Back to Journals » International Medical Case Reports Journal » Volume 16
Idiopathic Granulomatous Mastitis as an Unusual Cause of Erythema Nodosum in a Malagasy Woman
Authors Rakotoarisaona MF , Razafimaharo TI, Razanakoto NH, Sendrasoa FA , Ducournau A, Devalland C, Dupond AS, Ranaivo IM , Ramarozatovo LS, Rapelanoro Rabenja F
Received 12 January 2023
Accepted for publication 28 February 2023
Published 11 March 2023 Volume 2023:16 Pages 159—165
DOI https://doi.org/10.2147/IMCRJ.S403050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Video abstract presented by Mendrika Fifaliana Rakotoarisaona.
Views: 248
Mendrika Fifaliana Rakotoarisaona,1 Tsiory Irintsoa Razafimaharo,1 Naina Harinjara Razanakoto,1 Fandresena Arilala Sendrasoa,1 Anne Ducournau,2 Christine Devalland,3 Anne-Sophie Dupond,2 Irina Mamisoa Ranaivo,4 Lala Soavina Ramarozatovo,1 Fahafahantsoa Rapelanoro Rabenja1
1Department of Dermatology, University Hospital Joseph Raseta Befelatanana, Antananarivo, Madagascar; 2Department of Dermatology, Nord Franche-Comté Hospital, Trevenans, France; 3Department of Pathology, Nord Franche-Comté Hospital, Trevenans, France; 4Department of Dermatology, University Hospital Morafeno, Toamasina, Madagascar
Correspondence: Mendrika Fifaliana Rakotoarisaona, Department of Dermatology, University Hospital Joseph Raseta Befelatanana, Rue Dr Davioud Jacques, Antananarivo, 101, Madagascar, Tel/Fax +261 34 61947 34, Email [email protected]
Introduction: Idiopathic granulomatous mastitis (IGM) is a rare chronic inflammatory disease. Neoplastic and infectious etiologies must be ruled out. IGM is a diagnostic challenge for countries with high tuberculosis endemicity like Madagascar since it may clinically and radiologically mimic breast tuberculosis. We report a case of IGM associated with erythema nodosum in a Malagasy.
Case Report: A 29-year-old primiparous woman came to a dermatological consultation for typical erythema nodosum lesions that appeared one month after a breast swelling. She had no particular medical history. Examination revealed typical erythema nodosum lesions on the legs, voluminous tender mass in the right breast. Bacteriological samples and tuberculosis test were negative. Imaging showed mastitis on the right breast with no evidence of malignancy. Histology revealed a non-caseating granulomas on the lobule of the right breast. As part of an etiological work-up, COVID-19 serology was performed with a positive IgG antibody. The diagnosis of IGM associated with erythema nodosum was evocated. The evolution was favorable under systemic corticosteroid therapy.
Discussion: The cause of this uncommon lesion remains obscure. The extramammary localizations such as erythema nodosum and arthralgia suggest an autoimmune origin. This pathogenesis is also reinforced by a good response to systemic immunosuppression. In our patient, the etiological assessment of the mastitis revealed a chronic infection with SARS-CoV-2. Histopathology is the gold standard for the IGM diagnosis which demonstrates a lobulocentric granulomas without caseous necrosis. Oral corticosteroid therapy is the initial choice of treatment.
Conclusion: Now, with several cases of concomitant IGM and EN reported, dermatologists should be aware that erythema nodosum can be one of the presenting signs of IGM, since the two conditions appear to be associated. The particularity of our case lies in the incidental discovery of SARS-CoV-2 infection. Is a chronic granulomatous disease associated with SARS-CoV-2 infection, a coincidence?
Keywords: corticosteroid, erythema nodosum, granulomatous mastitis, Madagascar
Introduction
Idiopathic granulomatous mastitis (IGM) is an uncommon chronic inflammatory disease of the breast characterized by a non-caseating granulomas confined to breast lobules.1 IGM is a rare cause of erythema nodosum. Association of IGM and erythema nodosum is a rare feature, less than 30 cases have been reported in the literature.2,3 IGM may simulate malignancy or infection such as breast tuberculosis.4,5 Due to the fact that clinical manifestations and radiological images are similar to those of breast tuberculosis, IGM may be misdiagnosed and treated as tuberculosis especially in an endemic area.6 We report a case of MGI associated with erythema nodosum in a Malagasy woman in order to raise the diagnostic and therapeutic challenge that it represents.
