Back to Journals » Clinical Ophthalmology » Volume 13
Idiopathic epiretinal membrane surgery: safety, efficacy and patient related outcomes
Authors Iuliano L , Fogliato G, Gorgoni F, Corbelli E, Bandello F , Codenotti M
Received 14 April 2019
Accepted for publication 1 July 2019
Published 15 July 2019 Volume 2019:13 Pages 1253—1265
DOI https://doi.org/10.2147/OPTH.S176120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lorenzo Iuliano,1 Giovanni Fogliato,1 Francesca Gorgoni,2 Eleonora Corbelli,1 Francesco Bandello,1 Marco Codenotti1
1Ospedale San Raffaele Scientific Institute, Vita-Salute University, Milan, Italy; 2Ospedali Riuniti di Ancona, Università Politecnica Delle Marche, Ancona, Italy
Abstract: This review aims to give to the reader an overview selectively oriented on safety and efficacy of surgery, providing concise and direct answers about crucial questions of trainees and experts. Surgery for idiopathic epiretinal membrane (ERM) is a safe and effective procedure that can achieve long-term stable postoperative visual and anatomical improvement, with an overall low recurrence and complication rate. Young patients, with a short onset of symptoms and with better initial visual acuity achieve higher levels of visual outcome. The preoperative degree of metamorphopsia is the prognostic factor for their postoperative degree. Successful results may be obtained in eyes with specific optical coherence tomography criteria, such as thin ganglion cell layers, thin internal plexiform layer, longer photoreceptors outer segment, regular ellipsoid zone and cone outer segment tips line, and without ectopic inner foveal layer. Internal limiting membrane peeling demonstrates positive anatomical and functional outcomes, but final positions about its safety remain controversial.
Keywords: idiopathic epiretinal membrane, outcome, safety, efficacy, prognostic factor
Introduction
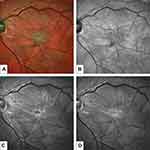
The challenge of idiopathic epiretinal membrane (ERM) treatment in the era of mini-invasive surgery is the correct surgical indication (Figure 1). Substantial technological innovations, including high-rate bilinear beveled cutters, improved visualization systems (3D of head-mounted helmets), enhanced light filters, high viscosity dies and premium forceps, are giving to the new era surgeon an astounding portfolio of tools to perform safer, cleaner and high quality procedure.
Furthermore, combined surgeries (phaco-vitrectomy) must face the high functional expectations of patients.
This review aims to give to the reader an overview selectively oriented on safety and efficacy of surgery, providing concise and direct answers about crucial questions of trainees and experts.
The research was performed by means of a focused PUMBED-based search of key words about each area and specific topic. Case reports or small series were omitted, and recently (last 10 years) published papers were favoured. Large studies or randomized clinical trials were preferred. We critically reviewed the search results, merging and integrating the information to achieve comprehensive considerations.
Safety
The safety of ERM surgery has been a striking issue since late ‘80s. Safety related argumentations can be addressed to general and specific risks.
General surgery related risks
Pars plana vitrectomy (PPV) with membrane peeling has been effectively used for the surgical treatment of ERM since 1978.1 General complications of PPV, regardless the disease for which it is performed, are:
- Retinal breaks and retinal detachment. Iatrogenic damages may be related to direct injuries or generated by vitreous traction. They have been reported respectively from 1.7% to 11% and from 6% to 8% after surgery for ERM2
- Cataract. Accelerated lens opacification is a well-known consequence of lens-sparing PPV. The rate of cataract progression was found to be higher in subjects aged more than 50,3 with similar rate after both 20- and 23-gauge techniques4
- Open-angle glaucoma. Increase of intraocular pressure (IOP) in the immediate postoperative period is a largely known event, which may be related to diverse mechanisms (buckling procedures, laser photocoagulation, use of tamponades, intraocular steroids, hemorrhages, inflammation).5 The association of open-angle glaucoma and vitrectomy has been postulated and widely investigated, owing this to the putative post-surgical trabecular oxidation and malfunction. However, this relationship was however proved to be weak6
- Endophthalmitis. Post vitrectomy endophthalmitis rate was found to be 0.05–0.07%. 23- and 25-gauge sutureless vitrectomy were found to have comparable endophthalmitis rates to sutured 20 g surgery, with rates of 0.03%, 0.13% and 0.02% respectively (with no statistically significant difference between the groups)7–9
Gauge-related safety
Large gauge vitrectomy was the first to be explored since late ‘80s. From 2004 small gauge (23-25-27) instruments became widely available.10 Microincision vitrectomy reduces surgical trauma, improves patient comfort, has faster postoperative healing and recovery, and shorter operating times. It is reasonable to question whether these advantages are related to increased surgical risks.
At first, it must be said that the trocars used for transconjunctival surgery insertion are not as sharp as blades used to make 20-gauge sclerotomies, therefore the forces required for their placement are substantially greater. Pressures as high as 63.7 mmHg have been measure during 25-gauge trocar insertion.11 This dynamics may result in vitreous traction (from either the insertion or removal of instruments or as a consequence of incarcerated vitreous) and retinal breaks. This hypothesis was largely investigated, but results remain controversial.12,13 It can be argued that probably other factors, such as surgeon experience and techniques, significantly influence the results.
With regard to the postoperative complications, it has been solidly reported that that the gauge choice does not influence the incidence of choroidal detachment, vitreous hemorrhage, and retinal detachment.14 Hypotony is reported to more frequent in small gauge vitrectomy, but is usually transient and is due to the fact that wounds seal within two weeks.15 The risk factors of intraoperative sclerotomy leakage requiring suture placement are prior vitrectomy, a young age at operation, and vitreous base dissection.16 Bipolar wet-field diathermy of sutureless sclerotomies is an effective method for ensuring a leaking sclerotomies closure.17
The gauge influence on the rate of endophthalmitis is imperative, considering the potential infections site entry in sutureless techniques (leaking sclerotomies, early postoperative vitreous wick, hypotony). Large series clarified that this risk is not increased in small gauge surgery.8,18
Procedure-related risks
To prevent ERM recurrence, peeling of internal limiting membrane (ILM) has been widely used as an additional procedure.19,20 Despite ILM peeling has debated functional benefits, its favorable effect to prevent recurrence has been extensively demonstrated.1 ILM peeling can cause functional and mechanical damage to the retina, temporally classified as “early” and “late”.
