Back to Journals » Cancer Management and Research » Volume 11
Identifying risk factors for high-dose methotrexate-induced toxicities in children with acute lymphoblastic leukemia
Authors Li X, Sui Z, Jing F, Xu W, Li X, Guo Q, Sun S, Bi X
Received 7 March 2019
Accepted for publication 6 June 2019
Published 5 July 2019 Volume 2019:11 Pages 6265—6274
DOI https://doi.org/10.2147/CMAR.S207959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Xiao Li,1 Zhongguo Sui,1 Fanbo Jing,1 Wen Xu,1 Xiangpeng Li,1 Qie Guo,1 Shuhong Sun,1 Xiaolin Bi2
1Department of Clinical Pharmacy, The Affiliated Hospital of Qingdao University, Qingdao, Shandong 266003, People’s Republic of China; 2Department of Nutrition, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, 266003, People’s Republic of China
Background: Whether monitoring of the methotrexate (MTX) concentrations after high-dose MTX (HD-MTX) infusion can predict toxicities is still controversial, especially when HD-MTX therapy is used in the treatment of children with acute lymphoblastic leukemia (ALL), which is different than the previous schedules. The relationship between patient characteristics and severe adverse events (AEs) has yet to be determined.
Objective: To analyze the relationship between the MTX concentration and toxicities and to identify the risk predictors from patient characteristics for severe AEs during HD-MTX therapy in children with ALL.
Methods: We conducted a retrospective study on children with ALL who were treated with 388 HD-MTX infusions. The chi-square test and univariate and logistic regression analyses were used to analyze the relationship between the MTX concentrations and toxicities and to identify predictors for severe AEs.
Results: Febrile neutropenia (P=0.000) and vomiting (P=0.034) were more likely to occur if the infusion had an MTX level ≥1 μmol/L at 44 h, but other toxicities had no correlations with MTX concentration. Predictive factors for toxicities were as follows: higher risk stratification and higher values of albumin (ALB) for leucopenia, higher values of white blood cell count (WBC) for anemia, higher values of ALB and creatinine (Cr) for neutropenia, higher risk stratification and higher 44-h MTX concentration for febrile neutropenia, higher values of alanine transferase (ALT) for elevated ALT, higher values of ALT for elevated aspartate transferase (AST), and higher values of total bilirubin (TBil) for vomiting.
Conclusion: Routine monitoring of 44-h MTX concentrations is essential to identify patients at high risk of developing febrile neutropenia and vomiting. This study may provide a reference for clinicians to distinguish patients with a relatively high risk of severe AEs based on certain characteristics before HD-MTX infusion.
Keywords: high-dose methotrexate, methotrexate concentration, toxicities, patient characteristics, risk predictors, acute lymphoblastic leukemia
Introduction
Acute lymphoblastic leukemia (ALL) is a relatively infrequent malignant hematopoietic neoplasm in children.1 Methotrexate (MTX) is an effective drug for the treatment of ALL. Antineoplastic activity is enhanced when MTX is used at a high dose. High-dose MTX (HD-MTX), defined as MTX doses≥1 g/m2, is a useful therapy for central nervous system prophylaxis in the treatment of children with ALL.2–5 The administration of calcium folinate (CF) following HD-MTX treatment is essential to prevent severe myelosuppression and oral mucositis.6,7 However, excessive CF rescue reduces both the toxicity and the desired antitumor effect of MTX.8,9 Therefore, the balance between efficacy and adverse events (AEs) is one of the major clinical challenges to achieve a high cure rate of the disease. Monitoring the serum concentrations of HD-MTX is a well-accepted method to identify patients who are at high risk for severe toxic effects of the drug10 and to prevent toxic effects by early administration of a modified dose of CF in patients with evidence of abnormal plasma MTX clearance.6,7
The plasma concentration of MTX and the duration of a concentration above a certain threshold are important for the development of toxicity in the treatment of children with ALL.11–14 However, currently, HD-MTX therapy is administered with 24-h continuous infusion and as part of a multiagent anticancer chemotherapy, which is different from the schedules used when these studies were originally performed.6,15,16