Case Report
A 29-year-old primiparous Malagasy woman came for a consultation to the dermatological clinic of the Nord Franche-Comté Hospital in June 2020 for painful nodules on her legs typical of erythema nodosum. These lesions developed a month after a swelling of the right breast undergoing etiological investigation. She was not pregnant or lactating. She had last breastfed her child two years ago. She did not use oral contraceptives or any other medication. The patient had no history of tuberculosis infection or autoimmune disease or any family history of breast cancer. A contact with SARS-CoV-2 was noted in April 2020 for which she had not presented any symptoms.
On clinical examination, she was not febrile. Voluminous painful tender mass (10x6 cm diameter) was located in upper inner of the right breast with retraction of the nipple (Figure 1). The contralateral breast was unremarkable. The right axillary lymphadenopathy was palpable. The dermatological examination revealed a typical erythema nodosum lesions characterized by multiple erythematous, painful, infiltrated nodules of varying size on the lower limbs (Figure 2). The remaining examination was unremarkable.
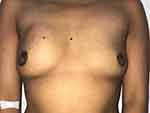 |
Figure 1 Tender mass of the right breast with mild nipple retraction. |
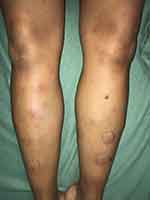 |
Figure 2 Erythematous indurated nodules over the lower extremities. |
Biological investigations for an underlying cause such as inflammatory tests (full blood count, erythrocyte sedimentation rate, C-reactive protein), autoimmune markers (antinuclear antibody, anti-DNA, anti-neutrophil cytoplasm antibody) and infective serologies (HIV, hepatitis B and C) were negative or within normal range. Serology SARS-CoV-2 IgG testing as part of a systematic work-up came back positive at over 100.
An ultrasound examination, performed twice, showed a hypoechoic mass on the right side, frankly hyper vascularized in favor of mastitis. Mammography did not reveal any suspicious mass or microcalcification. A purulent nipple discharge occurred during the mammography.
Breast biopsies showed a lobulocentric subacute mastitis with a non-caseating granulomatous inflammation and multinucleated giant cells (Figure 3a and b). There was no evidence of malignancy. Immunohistochemistry was normal. No organisms were identified on Grocott methanamine silver (GMS), Gram, Periodic acid-Schiff (PAS) and Ziehl-Neelsen stainings. Bacterial, fungal and mycobacterium cultures of the aspiration specimen were all negative.
TB quantiferon, polymerase chain reaction test and acid-fast bacilli staining were negative. On the other hand, blood calcium and angiotensin-converting enzyme level were within normal range. The chest X-ray did not show any hilar adenopathy or pulmonary parenchymal infiltrate. Either tuberculosis or sarcoidosis was excluded with these results.
Initially, an infectious origin was suggested. She was empirically treated with antibiotics: pristinamycin (7 days), amoxicillin-clavulanic acid (10 days), and trimethoprim-sulfamethoxazole (2 months). Then, a treatment with colchicine 1mg/day was administered as the disease worsened with discharge and fistulation of the swelling at the biopsy sites. Spectrometry of a pus sample identified a germ called Corynebacterium kroppenstedtii. The other two bacteriological control samples were aseptic.
The diagnosis of IGM associated with erythema nodosum was evocated. Daily prednisone 60 mg was started with a gradual taper 5 months. The evolution was rapidly favorable with complete regression of the breast swelling and erythema nodosum. No recurrence was reported within two years of follow-up.
