Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
If Not Insulin Resistance so What? – Comparison of Fasting Glycemia in Idiopathic Parkinson’s Disease and Atypical Parkinsonism
Authors Chmiela T , Węgrzynek J , Kasprzyk A, Waksmundzki D, Wilczek D, Gorzkowska A
Received 25 January 2022
Accepted for publication 29 March 2022
Published 9 May 2022 Volume 2022:15 Pages 1451—1460
DOI https://doi.org/10.2147/DMSO.S359856
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Tomasz Chmiela,1 Julia Węgrzynek,2 Amadeusz Kasprzyk,2 Damian Waksmundzki,2 Dawid Wilczek,2 Agnieszka Gorzkowska3
1Department of Neurology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 2Students’ Scientific Association, Department of Neurorehabilitation, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland; 3Department of Neurorehabilitation, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland
Correspondence: Tomasz Chmiela, Department of Neurology, Faculty of Medical Sciences in Katowice, Medical University of Silesia, Katowice, Poland, Tel +48 32 789 46 01, Fax +48 32 789 45 55, Email [email protected]
Background: Parkinson’s disease (PD) is a synucleinopathy, which presents dysautonomia, as its common non-motor symptom. Some research suggests the existing interplay between the autonomic nervous system dysfunction and glucose metabolism dysregulation in PD.
Objective: To determine the prevalence of metabolic disorders with particular emphasis on glucose metabolism in patients with PD and atypical parkinsonism (AP).
Patients and Methods: A retrospective study was performed by analyzing 461 clinical data of consecutive patients diagnosed with PD, multiple system atrophy (MSA) and progressive supranuclear palsy (PSP) hospitalized from 2019 to 2021 in the authors’ institution. The study group included 350 patients (303 PD, 14 MSA, 33 PSP), aged 65.8 ± 9.7 years (42% were female). Laboratory results (fasting glycemia, lipid parameters, TSH, homocysteine and vitamin D3 levels) were collected. The patient’s clinical condition was assessed in III part of Unified Parkinson’s Disease Rating Scale (UPDRS p. III), Hoehn–Yahr scale, Mini Mental State Examination (MMSE) and Beck Depression Inventory (BDI).
Results: Impaired fasting glycemia (IGF) was more prevalent in PD than in the PSP (43.43% vs 18.18%; p = 0.043). Similarly, PD presented a higher level of fasting glycemia (102.4 ± 16.7 mg/dl vs 92.2 ± 16.1mg/dl; p = 0.042). According to lipid parameters, patients with PD showed lower LDL cholesterol (92.3 ± 44.3mg/dl vs 119 ± 61.0mg/dl; p = 0.016) and lower BMI compared to patients with PSP (26.1 ± 4.0kg/m2 vs 29.3 ± 4.4 kg/m2; p = 0.024), but there were no statistically significant differences in triglycerides (TG) and HDL cholesterol levels. Males with PD presented greater frequency of IFG (35.05% vs 50.6%; p = 0.042), higher fasting glycemia (99.1 ± 14.3mg/dl vs 103.7 ± 14.7mg/dl; p = 0.006), lower total cholesterol, HDL cholesterol, and BMI compared to women with PD.
Conclusion: Our investigation supports an association between synucleinopathies and glucose metabolism dysregulation.