Swelling of the arcuate nerve fiber layer (SANFL)
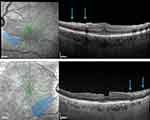
First described by Clarck, it is found in 31% of ILM-peeling eyes as hypoautofluorescent arcuate striae in the macular region on infrared and autofluorescence imaging, with corresponding hyperreflectant swelling on SD-OCT (Figure 2).21,22 Its pathogenesis is postulated to origin by a direct trauma to the inner retina or by a subclinical trauma due to injury to the Müller cell endplates, attached to the ILM. It disappears within the third month and is not associated to a visual acuity (VA) reduction.
Dissociated optic nerve fiber layer (DONFL)
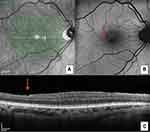
First described by Tadayoni and detected in about 57% of ILM-peeling eyes after 3 months, it consists of numerous arcuate striae within the posterior pole in the direction of the optic nerve fibers, darker than the surrounding retina on blue-filtered photographs and correspond to “dimples” in the inner retinal layer on OCT imaging (Figure 3A–C).23
Two mechanisms have been described:
- Cleavage (separation) of the optic nerve fiber bundles due to damage to the Müller cells, similarly to SANFL
- Exposition of the rough surface composed of optic nerve fibers surrounded by Müller cell processes after ILM removal.
The first hypothesis is supported by Spaide. He stated that the DONFL corresponds to inner retinal dimples that course along the path of the nerve fiber layer. The dimples seem to be the result of an interplay between trauma and healing processes constrained by nerve fiber layer, and are not associated with any loss of VA or changes in microperimetry.24
Dyes
The safety of dyes has struggled surgeons for decades.
Among the considerable amount of dyes used for macular surgery, at present, indocyanine green has been abandoned, owing to the in-vitro and in-vivo toxicity concerns.25,26
Trypan blue and brilliant blue are safe, and have respectively good and selective affinity for the ERM and the ILM.27–29 Both showed elevated biochemical and biological safety profiles, and are at present unanimously considered the gold standard for ERM surgery.30–33 There are available in the market conjugated dyes that join together trypan blue and brilliant blue, with adjunctive excipients providing unique physical properties.
Post-surgical macular edema
Cystoid macular edema (CME) is a well-known complication of anterior segment surgery (Irvine-Gass Syndrome). However, macular edema may appear after virtually any surgical eye procedure. Edemas occurring after ERM surgery present as persistent, and disappoint both the surgeon and the patient.
It has been argued that the rate of CME after vitrectomy for ERM could be higher in case of combined phaco-vitrectomy surgery, because of the release of pro-inflammatory mediators from the anterior segment. Despite this, evidence of increased CME rate with combined surgery are lacking.
A recent research reported an incidence of CME specifically after ERM peeling of 12.8%,34 which is higher than previous reports.35 Two pathogenetic mechanisms can be formulated:
- ILM peeling iatrogenically damages the Müller cells. The absence of leakage during fluorescein angiography confirms that blood-retinal barrier breakdown is absent, in lieu of a structural damage
- Inflammatory process due to a breakdown of the blood-retinal barrier.
The presence of preoperative intra-retinal cysts were associated with persistent CME following surgery.
Conclusions
Surgery for idiopathic ERM is a safe procedure, burdened by low complication rate. The gauge choice does not seem to influence the outcome, either in terms of efficacy or safety. The available dies also disclose a high safety profile. Despite ILM peeling demonstrates positive anatomical and functional outcomes, positions about its safety remain controversial. Hence no mandatory indications are given regarding its standard procedure.
Efficacy
The efficacy of surgical treatment for ERM has been extensively studied and is currently unanimously approved. Epiretinal membrane surgery is generally recommended when blurred vision or vision distortions are severe enough to interfere with binocular vision or daily activities.
Visual acuity
Since the ‘80s favorable outcomes were reported achieving good results simply removing the ERM: reduced metamorphopsia and improved VA were described in the majority (70% to 90%) of operated of patients.36–39 The VA improvement continued for the next 6–8 months and the best final VA might be obtained after 1 year.40 The chance to regain successful vision after surgery is increased in patients with a mild-to-moderate preoperative visual impairment (20/63 or better), and patients with better baseline VA can get a full visual recovery.41 In patients with longstanding ERMs, a complete recovery of vision is rare and retinal thickness and the macular profile rarely return to normal.42 Thus, early surgery is likely to decrease the risk of developing irreversible macular damage.
Eyes receiving ILM peeling as an adjunctive treatment showed better visual outcomes compared to non-peeled eyes, especially in the long term period.1
Macular morphology
ERM removal can lead to significant improvement of the macular morphology, in terms of reduced retinal thickness and restoration of the foveal contour. Since the early 90s, it was observed that by performing ILM peeling the retinal striae were more likely to disappear or flatten.43 After the introduction of vital dies, numerous studies confirmed that ILM removal during surgery for ERM was associated with better anatomical improvement compared to eyes were the ILM was left.44–48
Recurrence
The overall ERM recurrence rate after surgical removal is estimated to be from 1% to 16%, and is higher in secondary ERMs, owing this to the underlying causing condition.1,31,49 ILM removal during surgery for ERM was demonstrated to be associated a lower risk of recurrent ERMs.44–48
Metamorphopsia
Metamorphopsia is the major complaint of patients with ERM, even after successful surgery. They can be assessed qualitatively by Amsler grid test, or may be measured by mean of M-charts (Inami Co, Tokyo, Japan), or preferential hyperacuity perimetry (PHP).
The M-charts consist of 19 dotted vertical and horizontal lines with the dot intervals ranging from 0.2° to 2.0° of visual angle. The patient is asked to report the chart with the minimum angle of the dots in the line that appears straight, and this is taken as the metamorphopsia score (horizontal and vertical).
An anatomical correspondence of metamorphopsia was demonstrated, being correlated to the degree of distance of tangential retinal displacement.50 A correlation with OCT was also reported, as the degree of metamorphopsia, assessed with the M-charts, was significantly correlated with the Inner Nuclear Layer (INL) thickness, and that INL thickness was significantly correlated with tangential retinal displacement.51
The PHP was designed for a reproducible and quantitative assessment of metamorphopsia based on the visual function of hyperacuity, which was originally developed to monitor the progression of neovascular age-related macular degeneration.52 Significant reduction of metamorphopsia paralleled the improvement of VA and central foveal thickness were reported after idiopathic epiretinal membrane surgery.53
Microperimetry
A substantial number of studies investigated the correlation of macular morphology and function using spectral-domain optical coherence tomography (SD-OCT) and microperimetry.