Discussion
IGM was first described by Kessler and Wolloch in 1972 as “a rare benign chronic inflammatory disease of the breast of unknown etiology”.6 It is a subtype of panniculitis characterized by non-caseating granulomatous inflammation located in the lobules.7 IGM is a rare cause of mastopathy. The association of IGM and erythema nodosum is an even rarer entity. The first case was described by Adams in 1987.8
IGM usually occurs in healthy women of childbearing age like our patient and exceptionally adolescent girls, menopausal women and men. A mean age of 33.5 years was reported with an extreme of 17 and 78 years.1,8,9
Clinical manifestations include unilateral or bilateral breast pain, swelling, and indurated mass formation associated with homolateral axillary lymphadenopathy. A skin or a nipple retraction may be present as reported in our patient.10 Histology helps to rule out differential diagnosis and to confirm the diagnosis of IGM.2,3 It typically demonstrates granulomas located in the center of the breast lobule with dense neutrophilic infiltration that may form micro-abscesses. It consists on non-caseating granulomas with multinucleated giant cells and epithelioid histiocytes. The presence of the granulomas in the center of the lobules and the absence of caseous necrosis can help to distinguish IGM from other granulomatous diseases.7,11,12
The pathogenesis of this uncommon lesion remains obscure. Several hypotheses have been put forward, such as pregnancy, breastfeeding, contraceptive pills, smoking, α1-antitrypsin deficiency, hyperprolactinemia.13–19 Some authors have suggested that IGM is a local granulomatous inflammatory response to the extravasation of glandular secretions into the lobular connective tissue after a traumatic, toxic or infectious trigger.20 Several reports have proposed that IGM might have an autoimmune disease component.21–23 The extra-mammary involvement of IGM (arthralgia, erythema nodosum, episcleritis, suppurative hidradenitis), its association with autoimmune diseases (Sjogren’s disease) and the presence of histological analogies with lesions observed in thyroiditis or granulomatous orchitis could raise the suspicion of an autoimmune origin.2,24,25 This proposed pathogenesis is also supported by a good response to systemic immunosuppression. Konstantinos et al proposed that the described phenotype represents an underrecognized systemic autoimmune disease that could be designated by the acronym “GMENA” (granulomatous mastitis, erythema nodosum, arthritis) syndrome.26 Koksal et al reported the role of proinflammatory cytokines including IL-8 and IL-17 in the etiopathogenesis of IGM.27 Infection has been also suggested as another trigger for IGM. Some studies reported the role of Corynebacterium in the formation of micro-abscesses during IGM.28,29 In our patient, Corynebacterium kroppenstedtii was found in a single local bacteriological sample. The control samples (repeated three times) aimed to confirm its role in the occurrence of IGM came back negative. In the literature, the study of the role of this germ in IGM is controversial and conflicting. Taylor et al detected Corynebacterium in 41% of 34 isolated IGM lesions.29 A causal effect was not confirmed since Corynebacterium is a member of normal flora.7,29 On the contrary, some authors have suggested that C. kroppenstedtii may be involved in the occurrence of IGM as it was found in all bacteriological samples taken on several occasions.30–32 In our case, SARS-CoV-2 are considered as a possible etiologic agent and an initial trigger of IGM. Since the beginning of the COVID-19 pandemic, emergence of inflammatory and autoimmune disease was reported.33 Our patient had a history of contamination two months before the onset of clinical manifestations of IGM. In this context, as our patient did not have any autoimmune diseases and was in good health, a SARS-CoV-2 test was performed. The test came back positive thus, an IGM as a sequela of COVID-19 were suspected. In certain individuals, a longer phase of disease named long COVID can follow the acute phase of the infection. The long COVID of unknown etiology is probably associated with autoimmune dysregulation and can lead to the development of autoimmune phenomena and the new-onset of rheumatic autoimmune diseases.34 It is known that SARS-CoV-2 is responsible for uncontrolled dramatic release of proinflammatory cytokines often referred to as “cytokine storm” or “cytokine release syndrome” which promotes macrophage activation and accumulation resulting in the granulomas formation.35 On the other hand, recent data reported that in sarcoidosis and idiopathic granulomatous multisystem disease, SARS-CoV-2 may induce the non-caseating granulomas formation via the angiotensin-converting enzyme II (ACE II) and the innate immune system.33 However, the mechanisms underlying the association between autoimmune abnormalities and COVID-19 remain not well documented and most of the existing evidence was based on case reports and case series without a long-term follow-up.35
IGM, a benign breast disorder, remains a diagnosis of exclusion. Other granulomatous diseases should be first ruled out such as infectious (tuberculosis and non-tuberculosis mycobacterial infections), autoimmune diseases (sarcoidosis, granulomatosis with polyangiitis or Wegener's granulomatosis) and other conditions (diabetes, breast cytosteatonecrosis, foreign body reaction, granulomatous reaction to ductal ectasia or carcinoma).10,11,20,28,36
IGM posed a differential diagnosis problem with breast tuberculosis in our case. It was the first diagnosis suspected in our patient in view of the epidemiological context given that Madagascar is a tuberculosis endemic country. Although mammary tuberculosis is rare (0.06–0.1% of cases), it should be ruled out in patients living in an endemic country or in immunocompromised patients.37 Tuberculosis must be excluded and frequently requires more sensitive testing such as TB quantiferon or polymerase chain reaction of the core biopsy rather than the absence of acid-fast bacilli or culture.38
There is no consensus in the treatment of IGM. In moderate and severe forms, systemic corticosteroid therapy is the initial choice of treatment.39,40 Studies have also reported the effectiveness of colchicine and hydroxychloroquine. Alternative therapies include immunosuppressive drugs such as methotrexate and azathioprine.41 Surgery is indicated rather for diagnostic purposes than treatment of IGM because of the delayed healing of postoperative wounds and the high rate of recurrence, which can be more than 50% of cases.7,42,43 Alternatively, some investigators propose that IGM should be treated with wide local excision at the onset of disease, citing a lower chance of recurrence with surgical therapy.44–47 In our patient, colchicine was not effective and the second-line treatment was corticosteroid with a good response and no recurrence was reported over 2 years.