Keywords: Parkinson’s disease, atypical parkinsonism, diabetes mellitus, impaired fasting glycemia
Introduction
Parkinsonism is a term used to describe a complex motor syndrome that manifests as rigidity, tremors, bradykinesia, and postural instability. It was initially considered specific to Parkinson’s disease (PD), but later on, also to some other neurodegenerative diseases, like multiple system atrophy (MSA) or progressive supranuclear palsy (PSP). These symptoms can also be manifestations of other conditions, like infections, intoxications, iatrogenic conditions, and central nervous system (CNS) disorders, like hydrocephalus or infarction.1
The most common cause of parkinsonism and also the second most common neurodegenerative disease is PD, which includes about eighty percent cases of parkinsonism.2 PD is characterized by the abnormal accumulation of intraneuronal aggregated α-synuclein in Lewy bodies, which reduces neuronal viability and contributes to the loss of dopaminergic neurons in the substantia nigra.1–3. Numerous post mortem studies on dopaminergic grey matter neurons of PD patients and healthy subjects show many cellular mechanisms and complex interactions including impaired mitochondrial bioenergetics, abnormal regulation of calcium homeostasis, and impaired mitochondrial degradation4,5 which leads to degeneration and death of neurons resulting in disruption of dopaminergic transmission in the extrapyramidal system manifested as motor and non-motor clinical symptoms.6,7 Usually, attention is focused on the motor manifestation of PD. However, it is estimated that the non-motor symptoms occur by even 88% of PD patients and include neuropsychiatric symptoms such as dementia, mood disorders, apathy and depression, sleep disturbances including insomnia, REM behavior disorder, sleep-disordered breathing or restless legs syndrome, and many symptoms related to autonomic dysfunction.8,9
Dysautonomia is presented by about 30–40% of PD patients and has a significant impact on their life. The most common autonomic symptoms include constipation, micturition disorders, excessive perspiration, dysfunctions of sphincters and orthostatic hypotension.10,11
Epidemiological data suggest a connection between PD and impaired glucose metabolism. It is well established that non-neurological disorders, like diabetes mellitus (DM) can determine the severity and even development of neurodegeneration, and the presence of DM in such patients increases their risk of getting PD.12–16 Prediabetes features, such an impaired glucose tolerance and impaired fasting glucose (IFG) are common in patients with PD.17 Some research suggests that glucose metabolism dysregulation in this group of patients may develop in another mechanism than in prediabetes, in the general population.
The interplay between neurodegeneration and glucose dysregulation is complex and insulin resistance seems to be both cause and consequence of neurodegeneration. As one of the possible causes is considered dysautonomia, which occurs commonly in PD patients.18 Production and secretion of insulin by the pancreatic gland is modulated by the sympathetic nervous system. In addition, epinephrine and norepinephrine secretion depends on the activation of D2 receptors.19,20 This may explain the association between autonomic dysfunction and glucose metabolism dysregulation. However, there are not many studies describing this interdependence.18,21–24 Other research presents complex mechanisms that may be responsible for glucose dysregulation in PD. It is hypothesized that mitochondrial damage in PD occurs as a result of pentose phosphate pathway dysregulation, which contributes to increasing oxidative stress and changing glucose metabolism.25 Other suggestions relate to the involvement of dopaminergic neurons promoting feeding behaviors in the hypoglycemic state, mediated by insulin receptors in the substantia nigra and therefore dopaminergic neuronal loss may alter glycemic control.14 Among the several molecular mechanisms, which link PD and DM, amylin has acquired a particular interest, because of its aggregation properties. Amylin is co-secreted with natively unfolded insulin by pancreatic beta-cells, in response to blood glucose. Studies suggested that amylin could trigger the α-synuclein amyloid formation and increase the risk of PD12,26 And inhibition of amylin-induced aggregation in the brain may be an interesting target to slow the α-synuclein amyloid formation.
Aside from PD, there are multiple neurodegenerative diseases manifesting as a parkinsonian syndrome with specific symptoms and greater severity, which are qualified as atypical parkinsonisms. This term is used to describe distinct pathological entities like multiple system atrophy (MSA) and dementia with Lewy bodies, which are alpha-synucleinopathies, and also progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS), which are caused by accumulation of tau proteins.27–30
Autonomic dysfunction is the key non-motor feature of MSA. It is also one of the symptoms appearing in advanced dementia with Lewy bodies. In other parkinsonisms, like PSP and corticobasal degeneration, the most prominent non-motor symptoms comprise those deriving from the cognitive or neuropsychiatric domain, as opposed to alpha-synucleinopathies.31 There is some presumptive evidence that α-synuclein has sophisticated connections with peripheral glucose metabolism.32 However, there is a very small number of research31,33 describing glucose metabolism in atypical parkinsonisms.