Since the purpose of cytotoxic therapy is to provide the best possible therapeutic index, an improved understanding of predictors for the occurrence of severe AEs is necessary. Recently, some newer studies concluded that the risks of oral mucositis and hematological toxicity are associated with serum MTX concentrations.3,17 However, another study indicated the opposite conclusions: there was no significant relationship between the serum MTX concentration and oral mucositis.18 Additionally, another study indicated that some patients sometimes encounter severe AEs during HD-MTX therapy, even if serum MTX concentrations comply with recommended values, and concluded that the plasma concentration of MTX is not a predictive value for clinical AEs during HD-MTX therapy.19 Therefore, whether monitoring of the serum concentrations after HD-MTX is suitable as the predictor of severe AE occurrence is still controversial, especially when HD-MTX therapy is used in the treatment of children with ALL today, which is different than the previous schedules. In addition, other relevant clinical prediction factors for AEs in HD-MTX therapy in children with ALL include patient characteristics such as age, gender, body surface area, subtype of leukemia, kidney function tests, complete blood counts and liver function tests that have yet to be clearly determined.
In this study, to answer these clinical issues, we performed a retrospective study to analyze the relationship between the serum MTX concentrations and toxicities (hematological toxicities, transient liver dysfunctions, vomiting and oral mucositis). In addition, this study also aimed to identify predictors from variables extracted from patient characteristics for the occurrence of severe AEs during HD-MTX therapy in children with ALL.
Patients and methods
Patients
We conducted a retrospective noninterventional study on patients aged ≤14 years with ALL who were treated by HD-MTX and who had adequate medical records available for review between May 2015 and May 2018 at the Affiliated Hospital of Qingdao University, China. Children with newly diagnosed ALL who received HD-MTX treatment and had plasma MTX concentrations determined at 44 h after the start of treatment as a routine practice were enrolled in this study. This study was approved by the Institute Medical Ethics Committee of the Affiliated Hospital of Qingdao University and was conducted in accordance with the ethical principles of the Declaration of Helsinki. The requirement for informed consent was waived by the Institute Medical Ethics Committee because of the retrospective nature of this study, but patient confidentiality was protected.
HD-MTX administration
All of the included patients were treated on the 2015 Chinese children’s leukemia cooperation group-ALL protocol (CCCG-ALL-2015), which was based on the St. Jude Children’s Research Hospital Total XV Study. Low-severity children received 3 g/m2 of MTX, whereas moderate- and high-severity children received a 5 g/m2 dose. The first dose (1/10 of the full dose) was intravenously transfused within 0.5 h. The remainder of the full dose was infused during the following 23.5 h. Hydration was employed before HD-MTX administration and lasted for 4 days. Urinary alkalinization was performed before HD-MTX administration with 5% sodium bicarbonate and lasted for 3 days to ensure the urine pH was between 7 and 8.
CF rescuing regimen
At 42 h after MTX administration, the rescue was started every 6 h for 3 to 5 doses. For patients with absolute toxicity, the rescue was started 36 h after MTX administration. For patients with 44-h plasma MTX concentrations below 1.0 μmol/L, 15 mg/m2 was given, whereas for those with 44-h plasma MTX concentrations above 1.0 μmol/L, the rescue regimen was given according to the 44-h plasma MTX concentrations as previously reported.20,21 Briefly, the CF rescue dosage was increased 15 mg/m2 every 6 h per μmol/L increase in the 44-h plasma MTX concentration. If the 44-h plasma MTX concentration was higher than 5 μmol/L, the dosage of CF was calculated according to the following formula: [CF (mg) = body weight (kg) multiplied by 44-h plasma MTX concentration (μmol/L)]. The rescue continued until the plasma MTX concentration was <0.1 μmol/L. In addition, CF (3 mg/d) was distributed to the oral mucosa to prevent oral mucositis during chemotherapy after HD-MTX infusion.