The evolution of IGM disease is generally protracted, with a significant impact on quality of life due to cosmetic sequela. In moderate forms, a spontaneous remission can be obtained after 9 to 12 months of evolution.7 Pandey et al reported in a prospective study of 49 women that a complete remission was achieved from 3 to 18 months after steroid treatment.48 Our patient had a complete resolution to medical treatment after 5 months.
Conclusion
IGM is a benign disease and the awareness of this rare entity prevents delays in diagnosis and unnecessary treatment. Our case highlighted that IGM is a poorly understood disease that often evades to diagnosis. Although the etiology of IGM is unknown, it seems that IGM is an autoimmune disease of the breast. Now, with several cases of concomitant IGM and erythema nodosum reported, dermatologists should be aware that erythema nodosum can be one of the presenting signs of IGM since the two conditions appear to be associated. In the diagnostic workup, other granulomatous diseases should be ruled out. Sarcoidosis and tuberculosis were the most frequent diagnosis in the association of IGM and erythema nodosum. The particularity of our case lies in the fortuitous discovery of an infection with SARS-CoV-2. We presented a case of IGM associated with erythema nodosum as an interesting possible sequela of SARS-CoV-2 infection. Chronic granulomatous disease associated with SARS-CoV-2 infection, is it a coincidence? As the mechanisms underlying this association are poorly understood, further studies and longer follow-ups are needed to confirm the link between SARS-CoV-2 and the occurrence of IGM.
Ethical Considerations
No conflict of interest regarding ethics occurred during the formulation or development of this article. This article was prepared after obtaining the written and informed consent from the patient for the publication of this case report and the photographs. No institutional approval was required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ayeva-Derman M, Perrotin F, Lefrancq T, et al. Idiopathic granulomatous mastitis: review of the literature illustrated by 4 cases. J Gynecol Obstet Biol Reprod. 1999;28:800–807.
2. Ellie CEC, Soon Boon JW, Sue-Ann JEH. Idiopathic granulomatous mastitis and erythema nodosum: a unifying pathophysiology? Australas J Dermatol. 2020;62:e149–e153.