Therefore, the aim of this study was to explore the difference between the occurrence of metabolic disorders with particular emphasis on glucose metabolism in patients with PD and atypical parkinsonism (AP). To our knowledge, this is the first study confronting glucose metabolism dysregulation in these groups of patients.
Materials and Methods
We performed a retrospective analysis of all patients with diagnoses of PD, MSA, and PSP admitted to Central Clinical Hospital of the Medical University of Silesia in Katowice from January 2019 to November 2021. The initial group consisted of 461 patients, including 403 patients with PD, 17 patients with MSA, and 39 patients with PSP. The diagnosis was confirmed by neurologists experienced in diagnostics and treatment of neurodegenerative diseases. 111 patients met exclusion criteria, such as pre-existing diabetes mellitus and taking medications that affect glucose levels (n = 65); patients after implantation of deep brain stimulation of the subthalamic nucleus (DBS-STN) (n = 40), no glucose assessment (n = 65). (Figure 1.)
The study group consisted of 350 patients (147 females (42%) and 202 males (58%), aged from 35 to 84 years (mean age 65.8±9.7 years)). Among them were 303 (86.6%) patients with PD, 14 (4%) patients with MSA, and 33 (9.4%) patients with PSP.
In final group of 350 patients clinical data regarding gender, age, BMI (Body mass index), duration of the disease, assessment of motor status using III part of MDS-UPDRS scale (Movement Disorder Society – Unified Parkinson’s Disease Rating Scale), performed without DRT (dopamine replacement therapy) and after administration of a dose of levodopa, rating in Hoehn–Yahr scale, assessment of cognitive function with MMSE scale (Mini Mental State Examination) and CDT (Clock Drawing Test) and results of BDI (Beck Depression Inventory) were collected. Data on current treatment were also collected. The analysis of the laboratory results concerned inter alia glucose metabolism, lipid metabolism, thyroid function, homocysteine, and vitamin D3 levels.
The statistical analysis was performed with Statistica 13.3 (TIBCO Software Inc. (2017) Statistica (data analysis software system, version 13. http://statistica.io)). The quantitative variables are presented as an arithmetic mean and a standard deviation (normally distributed variable) or a median and the interquartile range (variables of not normal/skewed distribution). The normality of distribution was assessed with the Shapiro–Wilk test. Qualitative variables are presented as absolute values and percentages.
Due to the fact that the normal distribution in the analyzed groups was not confirmed, the intergroup differences for the quantitative variable were assessed with the U-Mann–Whitney or the Kruskal–Wallis test (variables of skewed distribution). In the case of statistically significant differences within many groups revealed by the Kruskal–Wallis test, a post-hoc type of analysis was performed. Fisher’s exact test or chi-square test were performed for qualitative variables.
Due to the retrospective character of the work and data anonymization, the Ethics Committee of the Medical University of Silesia waived the requirement to obtain ethical approval for this study.
Results
Characteristics of Groups Comparing PD Patients with Atypical Parkinsonisms
In PD patients, impaired fasting glycemia (IFG) occurred more frequently than in PSP patients (43.43% vs 18.18%, p = 0.043); similarly, PD patients had higher fasting blood glucose levels (102.4 ± 16.7 mg/dl vs 92.2 ± 16.1 mg/dl p = 0.042). Patients with PD were also characterized by lower levels of LDL cholesterol compared to patients with PSP (92.3 ± 44.3 mg/dl vs 119 ± 61.0 mg/dl, p = 0.016). There were no statistically significant differences in total cholesterol, HDL cholesterol or triglycerides levels. In terms of TSH, Homocysteine, and vitamin D3 levels. - there were no statistically significant differences.