Determination of plasma MTX concentration
Serum concentrations of MTX for ALL patients monitored at our hospital from May 2015 to May 2018 were acquired via the Viva-E® system (Siemens Healthcare, Forchheim, Germany) and were retrospectively collected. For patients with a 44-h plasma MTX concentration above 1.0 μmol/L, monitoring was continued every 24 h until the plasma MTX concentrations were <0.1 μmol/L.
Date collection
The following clinical data were collected by retrospective chart review before every infusion: age, gender, subtype of leukemia, and body surface area (BSA) calculated as reported.22 Patient characteristics, including complete blood counts, liver function tests and kidney function tests that included white blood cell count (WBC), hemoglobin (Hb), platelet (PLT) count, albumin (ALB), total bilirubin (TBil), alanine transferase (ALT) and creatinine (Cr) were also collected.
Toxicities
The toxicity (hematological toxicity, transient liver dysfunction, vomiting and oral mucositis) was assessed according to the National Cancer Institute Common Toxicity Criteria (NCI-CTCAE version 4.0). The hematological toxicity included the reduction in WBC, neutrophil count (NEUT), Hb and PLT count. Here, we also included febrile neutropenia as one of the hematological toxicities. Hepatic toxicity included increases in ALT, aspartate transferase (AST) and TBil.
Statistics
All data were analyzed with SPSS statistical software (version 19.0; SPSS Inc., Chicago, Illinois, USA). Collected data were expressed as the mean ± SD for continuous variables with a Gaussian distribution, while the median (min, max) was used for continuous variables without Gaussian distribution. The distribution of categorical variables was presented as frequencies and percentages. The 44-h plasma MTX concentrations were expressed as the median (min, max), and the comparisons were calculated using the chi-square test or Fisher’s exact test, as appropriate.
To identify potential predictors for toxicities, we used univariate analyses and multivariate binary logistic regression analyses. Univariate analyses were used to test the relationship between the patient characteristics and toxicities. In the univariate analysis, Pearson’s chi-square test and, if necessary, Fisher’s exact test were used to evaluate the correlation of toxicities with categorical variables, and the independent sample t-test (means, Gaussian populations) as well as the Mann-Whitney U-test (medians, non-Gaussian populations) were used to investigate the correlation of toxicities with continuous variables.
Patient variables with P-values less than 0.2 were entered stepwise into a multivariate binary logistic regression model.23 The binary variables for these analyses were male =0 and female =1 for sex; B-cell ALL =0, T-cell ALL =1 for subtype of leukemia; low risk =0, intermediate and high risk =1 for degree of risk. All tests were two-sided, and P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 104 children (59 boys and 45 girls) with newly diagnosed ALL who received HD-MTX treatment (388 infusions) and had plasma MTX concentrations determined 44 h after the start of treatment as a routine practice were enrolled in this study. According to subtype of leukemia, 93 patients were diagnosed as B-cell ALL, and 11 patients were diagnosed as T-cell ALL. The mean patient age at the time of HD-MTX treatment was 5.4±3.3 years (median: 4.5 years; range: 0.75–14.00 years). According to risk stratification, 56 patients were from the low-risk group, and 48 were from the intermediate- and high-risk groups. A total of 209 infusions of HD-MTX at 3 g/m2 were administered, while a total of 179 infusions of HD-MTX at 5 g/m2 were administered. The rest of the demographic and patient characteristics collected before every infusion are summarized in Table 1. All AEs categorized by differential grade are listed in Table 2. Anemia was the most common mild drug-induced toxicity (AEs > grade 1) (77.06%), followed by leucopenia (76.55%) and neutropenia (62.63%) (Table 2). Neutropenia was the most common severe drug-induced toxicity (AEs ≥ grade 3) (26.54%), followed by leucopenia (24.49%) and febrile neutropenia (10.83%) (Table 2).