3. Jacquin-Porretaz C, Devalland C, Delapparent T, et al. Idiopathic granulomatous mastitis associated with erythema nodosum. Ann Dermatol Venereol. 2019;146(8–9):571–576. doi:10.1016/j.annder.2019.04.023
4. Pereira FA, Mudgil AV, Macias ES, et al. Idiopathic granulomatous lobular mastitis. Int J Dermatol. 2012;51(2):142–151. doi:10.1111/j.1365-4632.2011.05168.x
5. Imoto S, Kitaya T, Kodama T. Idiopathic granulomatous mastitis: case report and review of the literature. Jpn J Clin Oncol. 1997;27:274–277. doi:10.1093/jjco/27.4.274
6. Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically stimulating carcinoma. Am J Clin Pathol. 1972;58:642–646. doi:10.1093/ajcp/58.6.642
7. Vural S, Ertop P, Ceyhan K, et al. An unusual cause of oligoarthritis and erythema nodosum: idiopathic granulomatous mastitis. Arch Rheumatol. 2017;32(1):71–75. doi:10.5606/ArchRheumatol.2017.5952
8. Adams DH, Hubscher SG, Scot DG, et al. Granulomatous mastitis: a rare cause of erythema nodosum. Postgrad Med J. 1987;63(741):581–582. doi:10.1136/pgmj.63.741.581
9. Omranipour R, Mohammadi SF, Samimi P. Idiopathic granulomatous lobular mastitis: report of 43 cases from Iran; introducing a preliminary clinical practice guideline. Breast Care. 2013;8:439–443. doi:10.1159/000357320
10. Fruchter R, Castilla C, Ng E, et al. Erythema nodosum in association with idiopathic granulomatous mastitis: a case series and review of the literature. J Eur Acad Dermatol Venereol. 2017;31:391–393. doi:10.1111/jdv.14194
11. Tse GM, Poon CS, Ramachandram K, et al. Granulomatous mastitis: a clinicopathological review of 26 cases. Pathology. 2004;36:254–257.
12. Lee JH, Oh KK, Kim EK, et al. Radiologic and clinical features of idiopathic granulomatous lobular mastitis mimicking advanced breast cancer. Yonsei Med J. 2006;47:78–84. doi:10.3349/ymj.2006.47.1.78
13. Altintoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases. 2014;2:852–858. doi:10.12998/wjcc.v2.i12.852
14. Benson JR, Dumitru D. Idiopathic granulomatous mastitis: presentation, investigation and management. Future Oncol. 2016;12:1381–1394. doi:10.2217/fon-2015-0038
15. Altintoprak F, Karakece E, Kivilcim T, et al. Idiopathic granulomatous mastitis: an autoimmune disease? Sci World J. 2013;2013:1–5. doi:10.1155/2013/148727
16. Sheybani F, Naderi HR, Gharib M, et al. Idiopathic granulomatous mastitis: long-discussed but yet to be known. Autoimmunity. 2016;49:236–239. doi:10.3109/08916934.2016.1138221
17. Ozel L, Unal A, Unal E, et al. Granulomatous mastitis: is it an autoimmune disease? Diagnostic and therapeutic dilemmas. Surg Today. 2012;42:729–733. doi:10.1007/s00595-011-0046-z
18. Lin CH, Hsu CW, Tsao TY, et al. Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol. 2012;7:2. doi:10.1186/1746-1596-7-2
19. Jeon J, Lee K, Kim Y, Chun YS, Park HK. Retrospective analysis of idiopathic granulomatous mastitis: its diagnosis and treatment. J Breast Dis. 2017;5:82–88. doi:10.14449/jbd.2017.5.2.82
20. Fletcher A, Magrath IM, Riddell RH, et al. Granulomatous mastitis: a report of seven cases. J Clin Pathol. 1982;35:941–945. doi:10.1136/jcp.35.9.941
21. Binesh F, Shiryazdi M, Bagher Owlia M, et al. Idiopathic granulomatous mastitis, erythema nodosum and bilateral ankle arthritis in an Iranian woman. BMJ Case Rep. 2013;2013:bcr2012007636–bcr2012007636. doi:10.1136/bcr-2012-007636
22. Alungal J, Abdulla MC, Narayan R. Idiopathic granulomatous mastitis with erythema nodosum and polyarthritis. Reumatismo. 2016;68:97–99. doi:10.4081/reumatismo.2016.844
23. Akın M, Karabacak H, Esendağlı G, et al. Coexistence of idiopathic granulomatous mastitis and erythema nodosum: successful treatment with corticosteroids. Turk J Med Sci. 2017;47:1590–1592. doi:10.3906/sag-1611-100
24. Pouchot J, Damade R, Barge J, et al. Idiopathic granulomatous mastitis and extra-mammary manifestations. Arch Pathol Lab Med. 1995;119:680.