Additionally, PD patients had a lower BMI than PSP patients (26.1 ± 4.0 kg/m2 vs 29.3 ± 4.4 kg/m2; p = 0.024).
As expected, patients with atypical parkinsonisms were characterized by a shorter disease duration (9.0 ± 5.8 years (PD) vs 4.4 ± 3.5 years (MSA) vs 4.2 ± 2.6 years (PSP); p = 0.000), a worse response to dopaminergic treatment expressed as percentage improvement assessed in part III of the MDS-UPDRS scale (OFF vs ON state) (52.4 ± 18.4% (PD); 16.2 ± 12% (MSA); 6.3 ± 10.0% (PSP) p = 0.000); patients with PSP showed more severe cognitive impairment compared to PD patients: MMSE (25.9 ± 6.3 vs 23.0 ± 6.3; p = 0.002) TRZ (9.1 ± 1.6 vs 7.3 ± 2.6 p = 0.010).
There were no differences between the groups regarding the age or gender of the patients. Detailed data are summarized in Tables 1 and 2.
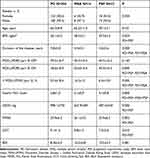 |
Table 1 Demographic and Clinical of a Final Analyzed Group Broken Down by Diagnosis. Kruskal–Wallis Test for Quantitative Variables and Fisher’s Exact Test for Qualitative Variables |
 |
Table 2 Laboratory Characteristic of a Final Analyzed Group Broken Down by Diagnosis. Kruskal–Wallis Test for Quantitative Variables and Fisher’s Exact Test for Qualitative Variables |
Characteristics of Men and Women in the Final Group of Patients with PD
Among the patients with PD men had higher glycemia (99.1 ± 14.3 mg/dl vs 103.7 ± 14.7 mg/dl p = 0.006) and greater frequency of impaired fasting glycemia (35.05% vs 50.6%) p=0.042). They presented also lower total cholesterol (208.5 ± 49.7 mg/dl vs 196.9 ± 47.6 mg/dl; p = 0.040), lower HDL cholesterol (64.3 ± 16.0 mg/dl vs 57.1 ± 12.2 mg/dl; p = 0.008), as well as a lower BMI (26.9 ± 3.8 vs 24.9 ± 4.1; p = 0.002). Women had a higher mean BDI score (10.1 ± 6.5 vs 7.1 ± 5.1; p = 0.000). Detailed data are summarized in Tables 3 and 4.
 |
Table 3 Demographic and Clinical Characteristic of a PD Patients Group Broken Down by Gender. U-Mann–Whitney Test for Quantitative Variables and Fisher’s Exact Test for Qualitative Variables |
 |
Table 4 Laboratory Characteristic of a PD Patients Group Broken Down by Gender. U-Mann–Whitney Test for Quantitative Variables and Fisher’s Exact Test for Qualitative Variables |
Discussion
Parkinson’s disease (PD) and diabetes mellitus (DM) are two important aging-associated disorders. DM is considered an epidemic in modern societies. World Health Organization (WHO) estimated that in 2019, diabetes was the ninth leading cause of death, and 1.5 million deaths were directly caused by diabetes.34 Prediabetes is a condition preceding diabetes mellitus type 2 (DMT2). It is caused by disturbances in signaling pathways leading to receptors’ resistance to insulin actions in the body cells. Prediabetes is defined as impaired fasting glycemia (IFG) or impaired glucose tolerance, depending on hepatic or muscles resistance.35,36 Occurrence of IFG in the general population, aged 50–65, based on Croatian study, was assessed as 15.7%.37 PD affects at least 1% of the population above age 60.38 Our study results have shown that IFG occurred in 43.43% of patients with PD. There is no other data approximating IFG prevalence in this population to compare. There is strong evidence suggesting that DM can be a risk factor for PD. Moreover, DM is considered to be a modifying factor for motor and non-motor factors in PD.12 Nevertheless there are several epidemiological and experimental studies that revealed the association between DM and PD and suggest that PD is a possible cause of glucose metabolism dysregulation.14–19