 |
Table 1 Patient demographics and baseline characteristics |
 |
Table 2 Number of courses per grade of adverse events (n=388) |
The correlation between plasma MTX concentration at 44 h and toxicities
The correlation between plasma MTX concentration and toxicities induced by MTX, including hematological toxicity, transient liver dysfunction, vomiting and oral mucositis, was statistically analyzed to determine which toxicity was associated with 44-h plasma MTX concentration. In general, AEs ≥ grade 3 were collected for analysis, but vomiting and oral mucositis ≥ grade 2 were used for analysis because the number of vomiting incidents ≥ grade 3 occurred in only 4 infusions, and oral mucositis ≥ grade 3 was observed in only 16 infusions. In this study, we excluded reduced PLT count from the analysis of the hematological toxicity and reduction in TBil from the analysis of hepatic toxicity because a reduction in PLT count ≥ grade 3 was observed in only one infusion, and a reduction in TBil ≥ grade 3 also occurred in only one infusion. The 44-h plasma MTX concentrations in all 388 infusions are described in Figure 1. As described in Figure 1, most infusions had an MTX level <1 μmol/L; only 37 infusions had an MTX level ≥1 μmol/L at 44 h. The correlation between 44-h plasma MTX concentrations and MTX-related toxicities is shown in Table 3. As shown in Table 3, febrile neutropenia ≥ grade 3 (P=0.000) and vomiting ≥ grade 2 (P=0.034) were more likely to occur if the infusion had an MTX level ≥1 μmol/L at 44 h, but other hematological toxicities, including leucopenia, neutropenia and anemia, had no correlations with MTX levels. Hepatic toxicity and oral mucositis had no correlations with MTX levels.
 |
Table 3 Relationship between 44 hr plasma MTX concentration and MTX-related toxicities |
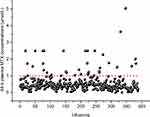 |
Figure 1 The 44-h plasma MTX concentrations in all 388 infusions. Abbreviation: MTX, methotrexate. |
Univariate and multivariate analysis of predictive factors for MTX-related toxicities in HD-MTX regimens
Univariate analyses were used to test the relationship between the patient characteristics and toxicities. The selected patient characteristics were age, gender, BSA, subtype of leukemia, WBC, Hb, PLT, ALB, TBil and Cr. In general, AEs ≥ grade 3 were collected for analysis, but vomiting and oral mucositis ≥ grade 2 were collected for the same reason in the analysis of the correlation between plasma MTX concentration and toxicities. Patient variables with P-values less than 0.2 were entered stepwise into a multivariate binary logistic regression model to identify the predictive factors for occurrence of AEs (≥ grade 3) before every HD-MTX regimen (Tables S1–S8). The predictive factors for the occurrence of hematological toxicity (≥ grade 3) before every HD-MTX regimen in the HD-MTX therapy of children with ALL are shown in Table 4. The correlation between patient characteristics before every HD-MTX regimen and hepatic toxicity (≥ grade 3) in the HD-MTX therapy of children with ALL is shown in Table 5. The predictive factors for the occurrence of vomiting and oral mucositis (≥ grade 2) before every HD-MTX regimen in the HD-MTX therapy of children with ALL are shown in Table 6.
 |
Table 4 Results of logistic regression analysis for MTX-related hematological toxicities (leucopenia, anemia, neutropenia and febrile neutropenia) |
 |
Table 5 Results of logistic regression analysis for MTX-induced transient liver dysfunction toxicities (elevated ALT and AST) |
 |
Table 6 Results of logistic regression analysis for MTX-induced toxicities (vomiting and oral mucositis) |
As shown in Table 4, the predictive factors for the occurrence of hematological toxicity were as follows: higher risk stratification [odds ratio (OR) =2.013, confidence interval (CI) =1.162–3.486; P=0.013] for leucopenia, higher values of ALB [OR =1.084, CI =1.003–1.171; P=0.041] for leucopenia, higher values of WBC [OR =1.252, CI =1.027–1.525; P=0.026] for anemia, higher values of ALB [OR =1.101, CI =1.019–1.189; P=0.015] for neutropenia, higher values of Cr [OR =1.021, CI =1.001–1.042; P=0.038] for neutropenia, higher risk stratification [OR =2.379, CI =1.124–5.034; P=0.026] for febrile neutropenia, and higher 44-h plasma MTX concentration [OR =2.786, CI =1.613–4.813; P=0.000] for febrile neutropenia. Protective factors for the occurrence of hematological toxicity were higher values of WBC [OR =0.608, CI =0.471–0.786; P=0.000] for leucopenia, higher PLT count [OR =0.996, CI =0.994–0.999; P=0.003] for leucopenia, higher Hb count [OR =0.855, CI =0.807–0.907; P=0.000] for anemia, and higher values of WBC [OR =0.556, CI =0.428–0.723] for neutropenia.