The macular sensitivity continues to improve after surgery over the long-term period, even in cases where retinal thickness and VA stop to improve.54 The disruptions of the ellipsoid zone seems to be a potential predictor for poor visual recovery and paracentral microscotomas in eyes undergoing macular surgery, ranging between 16.6% and 56.2% of cases.55–59 Some authors supposed a correlation between functional postoperative microperimetry alterations and potential mechanical trauma induced by end-gripping forceps during ILM peeling.60,61
Stereopsis
Loss of binocularity can occur with a unilateral ERM, due to aniseikonia and metamorphopsia. Stereopsis is functionally associated with various OCT parameters, including central foveal and paracentral thickness.62 Some degrees of recovery have been observed after surgery, although not to a normal level.63 Functional tests, such as Titmus and TNO stereotests, indeed demonstrated that stereopsis improved after ERM removal, albeit not to a normal level.
Conclusions
Vitrectomy with ERM removal associated with ILM peeling is undoubtedly an effective treatment for idiopathic ERM. It can achieve long-term stable postoperative visual and anatomical improvement, with an overall low recurrence rate.
Patient related outcomes
Age and gender
The influence of age on postoperative VA was extensively investigated.63–67 Only one study found that a younger age (average 63.1) was statistically significantly associated with visual improvement (0.3 logMAR or more from baseline) in patients with hyperfluorescent lines on fundus autofluorescence (FAF) suggestive of retinal displacement.66
A statistically significant effect of gender on postoperative VA was not found.64
Duration of symptoms
Duration of symptoms negatively correlates with postoperative VA and with VA improvement.63,68 Thus, the longer the symptoms were present, the lower the postoperative VA and the less functional gain.
Preoperative visual acuity
A better preoperative VA is associated with better postoperative VA.58,63,64,67,69–72
Sub-analyses were able to show a statistically significant higher VA gain in subjects with poorer preoperative VA after six and twelve months.58,66,69 Hence, patients with better initial VA achieve higher levels of visual outcome but those with poorer pre-operative VA show a greater change in VA following ERM surgery.73
The results of surgery improved over the time period of the study,73 and a 12 months-postoperative follow-up period may be sufficient to assess the improvements induced by the ERM surgery.
Metamorphopsia
Metamorphopsia is the key symptom related to ERM, which leads to vision quality deterioration and loss of binocularity.
Studies with M-charts showed that the preoperative vertical metamorphopsia score was correlated with postoperative VA and with the improvement of VA. A higher metamorphopsia score was associated with worse postoperative VA and with less improvement of VA.70
One study with PHP confirmed that the significant predictors for postoperative metamorphopsia outcome were the degree of preoperative metamorphopsia. Significant reduction of metamorphopsia is coupled with VA improvement and central foveal thickness (CFT).74
Preoperative metamorphopsia scores and preoperative VA are independent and not correlated.64,70 Therefore, the preoperative degree of metamorphopsia is the prognostic factor for their postoperative degree, suggesting that surgery should be performed before development of severe grades of visual deterioration.75
The parafoveal inner nuclear layer (INL) is the only retinal structural parameter that was found to be associated with VA and metamorphopsia. Preoperative INL thickness was closely associated with preoperative, postoperative VA, and preoperative metamorphopsia.76,77
OCT parameters
Macular thickness
The macular thickness, both intended as CFT and average macular thickness, has been broadly investigated as a prognostic factor in idiopathic ERM surgery, but results are controversial.
Some studies analyzed the correlation between macular thickness and postoperative VA. They found a statistically significant worse postoperative VA in patients with higher preoperative CFT values in a univariable analysis.65,69,76,78 Further multivariate analysis performed with age, IS/OS integrity and multifocal electroretinogram (mfERG) confuted these results.65
Other studies focused on the VA improvement (more than the pure postoperative VA).55,58,79 They achieved conflicting results, since only Mitamura found that greater preoperative CFT is related to less improvement in VA.55
Ganglion cells
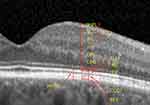
Ganglion cells are assessed by OCT and may be segmented in (Figure 4):
- Retinal nerve fiber layer (RNFL), which comprises the ganglion cell axons
- Ganglion cell layer (GCL), that includes the ganglion cell bodies
- Inner plexiform layer (IPL), made by the synaptic connections between ganglion cells and bipolar cells
Ganglion cells and VA are functionally associated.
The ganglion cell complex (GCC=RNFL+GCL+IPL) thickness has been found to be higher in eyes with idiopathic ERM, and after surgery turns back to similar values of healthy eyes. Post-operative GCC reduction is proportionally higher than thinning of the whole retina, and this reduction is correlated with visual restoration.80
The preoperative thickness of the GCL+IPL was significantly associated with post-operative VA. The thinner the inner layer, the better the postoperative VA. No associations were found with improvement in VA.76
Inner nuclear layer
The inner nuclear layer (INL) comprises the bodies of the bipolar cells.
The INL thickness was found to be a good indicator of metamorphopsia both before and after surgery, and a predictor of postoperative metamorphopsia.77 The parafoveal INL is also significantly associated with the postoperative VA.76
Photoreceptor outer segment (PROS)
The PROS length is the distance between the IS/OS junction and the RPE.
The PROS length was found to be a valuable prognostic factor for the postoperative VA.75 This was also confirmed in a multiple regression analysis, including age and preoperative VA.67
External limiting membrane (ELM)
No statistically significant association between ELM integrity and postoperative VA was found
Ellipsoid zone (EZ)
The ellipsoid zone represents the inner/outer segments junction of photoreceptors. This hyper-reflective band at OCT is strongly associated with the functional status and is a valuable prognostic factor for VA after surgery.
Eyes with a preoperative disruption of the EZ disclose a worse postoperative VA.55,58,64,65,81 This effect on postoperative VA remains stable in the long-term follow-up.58,65 Accordingly, a preoperative continuous EZ is associated with higher gain in VA after surgery.58,59,81
Preoperative disrupted EZ may undergo an anatomical and functional recovery even after more than 1 year postoperatively. However subjects with persistent EZ irregularities might have a limited visual improvement.82,83
Cone outer segment tips (COST)
The COST line, also known as Verhoeff membrane, is a hyper-reflective line visible at OCT between the EZ and the RPE.