Atypical parkinsonism (AP) is a diverse group of disorders, presenting specific symptoms and different courses from PD. It is estimated that the percentages of AP conditions such as multiple system atrophy (MSA), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and dementia with Lewy bodies (DLB) among parkinsonian syndromes comprise 10–15% of patients.37 We have included to the study all consecutive patients from 2019 to 2021. Among this population, the percentage of patients with AP has been 13.39% (similarly to general estimation). AP has been characterized by greater severity coexisting with worse dopaminergic treatment response and shorter survival of patients.38–40 Our study has shown consistent results. Patients diagnosed with MSA and PSP have presented worse response to dopaminergic treatment and higher scores on the H-Y scale with a shorter average disease duration. PSP predisposes to early gait disturbances and falls, which determines the worse physical condition of these patients.41 That explains the highest score on the H-Y scale, in comparison to all three groups of our patients. Likewise, results of a test assessing neuropsychiatric condition that has been worse in tauopathy.25
According to metabolic parameters, there is a small amount of research comparing PD and other parkinsonisms, so our knowledge is still incomplete in this regard. In general, population causes and risk factors of metabolic diseases are commonly known. For well over half a century, the link between insulin resistance and DMT2 has been recognized.35 At present, a well-known topic is Metabolic Syndrome (MeS), in which diabetes is an important component. Moreover, MeS is strongly associated with an increased risk for the development of atherosclerotic and nonatherosclerotic cardiovascular disease. MeS is defined by the latest three of the following criteria: central obesity, reduced HDL cholesterol, elevated TG, arterial hypertension, and a previously mentioned IFG. The commonly known fact is that insulin resistance has been associated with central obesity as well.35,36
Our study, focused on metabolic disturbances in patients diagnosed with PD, has shown that typical risk factors related to insulin resistance have not occurred in this group of patients. In PD patients, IFG has occurred more frequently (43.43%) than in PSP patients (18.18%), and a higher level of fasting glycemia (102.4 vs 92.2 mg/dl) has occurred, in spite of no statistically significant differences in total cholesterol, HDL cholesterol, or TG levels.
However, patients with PD have also been characterized by reduced levels of LDL cholesterol compared to patients with PSP (92.3 mg/dl vs 119 mg/dl), and lower BMI (26.1 vs 29.3 kg/m2). Higher levels of LDL cholesterol often occur in prediabetes or DMT2. Some research studies suggest that isolated IFG is related to increased apolipoprotein B and total LDL particles.42,43 BMI represents a common risk factor for insulin resistance. Furthermore, increasing BMI is related to prediabetes.
IFG is a type of prediabetes, in which blood glucose levels during fasting are consistently above the normal range, but below the cut-off point for a diagnosis of DM. Together with impaired glucose tolerance (IGT), IFG is a sign of insulin resistance, and also one of the conditions associated with MeS.44–47 Patients with MSA had similar laboratory results to the ones with PD. However, there were no statistically significant differences between the MSA and PSP groups, probably due to small sample size of MSA patients.