As shown in Table 5, predictive factors for the occurrence of hepatic toxicity were higher values of ALT [OR =1.018, CI =1.009–1.027; P=0.000] for elevated ALT and higher values of ALT [OR =1.013, CI =1.003–1.022; P=0.008] for elevated AST.
As shown in Table 6, this analysis revealed only one predictive factor for the occurrence of vomiting: higher values of TBil [OR =1.077, CI =1.004–1.157; P=0.040]; there was no predictive factor for the occurrence of oral mucositis.
Discussion
Currently, ALL is nearly curable.17 Intravenous HD-MTX is a useful component in the treatment of children with ALL.24 However, MTX was recently associated with severe AEs in a considerable number of patients despite CF rescue, hydration and urinary alkalinization.25 In this study, reversible myelosuppression was the most common side effect, which was consistent with a previous study.26 Neutropenia was the most common severe drug-induced side effect (≥ grade 3) (26.54%), followed by leucopenia (24.49%) and febrile neutropenia (10.83%). No life-threatening AEs were observed. It is worth noting that the occurrence of severe oral mucositis was rare (4.13%) in our study, which was different from previous studies.18,27 Our present finding is most likely a result of the fact that CF (3 mg/d) was also distributed to the oral mucosa to prevent oral mucositis during chemotherapy after HD-MTX infusion, except for intravenous injection.
The plasma concentration of MTX is important for the development of toxicity in the treatment of children with ALL.11–14 However, today, HD-MTX therapy for the treatment of children with ALL is administered with 24-h continuous infusion, CF rescue, hydration and urinary alkalinization and as part of a multiagent anticancer chemotherapy that is different from the previous therapies used when these studies were performed.6,15,16 Evaluating the association between the plasma concentration of MTX and the toxicities induced by MTX in the HD-MTX therapy of children with ALL is important. In this study, febrile neutropenia ≥ grade 3 (P=0.000) and vomiting ≥ grade 2 (P=0.034) were more likely to occur if the infusion had an MTX level ≥1 μmol/L at 44 h, meaning that MTX level ≥1 μmol/L at 44 h was the threshold of MTX concentration, which was related to febrile neutropenia ≥ grade 3 and vomiting ≥ grade 2. The results indicate that monitoring the plasma concentration of MTX at 44 h is important for adjusting the dose of CF to decrease the MTX-related toxicities in the original treatment protocol of HD-MTX in all children with ALL. Interestingly, a recent study of HD-MTX treatment in children with ALL showed relationships between polymorphisms in folate-related genes and toxicities.28 However, routine monitoring of plasma concentration of MTX still has an important role in adjusting the dose of CF to decrease the MTX-related toxicities in practice before individual prediction of MTX-related toxicities based on pretreatment genotyping reach a practical use.26,29 In addition, we concluded that other toxicities, such as hepatic toxicity, oral mucositis, leucopenia, neutropenia and anemia, had no correlations with MTX levels at 44 h. This is in accordance with the findings that there was no significant relationship between the serum MTX concentration and clinical AEs during HD-MTX therapy.18,19 However, some studies have demonstrated that the risk of oral mucositis and hematological toxicity is associated with serum MTX concentrations.3,17 Nevertheless, remarkably, those previous studies still simultaneously employ 7-h continuous infusion and 24-h continuous infusion17 and took in only 28 cases of children with ALL and osteosarcoma.3 Therefore, a standardized treatment protocol and a homogenous diagnosis for all children with ALL ensure that the results of our study are credible. We concluded that a high 44-h plasma MTX concentration was associated with febrile neutropenia and vomiting, although it was not associated with mucositis and neutropenia. A review summarized that, in most cases, reversible myelosuppression, mucositis and hepatotoxicity can be prevented by leucovorin rescue.26 The mechanism of our present finding is likely a result of the fact that children with high 44-h plasma MTX concentrations were adequately managed by escalating the dose of leucovorin rescue to decrease toxicities such as myelosuppression, mucositis and hepatotoxicity, but not to decrease toxicities such as mucositis and neutropenia.