A preoperative continuous COST line is associated with better VA after surgery.79 The preoperative COST line defect size is strongly correlated with the postoperative VA.84
Ectopic inner foveal layer (EIFL)
Continuous EIFL in idiopathic ERMs is a newly described OCT finding associated with significant vision loss.85 The presence of EIFL is significantly associated with lower preoperative and postoperative VA, and its thickness is negatively correlated with preoperative VA. Postoperatively, the EIFL thickness decreases significantly, but the thinning has no direct effect on VA change. Thus the EIFL presence should be considered a negative prognostic factor for postoperative anatomical and functional recovery.86
Other
Preoperative foveal contour on OCT is found not to affect postoperative VA.64,72 The ILM profile evaluated on OCT (normal, or mildly or severely distorted) does not affect the postoperative VA.64
The presence of retinal cysts or macular pseudoholes do not influence the postoperative VA.58
Fundus autofluorescence (FAF)
Photoreceptor cell loss due to the ERM will theoretically lead to decreased lipofuscin levels and, as a consequence, to reduced foveal autofluorescence.
Preoperative foveal FAF and postoperative VA are correlated. Eyes with an enlarged hypofluorescent area encompassing the foveal and parafoveal area have lower VA after surgery.72
Electrophysiology
Electrophysiology has been employed to investigate the macular functional defects in eyes with idiopathic ERM, and to further assess the post-surgical changes.
Electroretinogram (ERG) is negatively affected by the ERM presence, and the VA decrease is related to the dysfunction of both preganglionic (abnormal focal ERG) and ganglionic (abnormal pattern ERG) macular elements.87
Surgery not only provides morphological and VA gain, but is also associated with functional improvement of both outer and innermost macular retinal layers, leading to a related increase in VA.88 The amplitude and the implicit time of the positive peak were shown to be correlated with the postoperative VA.65
It has been shown that N95 amplitude in pattern ERG is a predictor of visual outcomes, confirming the correlation of VA with the function of the ganglion cell layer, which is the closest cell layer to be affected by ERM.89
Preoperative morphologically thickened and electroretinographically reduced retinas have a greater likelihood of being affected by an irreversible photoreceptor damages.68
Rehabilitation
Biofeedback training by means of a microperimeter has been reported as an efficient method to improve the visual performance of patients with different macular diseases. Biofeedback can be used to train subjects who have lost foveal fixation, to relocate their preferred locus into an area with better sensitivity. The instruments uses the cerebral plasticity and neurosensory adaptation capabilities to improve the visual performance.90,91
Biofeedback training with the MAIA device was reported to improve VA in patients with insufficient recovery after successful macular hole surgery.91 Currently there are no reports of biofeedback in patients treated for idiopathic ERM.
Quality of life
The National Eye Institute 25-Item Visual Function Questionnaire (NEI VFQ-25) is a vision-related-quality of life instrument designed to assess patients’ perception of their visual function and quality of life.
Quality of life is significantly impaired in patients with idiopathic ERM. Specifically, it is the severity of metamorphopsia that strongly influences their visual performances.92
ERM surgery remarkably improves patients’ subjective perception of visual function (indicated by higher scores in VFQ-25) and improved metamorphopsia, even in the absence of significant VA improvement.93 In the immediate postoperative period, the improved VA was the most important factor related to the improved quality of life, although the simultaneous cataract surgery might have had a confounding effect. The improved metamorphopsia was the important factor associated with improved quality of life in the late postoperative follow-up.94
Conclusions
The patient outcomes may be predicted through some prognostic factors.
Young patients, with a short onset of symptoms and with better initial VA achieve higher levels of visual outcome. The preoperative degree of metamorphopsia is the prognostic factor for their postoperative degree. Some OCT parameters enable the surgeon to better predict the functional outcome. Among these, successful results may be obtained in eyes with thin ganglion cell layers, thin IPL, longer PROS, regular EZ and COST lines, and without EIFL. Despite being rarely used as routine pre-operative examinations, eyes with large hypofluorescent foveal areas and reduced mfERG, present worse outcomes.
Conclusions
The scenario of demanding patients and safe surgery is struggling surgeons, who should face with unfavorable outcomes of unhappy patients. Virtually any ERM can be peeled, so what the surgeon must know is the correct prognosis to be formulated. The surgical indication, together with the expression (and the communication) of a correct prognosis is the key aspect for satisfied patients.
This short review aims to concisely summarize the literature regarding safety, efficacy and patient related outcomes of idiopathic ERM surgery.
It can be finally stated that surgery for idiopathic ERM is a safe procedure, burdened by low complication rate. Despite ILM peeling demonstrates positive anatomical and functional outcomes, positions about its safety remain controversial. Hence no mandatory indications are given regarding its standard procedure. Regarding the efficacy, vitrectomy with ERM removal associated with ILM peeling is undoubtedly an effective treatment for idiopathic ERM. It can achieve long-term stable postoperative visual and anatomical improvement, with an overall low recurrence rate. As for the prognostic factors, young patients, with a short onset of symptoms and with better initial VA achieve higher levels of visual outcome. The preoperative degree of metamorphopsia is the prognostic factor for their postoperative degree. Moreover, some OCT parameters enable the surgeon to better predict the functional outcome. Among these, successful results may be obtained in eyes with thin ganglion cell layers, thin IPL, longer PROS, regular EZ and COST lines, and without EIFL (Table 1).
 |
Table 1 Prognostic factors for idiopathic epiretinal membrane surgery |
The principal limitation of this review is the lack of quantitative data and statistical considerations, as a direct comparisons among studies or meta-analytical inferences were besides the aims of this comprehensive review. Therefore all presented data are intentionally left (and should be intended) as general, without reporting any specific threshold or correlation coefficient. The intent was to provide a brief overview of these factors, in order to have a quick and comprehensive overview. The aim was to have a generic profile of “good” or “bad” factors, enabling the clinicians to combine them in each specific case.
Being aware of these aspects is of great support in the patient-surgeon relationship, because sometimes an aware patient with a minimal improvement is happier than an unaware patient with a better outcome.