It is assessed that PD occurs 1.5 more frequent by men than women, what has been shown in our study48 Comparing results between men and women, only in a group of patients with PD, we have received a similar tendency to higher fasting and greater frequency of IFG, in spite of lower BMI in the male group of patients. Unlike a meta-analysis by You et al,15 in this study, the male patients with PD had higher fasting glycemia and higher frequency of IFG than the females. However, in the general population, IFG is more prevalent in males, so the presented results are adequate.49,50
Similarly, lipid profiles are more unfavorable in post-menopausal females in the general population.51
Results of the conducted study suggest that besides the known role of glucose dysregulation in the development of neurodegeneration, there exist different mechanisms that contribute to DM by PD (opposite to PSP, and probably to the general population). Some research has shown the role of α-synuclein in glucose regulation, especially in beta-cell function and glucose utilization in peripheral tissues.19,32 It may confirm the association between synucleinopathies and specific glucose dysregulation. The mechanism may be related to autonomic dysfunction which occurs frequently in PD and MSA, unlike PSP. It has been estimated that autonomic disorders may be present in about 60% of patients with α-synucleinopathies, while only in 30% of patients with PSP or CBS.10
A similar study conducted to check factors related to glucose dysregulation in patients diagnosed with PD may confirm this suggestion, because it showed higher blood glucose levels associated with higher severity of dysautonomia, which was checked using Scales for Outcomes in Parkinson’s Disease – Autonomic Dysfunction. It is a questionnaire for the assessment of autonomic dysfunction in patients with PD.18 Nevertheless, there is still not enough research to prove a strong association and explain clearly all relevant mechanisms. In particular, there are no other studies comparing glucose dysregulation in synucleinopathies and other parkinsonisms, which seems to be the right direction for subsequent research.
Our study has limitations. First of all, the sample size of AP subtypes is relatively small, but it is worth noticing that AP are rare. We analyzed patients consecutively admitted to our department from 2019 to 2021, and in the single site setting, collecting a bigger group of patients with AP may otherwise take many years. Secondly, due to the retrospective nature of our study, we were unable to collect data on the physical activity of the patients.
Conclusion
Impaired fasting glucose (IFG) in Parkinson’s Disease is common in PD and MSA, but not in PSP, and thus, IFG seems to be a clinical feature of synucleinopathies. In this study, IFG has been more prevalent in synucleinopathies. These groups of patients have demonstrated a higher fasting glycemia. These results have not been related to typical metabolic risk factors including high BMI and high LDL cholesterol. Considering all this information, and data from studies carried up to this date, it has been suggested that there is an association between glucose metabolism dysregulation and synucleinopathies with autonomic dysfunction, as one of the possible mechanisms.
However, more research needs to be conducted to explain this mechanism, because it may be crucial for potential designing future therapeutic strategies for patients suffering from PD, prediabetes, and DM.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study, due to the retrospective character of the work and data anonymization. The Ethics Committee of the Medical University of Silesia waived the requirement to obtain the ethical approval for this study.
Informed Consent Statement
Patient consent was waived due to the retrospective character of the work and data anonymization.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Funding
This research received no external funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi:10.1016/S0140-6736(14)61393-3
2. Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27–42. doi:10.1111/ene.14108
3. Darden L. Mechanisms and models. Cambridge Companion to Philos Biol. 2007;39:139–159.
4. Zheng B, Liao Z, Locascio JJ, et al. PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med. 2010;2:52ra73.
5. Seibler P, Graziotto J, Jeong H, et al. Mitochondrial parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci. 2021;3:5970–5976.
6. Guzman JN, Sanchez-Padilla J, Wokosin D, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468(7324):696–700. doi:10.1038/nature09536
7. Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150:963–976. doi:10.1038/sj.bjp.0707167
8. Barbosa ER. Premotor manifestations of Parkinson’s disease. Rev Neurol. 2010;50:65–80.
9. Kim HS, Cheon SM, Seo JW, et al. Nonmotor symptoms more closely related to Parkinson’s disease: comparison with normal elderly. J Neurol Sci. 2013;324:70–73. doi:10.1016/j.jns.2012.10.004
10. Grażyńska A, Urbaś W, Antoniuk S, et al. Comparative analysis of non-motor symptoms in patients with Parkinson’s Disease and atypical parkinsonisms. Clin Neurol Neurosurg. 2020;197:106088.