Since the purpose of cytotoxic therapy is to provide the best possible therapeutic index, an improved understanding of the predictors of severe AEs is necessary. Although some reports have described the relationship between the effectiveness and the dosage of MTX used in the HD-MTX infusion,30 as well as patient characteristics and plasma MTX concentration,20 this is a well-designed study that evaluated the relationship between patient characteristics and severe AEs induced by MTX. Univariate analyses were used to test the relationship between patient characteristics and toxicities. The selected patient characteristics were age, gender, BSA, subtype of leukemia, WBC, Hb, PLT, ALB, TBil and Cr. Patient characteristics with P-values less than 0.2 were entered stepwise into a multivariate binary logistic regression model to identify the predictive factors for the occurrence of severe AEs before every HD-MTX regimen. MTX dosage was not included in the multivariate binary logistic regression because it was not an independent predictor (the dosage was adjusted based on BSA and risk stratification of the patients). The model is feasible, as these characteristics are routinely assessed before every HD-MTX infusion in our center. Thus, the model may help identify patients with a high risk of severe AEs in HD-MTX infusions and improve early prevention. We found that the predictive factors for the occurrence of hematological toxicity were as follows: higher risk stratification was a significant predictor for leucopenia and febrile neutropenia, higher values of ALB were a significant predictor for leucopenia and neutropenia, higher values of WBC were a significant predictor for anemia, higher values of Cr were a significant predictor for neutropenia, and higher 44-h plasma MTX concentration was a significant predictor for febrile neutropenia. Since higher 44-h plasma MTX concentration and higher risk stratification were identified as significant predictors for the occurrence of febrile neutropenia, monitoring for signs of febrile neutropenia should be prioritized, especially when patients with higher risk stratification have a higher 44-h plasma MTX concentration. Higher values of ALB and Cr were significant predictors of neutropenia, which may provide some reference for clinicians to distinguish those patients with a relatively high risk of neutropenia, especially when patients had higher values of ALB and Cr at the same time before HD-MTX infusion.
This analysis also revealed predictive factors for the occurrence of hepatic toxicity: higher values of ALT were a significant predictor for elevated ALT and elevated AST, which clearly indicated that mild abnormal liver function before HD-MTX infusion is the predictive factor for the occurrence of hepatic toxicity. This analysis revealed that higher values of TBil were the only predictive factor for the occurrence of vomiting. The results may help identify patients with mild abnormal liver function before HD-MTX infusion who easily suffer from hepatic toxicity and vomiting after HD-MTX infusions.
Several noteworthy limitations should be mentioned. First, this study employed a retrospective design with a limited sample size and the possibility of having other unknown factors. Second, our findings are limited to our center, as the study was based on a single center rather than multiple centers. Third, due to the deficiency of the test of renal function after HD-MTX infusion, we were not able to assess the risk factors for the occurrence of renal toxicity (≥ grade 3), which were analyzed in many other studies.2,31,32 Despite these limitations, this study still provided a reference for clinicians to improve the understanding of the predictors for the occurrence of severe AEs and early prevention.