Disclosure
Professor Francesco Bandello reports personal fees from Allergan, Bayer, Boehringer-Ingelheim, Fidia Sooft, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Thrombogenics, and Zeiss, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Chang W-C, Lin C, Lee C-H, Sung T-L, Tung T-H, Liu J-H. Vitrectomy with or without internal limiting membrane peeling for idiopathic epiretinal membrane: a meta-analysis. PLoS One. 2017;12(6):e0179105. doi:10.1371/journal.pone.0179105
2. Kim C-S, Kim K-N, Kim W-J, Kim J-Y. Intraoperative endolaser retinopexy around the sclerotomy site for prevention of retinal detachment after pars plana vitrectomy. Retina Phila Pa. 2011;31(9):1772–1776. doi:10.1097/IAE.0b013e31820b6129
3. Melberg NS, Thomas MA. Nuclear sclerotic cataract after vitrectomy in patients younger than 50 years of age. Ophthalmology. 1995;102(10):1466–1471.
4. Pielen A, Guerra NIP, Böhringer D, et al. Intra- and postoperative risks and complications of small-gauge (23-G) versus conventional (20-G) vitrectomy for macular surgery. Eur J Ophthalmol. 2014;24(5):778–785. doi:10.5301/ejo.5000461
5. Costarides AP, Alabata P, Bergstrom C. Elevated intraocular pressure following vitreoretinal surgery. Ophthalmol Clin N Am. 2004;17(4):507–512. doi:10.1016/j.ohc.2004.06.007
6. Yu AL, Brummeisl W, Schaumberger M, Kampik A, Welge-Lussen U. Vitrectomy does not increase the risk of open-angle glaucoma or ocular hypertension–a 5-year follow-up. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2010;248(10):1407–1414. doi:10.1007/s00417-010-1409-7
7. Cohen SM, Flynn HW, Murray TG, Smiddy WE. Endophthalmitis after pars plana vitrectomy. The Postvitrectomy Endophthalmitis Study Group. Ophthalmology. 1995;102(5):705–712.
8. Scott IU, Flynn HW, Dev S, et al. Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: incidence and outcomes. Retina Phila Pa. 2008;28(1):138–142. doi:10.1097/IAE.0b013e31815e9313
9. Shi X, Zhao H, Wei W. Analysis of post-operative endophthalmitis after pars plana vitrectomy: a 10-year experience at a single center. Chin Med J (Engl). 2013;126(15):2890–2893.
10. Thompson JT. Advantages and limitations of small gauge vitrectomy. Surv Ophthalmol. 2011;56(2):162–172. doi:10.1016/j.survophthal.2010.08.003
11. Dalma-Weiszhausz J, Gordon-Angelozzi M, Ustariz-Gonzalez O, Suarez-Licona AM. Intraocular pressure rise during 25-gauge vitrectomy trocar placement. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2008;246(2):187–189. doi:10.1007/s00417-007-0713-3
12. Sandinha T, de Souza C, Essex R, Kelly T-L, Lake S, Phillips R. Revisiting transconjunctival sutureless 25-gauge vitrectomy: still worthwhile? Clin Experiment Ophthalmol. 2009;37(7):649–653. doi:10.1111/j.1442-9071.2009.02116.x
13. Scartozzi R, Bessa AS, Gupta OP, Regillo CD. Intraoperative sclerotomy-related retinal breaks for macular surgery, 20- vs 25-gauge vitrectomy systems. Am J Ophthalmol. 2007;143(1):155–156. doi:10.1016/j.ajo.2006.07.038
14. Zhang Z-H, Liu H-Y, Wimpissinger B, Avitabile T, Xu X, Liu K. Transconjunctival sutureless vitrectomy versus 20-gauge vitrectomy for vitreoretinal surgery: a meta-analysis of randomized controlled trials. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2013;251(3):681–688. doi:10.1007/s00417-012-2077-6
15. Küçük E, Yılmaz U, Zor KR, Kalaycı D, Sarıkatipoğlu H. Risk factors for suture requirement and early hypotony in 23-gauge vitrectomy for complex vitreoretinal diseases. Int Ophthalmol. 2017;37(4):989–994. doi:10.1007/s10792-016-0361-x
16. Woo SJ, Park KH, Hwang J-M, Kim JH, Yu YS, Chung H. Risk factors associated with sclerotomy leakage and postoperative hypotony after 23-gauge transconjunctival sutureless vitrectomy. Retina Phila Pa. 2009;29(4):456–463. doi:10.1097/IAE.0b013e318195cb28
17. Reibaldi M, Longo A, Reibaldi A, et al. Diathermy of leaking sclerotomies after 23-gauge transconjunctival pars plana vitrectomy: a prospective study. Retina Phila Pa. 2013;33(5):939–945. doi:10.1097/IAE.0b013e3182725d65
18. Parolini B, Romanelli F, Prigione G, Pertile G. Incidence of endophthalmitis in a large series of 23-gauge and 20-gauge transconjunctival pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2009;247(7):895–898. doi:10.1007/s00417-009-1063-0
19. Díaz-Valverde A, Wu L. To peel or not to peel the internal limiting membrane in idiopathic epiretinal membranes. Retina Phila Pa. 2018;38(Suppl 1):S5–S11. doi:10.1097/IAE.0000000000001906
20. Azuma K, Ueta T, Eguchi S, Aihara M. Effects of internal limiting membrane peeling combined with removal of idiopathic epiretinal membrane: a systematic review of literature and meta-analysis. Retina Phila Pa. 2017;37(10):1813–1819. doi:10.1097/IAE.0000000000001537
21. Clark A, Balducci N, Pichi F, et al. Swelling of the arcuate nerve fiber layer after internal limiting membrane peeling. Retina Phila Pa. 2012;32(8):1608–1613. doi:10.1097/IAE.0b013e3182437e86
22. Pichi F, Lembo A, Morara M, et al. Early and late inner retinal changes after inner limiting membrane peeling. Int Ophthalmol. 2014;34(2):437–446. doi:10.1007/s10792-013-9831-6
23. Tadayoni R, Paques M, Massin P, Mouki-Benani S, Mikol J, Gaudric A. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology. 2001;108(12):2279–2283.