11. Merola A, Romagnolo A, Rosso M, et al. Autonomic dysfunction in Parkinson’s disease: a prospective cohort study. Mov Disord. 2018;33:391–397. doi:10.1002/mds.27268
12. Labandeira CM, Fraga-Bau A, Arias Ron D, et al. Diabetes, insulin and new therapeutic strategies for Parkinson’s disease: focus on glucagon-like peptide-1 receptor agonists. Front Neuroendocrinol. 2021;62:100914. doi:10.1016/j.yfrne.2021.100914
13. Hu G, Jousilahti P, Bidel S, Antikainen R. Type 2 diabetes and the risk of Parkinson’s Disease. Diabetes Care. 2007;30:842–847. doi:10.2337/dc06-2011
14. Xu Q, Park Y, Huang X, et al. Diabetes and risk of Parkinson’s disease. Diabetes Care. 2011;34(4):910–915. doi:10.2337/dc10-1922
15. Yue X, Li H, Yan H, et al. Risk of Parkinson disease in diabetes mellitus: an updated meta-analysis of population-based cohort studies. Medicine. 2016;95:3549. doi:10.1097/MD.0000000000003549
16. Yang YW, Hsieh TF, Li CI, et al. Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine. 2017;15(1):96. doi:10.1186/s12916-017-0859-8
17. Chung HS, Lee JS, Kim JA, et al. Fasting plasma glucose variability in midlife and risk of Parkinson’s disease: a nationwide population-based study. Diabetes Metab. 2021;47:101195.
18. Marques A, Dutheil F, Durand E, et al. Glucose dysregulation in Parkinson’s disease: too much glucose or not enough insulin? Park Relat Disord. 2018;55:122–127. doi:10.1016/j.parkreldis.2018.05.026
19. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14(1):45–54. doi:10.1016/j.cmet.2011.05.008
20. Steinbusch L, Labouèbe G, Thorens B. Brain glucose sensing in homeostatic and hedonic regulation. Trends Endocrinol Metab. 2015;26:455–466.
21. Van Woert MH, Mueller PS. Glucose, insulin, and free fatty acid metabolism in Parkinson’s disease treated with levodopa. Clin Pharmacol Ther. 1971;12:360–367. doi:10.1002/cpt1971122part2360
22. Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain. 2013;136:374–384. doi:10.1093/brain/aws009
23. Sandyk R. The relationship between diabetes mellitus and Parkinson’s disease. Intern J Neurosci. 1993;69:125–130. doi:10.3109/00207459309003322
24. Scigliano G, Musicco M, Soliveri P, et al. Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke. 2006;37:1184–1188. doi:10.1161/01.STR.0000217384.03237.9c
25. Dunn L, Allen GFG, Mamais A, et al. Dysregulation of glucose metabolism is an early event in sporadic Parkinson’s disease. Neurobiol Aging. 2014;35:1111–1115. doi:10.1016/j.neurobiolaging.2013.11.001
26. Sharma MK, Jalewa J, H¨olscher C. Neuroprotective and anti-apoptotic effects of liraglutide on SH-SY5Y cells exposed to methylglyoxal stress. J Neurochem. 2014;128:459–471. doi:10.1111/jnc.12469
27. Levin J, Kurz A, Arzberger T, et al. The differential diagnosis and treatment of atypical parkinsonism. Dtsch Arztebl Int. 2016;113:61–69. doi:10.3238/arztebl.2016.0061
28. Fanciulli A, Stankovic I, Krismer F, et al. Multiple system atrophy. Int Rev Neurobiol. 2019;149:137–192. doi:10.1016/bs.irn.2019.10.004
29. Fabbrini G, Fabbrini A, Suppa A. Progressive supranuclear palsy, multiple system atrophy and corticobasal degeneration. Handb Clin Neurol. 2019;165:155–177.