Conclusion
The results of our study suggest that routine monitoring of plasma MTX levels at 44 h after HD-MTX infusion is essential to identify patients at a high risk of developing febrile neutropenia and vomiting. The study developed and validated a feasible risk model to test the predictive factors for the occurrence of severe AEs before every HD-MTX infusion. The study provides a reference for clinicians to distinguish those patients with a relatively high risk of severe AEs before HD-MTX infusion.
Acknowledgment
This work was supported by the Natural Science Foundation of Shandong Province (grant No. ZR2019BH026).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xing C, Liang B, Wu J, et al. Prognostic significance of leukopenia during the induction phase in adult B cell acute lymphoblastic leukemia. Cancer Manag Res. 2018;10:625–635. doi:10.2147/CMAR.S158359
2. May J, Carson KR, Butler S, Liu W, Bartlett NL, Wagner-Johnston ND. High incidence of methotrexate associated renal toxicity in patients with lymphoma: a retrospective analysis. Leuk Lymphoma. 2014;55(6):1345–1349. doi:10.3109/10428194.2013.840780
3. Cheng KK. Association of plasma methotrexate, neutropenia, hepatic dysfunction, nausea/vomiting and oral mucositis in children with cancer. Eur J Cancer Care (Engl). 2008;17(3):306–311. doi:10.1111/j.1365-2354.2007.00843.x
4. Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med. 1998;338(8):499–505. doi:10.1056/NEJM199802193380803
5. Abromowitch M, Ochs J, Pui CH, et al. High-dose methotrexate improves clinical outcome in children with acute lymphoblastic leukemia: st. Jude total therapy study X. Med Pediatr Oncol. 1988;16(5):297–303.
6. Joannon P, Oviedo I, Campbell M, Tordecilla J. High-dose methotrexate therapy of childhood acute lymphoblastic leukemia: lack of relation between serum methotrexate concentration and creatinine clearance. Pediatr Blood Cancer. 2004;43(1):17–22. doi:10.1002/pbc.20032
7. Rask C, Albertioni F, Bentzen SM, Schroeder H, Peterson C. Clinical and pharmacokinetic risk factors for high-dose methotrexate-induced toxicity in children with acute lymphoblastic leukemia–a logistic regression analysis. Acta Oncol (Madr). 1998;37(3):277–284.
8. Borsi JD, Wesenberg F, Stokland T, Moe PJ. How much is too much? Folinic acid rescue dose in children with acute lymphoblastic leukaemia. Eur J Cancer. 1991;27(8):1006–1009.
9. Browman GP, Goodyear MD, Levine MN, Russell R, Archibald SD, Young JE. Modulation of the antitumor effect of methotrexate by low-dose leucovorin in squamous cell head and neck cancer: a randomized placebo-controlled clinical trial. J Clin Oncol. 1990;8(2):203–208. doi:10.1200/JCO.1990.8.2.203
10. Moore MJ, Erlichman C. Therapeutic drug monitoring in oncology. Problems and potential in antineoplastic therapy. Clin Pharmacokinet. 1987;13(4):205–227. doi:10.2165/00003088-198713040-00001
11. Sirotnak FM, Moccio DM. Pharmacokinetic basis for differences in methotrexate sensitivity of normal proliferative tissues in the mouse. Cancer Res. 1980;40(4):1230–1234.
12. Evans WE, Pratt CB, Taylor RH, Barker LF, Crom WR. Pharmacokinetic monitoring of high-dose methotrexate. Early recognition of high-risk patients. Cancer Chemother Pharmacol. 1979;3(3):161–166.
13. Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297(12):630–634. doi:10.1056/NEJM197709222971203
14. Perez C, Wang YM, Sutow WW, Herson J. Significance of the 48 hr plasma level in high-dose methotrexate regimens. Cancer Clin Trials. 1978;1(2):107–111.