24. Spaide RF. “Dissociated optic nerve fiber layer appearance” after internal limiting membrane removal is inner retinal dimpling. Retina Phila Pa. 2012;32(9):1719–1726. doi:10.1097/IAE.0b013e3182671191
25. Enaida H, Sakamoto T, Hisatomi T, Goto Y, Ishibashi T. Morphological and functional damage of the retina caused by intravitreous indocyanine green in rat eyes. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2002;240(3):209–213. doi:10.1007/s00417-002-0433-7
26. Sippy BD, Engelbrecht NE, Hubbard GB, et al. Indocyanine green effect on cultured human retinal pigment epithelial cells: implication for macular hole surgery. Am J Ophthalmol. 2001;132(3):433–435. doi:10.1016/s0002-9394(01)01061-3
27. Kawahara S, Hata Y, Miura M, et al. Intracellular events in retinal glial cells exposed to ICG and BBG. Invest Ophthalmol Vis Sci. 2007;48(10):4426–4432. doi:10.1167/iovs.07-0358
28. Ueno A, Hisatomi T, Enaida H, et al. Biocompatibility of brilliant blue G in a rat model of subretinal injection. Retina Phila Pa. 2007;27(4):499–504. doi:10.1097/IAE.0b013e318030a129
29. Lüke M, Januschowski K, Beutel J, et al. Electrophysiological effects of Brilliant Blue G in the model of the isolated perfused vertebrate retina. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2008;246(6):817–822. doi:10.1007/s00417-007-0761-8
30. Shukla D, Kalliath J, Neelakantan N, Naresh KB, Ramasamy K. A comparison of brilliant blue G, trypan blue, and indocyanine green dyes to assist internal limiting membrane peeling during macular hole surgery. Retina Phila Pa. 2011;31(10):2021–2025. doi:10.1097/IAE.0b013e318213618c
31. Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Double staining with brilliant blue G and double peeling for epiretinal membranes. Ophthalmology. 2009;116(7):1370–1376. doi:10.1016/j.ophtha.2009.01.024
32. Manousaridis K, Peter S, Mennel S. 20 g PPV with indocyanine green-assisted ILM peeling versus 23 g PPV with brilliant blue G-assisted ILM peeling for epiretinal membrane. Int Ophthalmol. 2016;36(3):407–412. doi:10.1007/s10792-015-0148-5
33. Baba T, Hagiwara A, Sato E, Arai M, Oshitari T, Yamamoto S. Comparison of vitrectomy with brilliant blue G or indocyanine green on retinal microstructure and function of eyes with macular hole. Ophthalmology. 2012;119(12):2609–2615. doi:10.1016/j.ophtha.2012.06.048
34. Frisina R, Pinackatt SJ, Sartore M, et al. Cystoid macular edema after pars plana vitrectomy for idiopathic epiretinal membrane. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2015;253(1):47–56. doi:10.1007/s00417-014-2655-x
35. Sigler EJ, Randolph JC, Charles S. Delayed onset inner nuclear layer cystic changes following internal limiting membrane removal for epimacular membrane. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2013;251(7):1679–1685. doi:10.1007/s00417-012-2253-8
36. de Bustros S, Thompson JT, Michels RG, Rice TA, Glaser BM. Vitrectomy for idiopathic epiretinal membranes causing macular pucker. Br J Ophthalmol. 1988;72(9):692–695. doi:10.1136/bjo.72.9.692
37. Michels RG. Vitreous surgery for macular pucker. Am J Ophthalmol. 1981;92(5):628–639. doi:10.1016/s0002-9394(14)74654-9
38. Margherio RR, Cox MS, Trese MT, Murphy PL, Johnson J, Minor LA. Removal of epimacular membranes. Ophthalmology. 1985;92(8):1075–1083.
39. Poliner LS, Olk RJ, Grand MG, Escoffery RF, Okun E, Boniuk I. Surgical management of premacular fibroplasia. Arch Ophthalmol Chic Ill 1960. 1988;106(6):761–764. doi:10.1001/archopht.1988.01060130831033
40. Pesin SR, Olk RJ, Grand MG, et al. Vitrectomy for premacular fibroplasia. Prognostic factors, long-term follow-up, and time course of visual improvement. Ophthalmology. 1991;98(7):1109–1114.
41. Rice TA, De Bustros S, Michels RG, Thompson JT, Debanne SM, Rowland DY. Prognostic factors in vitrectomy for epiretinal membranes of the macula. Ophthalmology. 1986;93(5):602–610.
42. Geerts L, Pertile G, van de Sompel W, Moreels T, Claes C. Vitrectomy for epiretinal membranes: visual outcome and prognostic criteria. Bull Soc Belge Ophtalmol. 2004;(293):7–15.
43. Gaudric A, Fardeau C, Goberville M, Cohen D, Paques M, Mikol J. [Ablation of the internal limiting membrane, macular unfolding and visual outcome in surgery of idiopathic epimacular membranes]. J Fr Ophtalmol. 1993;16(11):571–576.
44. Haritoglou C, Gandorfer A, Gass CA, Schaumberger M, Ulbig MW, Kampik A. The effect of indocyanine-green on functional outcome of macular pucker surgery. Am J Ophthalmol. 2003;135(3):328–337. doi:10.1016/s0002-9394(02)01969-4