25. Salesi M, Karimifar M, Salimi F, et al. A case of granulomatous mastitis with erythema nodosum and arthritis. Rheumatol Int. 2011;31:1093–1095. doi:10.1007/s00296-009-1273-0
26. Konstantinos P, Savvas A, Egli C, et al. Granulomatous mastitis, erythema nodosum and arthritis syndrome: case‑based review. Rheumatol Int. 2021;41:1175–1181. doi:10.1007/s00296-021-04820-8
27. Koksal H, Vatansev H, Artac H, et al. The clinical value of interleukins-8, −10, and −17 in idiopathic granulomatous mastitis. Clin Rheumatol. 2020;39:1671–1677. doi:10.1007/s10067-020-04925-8
28. Patel RA, Strickland P, Sankara IR, et al. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25:270–273. doi:10.1007/s11606-009-1207-2
29. Taylor GB, Paviour SD, Musaad S, et al. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology. 2003;35:109–119.
30. Paviour S, Musaad S, Roberts S, et al. Corynebacterium species isolated from patients with mastitis. Clin Infect Dis. 2002;35:1434–1440. doi:10.1086/344463
31. Hutton A, Elwasila SM, Sandkovsky G. A case of chronic granulomatous mastitis caused by Corynebacterium kroppenstedtii. Contagion. 2020;5:4.
32. Hida T, Minami M, Kawaguchi H, Oshiro Y, Kubo Y. Case of erythema nodosum associated with granulomatous mastitis probably due to Corynebacterium infection. J. Dermatol. 2014;41:821–823. doi:10.1111/1346-8138.12604
33. Mertz P, Jeannel J, Guffroy A, et al. Granulomatous manifestations associated with COVID19 infection: is there a link between these two diseases? Autoimmun Rev. 2021;20(6):102824. doi:10.1016/j.autrev.2021.102824
34. Fineschi S. Case report: systemic sclerosis after covid-19 infection. Front Immunol. 2021;12:686699. doi:10.3389/fimmu.2021.686699
35. Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592. doi:10.3390/cells10123592
36. Laas E, Touboul C, Kerdraon O, et al. Inflammatory and infectious breast mastitis outside of pregnancy and lactation: guidelines. J Gynecol Obstet Biol Reprod. 2015;44(10):996–1016. doi:10.1016/j.jgyn.2015.09.055
37. Tariq B, Badr S, Ebo Usman E, et al. Breast tuberculosis: about a case. Pan Afr Med J. 2017;28:183. doi:10.11604/pamj.2017.28.183.10742
38. Anita S, Leah M. Idiopathic granulomatous mastitis: a medical or surgical disease of the breast? ANZ J Surg. 2015;85(12):979–982. doi:10.1111/ans.12929
39. Néel A, Hello M, Cottereau A, et al. Long-term outcome in idiopathic granulomatous mastitis: a western multicentre study. QJM. 2013;106:433–441. doi:10.1093/qjmed/hct040
40. Lai EC, Chan WC, Ma TK, et al. The role of conservative treatment in idiopathic granulomatous mastitis. Breast J. 2005;11:454–456. doi:10.1111/j.1075-122X.2005.00127.x
41. Ocal K, Dag A, Turkmenoglu O, et al. Granulomatous mastitis: clinical, pathological features, and management. Breast J. 2010;16:176–182. doi:10.1111/j.1524-4741.2009.00879.x
42. Zabetian S, Friedman BJ, McHargue C. A case of idiopathic granulomatous mastitis associated with erythema nodosum, arthritis, and reactive cough. JAAD Case Rep. 2016;2:125–127. doi:10.1016/j.jdcr.2016.01.011
43. Diesing D, Axt-Fliedner R, Hornung D, et al. Granulomatous mastitis. Arch Gynecol Obstet. 2004;269:233–236. doi:10.1007/s00404-003-0561-2
44. Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011;17:661–668. doi:10.1111/j.1524-4741.2011.01162.x
45. Hugon-Rodin J, Plu-Bureau G, Hugol D, et al. Management of granulomatous mastitis: a series of 14 patients. Gynecol Endocrinol. 2012;28:921–924. doi:10.3109/09513590.2012.683075
46. Konan A, Kalyoncu U, Dogan I, et al. Combined long-term steroid and immunosuppressive treatment regimen in granulomatous mastitis. Breast Care. 2012;7:297–301. doi:10.1159/000341388
47. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798–802. doi:10.1177/000313480707300813
48. Pandey TS, Mackinnon JC, Bressler L, et al. Idiopathic granulomatous mastitis–a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J. 2014;20:258–266. doi:10.1111/tbj.12263
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

