 2010;196(1):56–59. doi:10.1016/j.toxlet.2010.03.1114
28. Lee JS, Jung WK, Jeong MH, Yoon TR, Kim HK. Sanguinarine induces apoptosis of HT-29 human colon cancer cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent pathway. Int J Toxicol. 2012;31(1):70–77. doi:10.1177/1091581811423845
29. Huh J, Liepins A, Zielonka J, Andrekopoulos C, Kalyanaraman B, Sorokin A. Cyclooxygenase 2 rescues LNCaP prostate cancer cells from sanguinarine-induced apoptosis by a mechanism involving inhibition of nitric oxide synthase activity. Cancer Res. 2006;66(7):3726–3736. doi:10.1158/0008-5472.CAN-05-4033
30. Sun M, Liu C, Nadiminty N, et al. Inhibition of Stat3 activation by sanguinarine suppresses prostate cancer cell growth and invasion. Prostate. 2012;72(1):82–89. doi:10.1002/pros.v72.1
31. Kim S, Lee TJ, Leem J, Choi KS, Park JW, Kwon TK. Sanguinarine-induced apoptosis: generation of ROS, down-regulation of Bcl-2, c-FLIP, and synergy with TRAIL. J Cell Biochem. 2008;104(3):895–907. doi:10.1002/jcb.21672
32. Jogie-Brahim S, Feldman D, Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr Rev. 2009;30(5):417–437. doi:10.1210/er.2008-0028
33. Li J, Jin D, Fu S, et al. Insulin-like growth factor binding protein-3 modulates osteoblast differentiation via interaction with vitamin D receptor. Biochem Biophys Res Commun. 2013;436(4):632–637. doi:10.1016/j.bbrc.2013.04.111
34. Tu W, Yang B, Leng X, et al. Testis-specific protein, Y-linked 1 activates PI3K/AKT and RAS signaling pathways through suppressing IGFBP3 expression during tumor progression. Cancer Sci. 2019;110(5):1573–1586. doi:10.1111/cas.13984
35. Shao YY, Huang CC, Lin SD, Hsu CH, Cheng AL. Serum insulin-like growth factor-1 levels predict outcomes of patients with advanced hepatocellular carcinoma receiving antiangiogenic therapy. Clin Cancer Res. 2012;18(14):3992–3997. doi:10.1158/1078-0432.CCR-11-2853
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.