45. Park DW, Dugel PU, Garda J, et al. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003;110(1):62–64.
46. Bovey EH, Uffer S, Achache F. Surgery for epimacular membrane: impact of retinal internal limiting membrane removal on functional outcome. Retina Phila Pa. 2004;24(5):728–735. doi:10.1097/00006982-200410000-00007
47. Kwok AK, Lai TY, Yuen KS. Epiretinal membrane surgery with or without internal limiting membrane peeling. Clin Experiment Ophthalmol. 2005;33(4):379–385. doi:10.1111/j.1442-9071.2005.01015.x
48. Rizzo S, Genovesi-Ebert F, Murri S, et al. 25-gauge, sutureless vitrectomy and standard 20-gauge pars plana vitrectomy in idiopathic epiretinal membrane surgery: a comparative pilot study. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2006;244(4):472–479. doi:10.1007/s00417-005-0173-6
49. Grewing R, Mester U. Results of surgery for epiretinal membranes and their recurrences. Br J Ophthalmol. 1996;80(4):323–326. doi:10.1136/bjo.80.4.323
50. Ichikawa Y, Imamura Y, Ishida M. METAMORPHOPSIA AND TANGENTIAL RETINAL DISPLACEMENT AFTER EPIRETINAL MEMBRANE SURGERY. Retina Phila Pa. 2017;37(4):673–679. doi:10.1097/IAE.0000000000001232
51. Ichikawa Y, Imamura Y, Ishida M. Inner nuclear layer thickness, a biomarker of metamorphopsia in epiretinal membrane, correlates with tangential retinal displacement. Am J Ophthalmol. 2018;193:20–27. doi:10.1016/j.ajo.2018.06.001
52. Loewenstein A, Malach R, Goldstein M, et al. Replacing the Amsler grid: a new method for monitoring patients with age-related macular degeneration. Ophthalmology. 2003;110(5):966–970. doi:10.1016/S0161-6420(03)00074-5
53. Richter-Mueksch S, Vécsei-Marlovits PV, Sacu SG, Kiss CG, Weingessel B, Schmidt-Erfurth U. Functional macular mapping in patients with vitreomacular pathologic features before and after surgery. Am J Ophthalmol. 2007;144(1):23–31. doi:10.1016/j.ajo.2007.03.045
54. Dal Vecchio M, Lavia C, Nassisi M, Grignolo FM, Fea AM. Microperimetric assessment after epiretinal membrane surgery: 4-year follow-up. J Ophthalmol. 2016;2016:7030791. doi:10.1155/2016/7030791
55. Mitamura Y, Hirano K, Baba T, Yamamoto S. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. Br J Ophthalmol. 2009;93(2):171–175. doi:10.1136/bjo.2008.146381
56. Inoue M, Morita S, Watanabe Y, et al. Inner segment/outer segment junction assessed by spectral-domain optical coherence tomography in patients with idiopathic epiretinal membrane. Am J Ophthalmol. 2010;150(6):834–839. doi:10.1016/j.ajo.2010.06.006
57. Oster SF, Mojana F, Brar M, Yuson RMS, Cheng L, Freeman WR. Disruption of the photoreceptor inner segment/outer segment layer on spectral domain-optical coherence tomography is a predictor of poor visual acuity in patients with epiretinal membranes. Retina Phila Pa. 2010;30(5):713–718. doi:10.1097/IAE.0b013e3181c596e3
58. Inoue M, Morita S, Watanabe Y, et al. Preoperative inner segment/outer segment junction in spectral-domain optical coherence tomography as a prognostic factor in epiretinal membrane surgery. Retina Phila Pa. 2011;31(7):1366–1372. doi:10.1097/IAE.0b013e318203c156
59. Mayer WJ, Vogel M, Neubauer A, et al. Pars plana vitrectomy and internal limiting membrane peeling in epimacular membranes: correlation of function and morphology across the macula. Ophthalmol J Int. 2013;230(1):9–17. doi:10.1159/000350233
60. Ripandelli G, Scarinci F, Piaggi P, et al. Macular pucker: to peel or not to peel the internal limiting membrane? A microperimetric response. Retina Phila Pa. 2015;35(3):498–507. doi:10.1097/IAE.0000000000000330
61. Deltour J-B, Grimbert P, Masse H, Lebreton O, Weber M. Detrimental effects of active internal limiting membrane peeling during epiretinal membrane surgery: microperimetric analysis. Retina Phila Pa. 2017;37(3):544–552. doi:10.1097/IAE.0000000000001179
62. Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. STEREOPSIS AND OPTICAL COHERENCE TOMOGRAPHY FINDINGS AFTER EPIRETINAL MEMBRANE SURGERY. Retina Phila Pa. 2015;35(7):1415–1421. doi:10.1097/IAE.0000000000000470
63. Asaria R, Garnham L, Gregor ZJ, Sloper JJ. A prospective study of binocular visual function before and after successful surgery to remove a unilateral epiretinal membrane. Ophthalmology. 2008;115(11):1930–1937. doi:10.1016/j.ophtha.2008.05.020
64. Falkner-Radler CI, Glittenberg C, Hagen S, Benesch T, Binder S. Spectral-domain optical coherence tomography for monitoring epiretinal membrane surgery. Ophthalmology. 2010;117(4):798–805. doi:10.1016/j.ophtha.2009.08.034
65. Kim JH, Kim YM, Chung EJ, Lee SY, Koh HJ. Structural and functional predictors of visual outcome of epiretinal membrane surgery. Am J Ophthalmol. 2012;153(1):103–110.e1. doi:10.1016/j.ajo.2011.06.021
66. Nitta E, Shiraga F, Shiragami C, Fukuda K, Yamashita A, Fujiwara A. Displacement of the retina and its recovery after vitrectomy in idiopathic epiretinal membrane. Am J Ophthalmol. 2013;155(6):1014–1020.e1. doi:10.1016/j.ajo.2013.01.021
67. Shiono A, Kogo J, Klose G, et al. Photoreceptor outer segment length: a prognostic factor for idiopathic epiretinal membrane surgery. Ophthalmology. 2013;120(4):788–794. doi:10.1016/j.ophtha.2012.09.044
68. Shimada Y, Sakurai S, Naito K, et al. Multifocal electroretinogram and optical coherent tomography: prediction of visual outcome after epiretinal membrane removal. Clin Exp Optom. 2011;94(3):296–301. doi:10.1111/j.1444-0938.2011.00604.x
69. Kunikata H, Abe T, Kinukawa J, Nishida K. Preoperative factors predictive of postoperative decimal visual acuity ≥ 1.0 following surgical treatment for idiopathic epiretinal membrane. Clin Ophthalmol Auckl NZ. 2011;5:147–154. doi:10.2147/OPTH.S15848
70. Kinoshita T, Imaizumi H, Okushiba U, Miyamoto H, Ogino T, Mitamura Y. Time course of changes in metamorphopsia, visual acuity, and OCT parameters after successful epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2012;53(7):3592–3597. doi:10.1167/iovs.12-9493