15. Christensen ML, Rivera GK, Crom WR, Hancock ML, Evans WE. Effect of hydration on methotrexate plasma concentrations in children with acute lymphocytic leukemia. J Clin Oncol. 1988;6(5):797–801. doi:10.1200/JCO.1988.6.5.797
16. Skarby T, Jonsson P, Hjorth L, et al. High-dose methotrexate: on the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol. 2003;51(4):311–320. doi:10.1007/s00280-002-0552-1
17. Xu W, Tang Y, Song H, Shi S, Yang S. Retrospective study on elimination delay of methotrexate in high-dose therapy of childhood acute lymphoblastic leukemia in China. J Pediatr Hematol Oncol. 2007;29(10):688–693. doi:10.1097/MPH.0b013e31814d6777
18. Maiguma T, Hayashi Y, Ueshima S, et al. Relationship between oral mucositis and high-dose methotrexate therapy in pediatric acute lymphoblastic leukemia. Int J Clin Pharmacol Ther. 2008;46(11):584–590.
19. Kanbayashi Y, Nomura K, Okamoto K, et al. Statistical examination to determine whether only 48-h value for serum concentration during high-dose methotrexate therapy is a predictor for clinical adverse events using ordered logistic regression analysis. Ann Hematol. 2010;89(10):965–969. doi:10.1007/s00277-010-0965-6
20. Relling MV, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12(8):1667–1672. doi:10.1200/JCO.1994.12.8.1667
21. Ye H, Gu L. [Advances in the study of acute lymphocytic leukemia treated by large dosage of methotrexate]. Zhonghua Xue Ye Xue Za Zhi. 1999;20(2):110–112.
22. Mosteller RD, Turksoy RN, Atkins L, McLaughlin C, Brown LG, Page DC. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi:10.1056/NEJM198707163170301
23. Moulla Y, Gradistanac T, Wittekind C, Eichfeld U, Gockel I, Dietrich A. Predictive risk factors for lymph node metastasis in patients with resected non-small cell lung cancer: a case control study. J Cardiothorac Surg. 2019;14(1):11. doi:10.1186/s13019-019-0831-0
24. Mahmoud LB, Mdhaffar M, Frikha R, et al. Use of MTHFR C677T polymorphism and plasma pharmacokinetics to predict methotrexate toxicity in patients with acute lymphoblastic leukemia. Adv Clin Exp Med. 2018;27(8):1061–1068. doi:10.17219/acem/69802
25. Ayad MW, El Naggar AA, El Naggar M. MTHFR C677T polymorphism: association with lymphoid neoplasm and effect on methotrexate therapy. Eur J Haematol. 2014;93(1):63–69. doi:10.1111/ejh.12302
26. Schmiegelow K. Advances in individual prediction of methotrexate toxicity: a review. Br J Haematol. 2009;146(5):489–503. doi:10.1111/j.1365-2141.2009.07765.x
27. Tiwari P, Thomas MK, Pathania S, et al. Serum creatinine versus plasma methotrexate levels to predict toxicities in children receiving high-dose methotrexate. Pediatr Hematol Oncol. 2015;32(8):576–584. doi:10.3109/08880018.2015.1087612
28. Yazicioglu B, Kaya Z, Guntekin Ergun S, et al. Influence of folate-related gene polymorphisms on high-dose methotrexate-related toxicity and prognosis in Turkish children with acute lymphoblastic leukemia. Turk J Haematol. 2017;34(2):143–150. doi:10.4274/tjh.2016.0007
29. Tsurusawa M, Gosho M, Mori T, et al. Statistical analysis of relation between plasma methotrexate concentration and toxicity in high-dose methotrexate therapy of childhood nonHodgkin lymphoma. Pediatr Blood Cancer. 2015;62(2):279–284. doi:10.1002/pbc.25305
30. Dordelmann M, Reiter A, Zimmermann M, et al. Intermediate dose methotrexate is as effective as high dose methotrexate in preventing isolated testicular relapse in childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 1998;20(5):444–450.
31. Green MR, Chamberlain MC. Renal dysfunction during and after high-dose methotrexate. Cancer Chemother Pharmacol. 2009;63(4):599–604. doi:10.1007/s00280-008-0772-0
32. Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11(6):694–703. doi:10.1634/theoncologist.11-6-694
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