30. Stamelou M, Hoeglinger GU. Atypical parkinsonism: an update. Curr Opin Neurol. 2013;26(4):401–405. doi:10.1097/WCO.0b013e3283632da6
31. Bhatia KP, Stamelou M. Nonmotor features in atypical Parkinsonism. Int Rev Neurobiol. 2017;134:1285–1301.
32. Wijesekara N, Ahrens R, Wu L, et al. α-synuclein regulates peripheral insulin secretion and glucose transport. Front Aging Neurosci. 2021;13:1–9. doi:10.3389/fnagi.2021.665348
33. Bassil F, Canron MH, Vital A, et al. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain. 2017;140:1420–1436. doi:10.1093/brain/awx044
34. World Health Organization. Global report on diabetes: Executive Summary; 2016.Available from: https://www.who.int/publications/i/item/9789241565257. Accessed May 4, 2022
35. Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61(4):778–779. doi:10.2337/db12-0073
36. Rochlani Y, Pothineni NV, Kovelamudi S, et al. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Vaccines. 2018;9:259–261.
37. Metelko Ž, Pavlić-Renar I, Poljičanin T, et al. Prevalence of diabetes mellitus in Croatia. Diabetes Res Clin Pract. 2008;81:263–267. doi:10.1016/j.diabres.2008.04.016
38. Martin WRW, Miles M, Zhong Q, et al. Is levodopa response a valid indicator of parkinson’s disease? Mov Disord. 2021;36:948–954. doi:10.1002/mds.28406
39. Hirschbichler ST, Erro R, Ganos C, et al. “Atypical” atypical parkinsonism: critical appraisal of a cohort. Park Relat Disord. 2017;37:36–42. doi:10.1016/j.parkreldis.2016.12.006
40. Hassan A, Sharma Kandel R, Mishra R, et al. Diabetes mellitus and parkinson’s disease: shared pathophysiological links and possible therapeutic implications. Cureus. 2020;12. doi:10.7759/cureus.9853
41. Painous C, Martí MJ, Simonet C, et al. Prediagnostic motor and non-motor symptoms in progressive supranuclear palsy: the step-back PSP study. Park Relat Disord. 2020;74:67–73. doi:10.1016/j.parkreldis.2020.03.003
42. Kansal S, Kamble TK. Lipid Profile in Prediabetes. J Assoc Physicians India. 2016;64:18–21.
43. Lorenzo C, Hartnett S, Hanley AJ, et al. Impaired fasting glucose and impaired glucose tolerance have distinct lipoprotein and apolipoprotein changes: the insulin resistance atherosclerosis study. J Clin Endocrinol Metab. 2013;98(4):1622–1630. doi:10.1210/jc.2012-3185
44. Martinez KE, Tucker LA, Bailey BW, LeCheminant JD. Expanded normal weight obesity and insulin resistance in US adults of the national health and nutrition examination survey. J Diabetes Res. 2017;2017:1–8. doi:10.1155/2017/9502643
45. Abbasi F, Brown BW, Lamendola C, et al. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–943. doi:10.1016/S0735-1097(02)02051-X
46. Al-Farai HH, Al-Aboodi I, Al-Sawafi A, et al. Insulin resistance and its correlation with risk factors for developing diabetes mellitus in 100 Omani medical students. Sultan Qaboos Univ Med J. 2014;14:393–396.
47. Ali A, Taj A, Ahmed MU, Tabrez E. Frequency of impaired fasting glucose in first degree relatives of type-ii diabetic patients and its association with body mass index. Pakistan J Med Sci. 2020;36:407–411.
48. Reekes TH, Higginson CI, Ledbetter CR, et al. Sex specific cognitive differences in Parkinson disease. Npj Park Dis. 2020;6:1–6.
49. Tonolo G. Sex-gender awareness in diabetes. Diabetology. 2021;2(2):117–122. doi:10.3390/diabetology2020010
50. Williams JW, Zimmet PZ, Shaw JE, et al. Gender differences in the prevalence of impaired fasting glycemia and impaired glucose tolerance in Mauritius Does sex matter? Diabet Med. 2003;20:915–920. doi:10.1046/j.1464-5491.2003.01059.x
51. Anagnostis P, Stevenson JC, Crook D, et al. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas. 2015;81:62–68. doi:10.1016/j.maturitas.2015.02.262
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