71. García-Fernández M, Castro Navarro J, González Castaño C, García Alonso A, Fonollá Gil M. Epiretinal membrane surgery: anatomic and functional outcomes. Arch Soc Espanola Oftalmol. 2013;88(4):139–144. doi:10.1016/j.oftal.2012.07.002
72. Brito PN, Gomes NL, Vieira MP, et al. Possible role for fundus autofluorescence as a predictive factor for visual acuity recovery after epiretinal membrane surgery. Retina Phila Pa. 2014;34(2):273–280. doi:10.1097/IAE.0b013e3182999a02
73. Dawson SR, Shunmugam M, Williamson TH. Visual acuity outcomes following surgery for idiopathic epiretinal membrane: an analysis of data from 2001 to 2011. Eye Lond Engl. 2014;28(2):219–224. doi:10.1038/eye.2013.253
74. Bae SH, Kim D, Park TK, Han JR, Kim H, Nam W. Preferential hyperacuity perimeter and prognostic factors for metamorphopsia after idiopathic epiretinal membrane surgery. Am J Ophthalmol. 2013;155(1):109–117.e3. doi:10.1016/j.ajo.2012.07.007
75. Kinoshita T, Imaizumi H, Miyamoto H, Katome T, Semba K, Mitamura Y. Two-year results of metamorphopsia, visual acuity, and optical coherence tomographic parameters after epiretinal membrane surgery. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2016;254(6):1041–1049. doi:10.1007/s00417-015-3147-3
76. Kim JH, Kang SW, Kong MG, Ha HS. Assessment of retinal layers and visual rehabilitation after epiretinal membrane removal. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2013;251(4):1055–1064. doi:10.1007/s00417-012-2120-7
77. Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Inner nuclear layer thickness as a prognostic factor for metamorphopsia after epiretinal membrane surgery. Retina Phila Pa. 2015;35(10):2107–2114. doi:10.1097/IAE.0000000000000602
78. Suh MH, Seo JM, Park KH, Yu HG. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol. 2009;147(3):473–480.e3. doi:10.1016/j.ajo.2008.09.020
79. Shimozono M, Oishi A, Hata M, et al. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am J Ophthalmol. 2012;153(4):698–704, 704.e1. doi:10.1016/j.ajo.2011.09.011
80. Pierro L, Iuliano L, Gagliardi M, Codenotti M, Ambrosi A, Bandello F. Role of ganglion cell complex in visual recovery following surgical internal limiting membrane peeling. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2015;253(1):37–45. doi:10.1007/s00417-014-2665-8
81. Cobos E, Arias L, Ruiz-Moreno J, et al. Preoperative study of the inner segment/outer segment junction of photoreceptors by spectral-domain optical coherence tomography as a prognostic factor in patients with epiretinal membranes. Clin Ophthalmol Auckl NZ. 2013;7:1467–1470. doi:10.2147/OPTH.S44837
82. Inoue M, Arakawa A, Yamane S, Kadonosono K. Long-term outcome of preoperative disrupted inner/outer segment junctions assessed using spectral-domain optical coherence tomography in patients with idiopathic epiretinal membrane. Ophthalmol J Int. 2012;228(4):222–228. doi:10.1159/000341606
83. Kim HJ, Kang J-W, Chung H, Kim HC. Correlation of foveal photoreceptor integrity with visual outcome in idiopathic epiretinal membrane. Curr Eye Res. 2014;39(6):626–633. doi:10.3109/02713683.2013.860990
84. Itoh Y, Inoue M, Rii T, Hirota K, Hirakata A. Correlation between foveal cone outer segment tips line and visual recovery after epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2013;54(12):7302–7308. doi:10.1167/iovs.13-12702
85. Govetto A, Lalane RA, Sarraf D, Figueroa MS, Hubschman JP. Insights into epiretinal membranes: presence of ectopic inner foveal layers and a new optical coherence tomography staging scheme. Am J Ophthalmol. 2017;175:99–113. doi:10.1016/j.ajo.2016.12.006
86. Govetto A, Virgili G, Rodriguez FJ, Figueroa MS, Sarraf D, Hubschman JP. Functional and anatomical significance of the ectopic inner foveal layers in eyes with idiopathic epiretinal membranes: surgical results at 12 months. Retina Phila Pa. 2019;39(2):347–357. doi:10.1097/IAE.0000000000001940
87. Parisi V, Coppè AM, Gallinaro G, Stirpe M. Assessment of macular function by focal electroretinogram and pattern electroretinogram before and after epimacular membrane surgery. Retina Phila Pa. 2007;27(3):312–320. doi:10.1097/01.iae.0000256039.59142.22
88. Lubiński W, Gosławski W, Krzystolik K, Mularczyk M, Kuprjanowicz L, Post M. Assessment of macular function, structure and predictive value of pattern electroretinogram parameters for postoperative visual acuity in patients with idiopathic epimacular membrane. Doc Ophthalmol Adv Ophthalmol. 2016;133(1):21–30. doi:10.1007/s10633-016-9543-0
89. Shin MK, Kim SI, Park SW, Byon IS, Kim HW, Lee JE. Evaluation of macular function using pattern electroretinogram in idiopathic epiretinal membrane. Asia-Pac J Ophthalmol. 2015;4(5):267–272. doi:10.1097/APO.0000000000000095
90. Ratra D, Gopalakrishnan S, Dalan D, Ratra V, Damkondwar D, Laxmi G. Visual rehabilitation using microperimetric acoustic biofeedback training in individuals with central scotoma. Clin Exp Optom. 2019;102(2):172–179. doi:10.1111/cxo.12834
91. Ueda-Consolvo T, Otsuka M, Hayashi Y, Ishida M, Hayashi A. Microperimetric biofeedback training improved visual acuity after successful macular hole surgery. J Ophthalmol. 2015;2015:572942. doi:10.1155/2015/572942
92. Okamoto F, Okamoto Y, Hiraoka T, Oshika T. Effect of vitrectomy for epiretinal membrane on visual function and vision-related quality of life. Am J Ophthalmol. 2009;147(5):869–874, 874.e1. doi:10.1016/j.ajo.2008.11.018
93. Ghazi-Nouri SMS, Tranos PG, Rubin GS, Adams ZC, Charteris DG. Visual function and quality of life following vitrectomy and epiretinal membrane peel surgery. Br J Ophthalmol. 2006;90(5):559–562. doi:10.1136/bjo.2005.085142
94. Matsuoka Y, Tanito M, Takai Y, Koyama Y, Nonoyama S, Ohira A. Visual function and vision-related quality of life after vitrectomy for epiretinal membranes: a 12-month follow-up study. Invest Ophthalmol Vis Sci. 2012;53(6):3054–3058. doi:10.1167/iovs.11-9153
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.