Back to Journals » Neuropsychiatric Disease and Treatment » Volume 12
Identifying predictive clinical characteristics of the treatment efficacy of mirtazapine monotherapy for major depressive disorder
Authors Tsutsumi T, Sugawara H, Ito R, Asano M, Shimizu S, Ishigooka J, Nishimura K
Received 16 May 2016
Accepted for publication 18 July 2016
Published 5 October 2016 Volume 2016:12 Pages 2533—2538
DOI https://doi.org/10.2147/NDT.S112901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Taro Kishi
Takahiro Tsutsumi,1 Hiroko Sugawara,1,2 Ryoko Ito,1 Mizuho Asano,1 Satoru Shimizu,3 Jun Ishigooka,1 Katsuji Nishimura1
1Department of Psychiatry, 2Support Center for Women Health Care Professionals and Research, 3Department of Research, Medical Research Institute, Tokyo Women’s Medical University, Tokyo, Japan
Background: Mirtazapine, which is classified as a noradrenergic and specific serotonergic antidepressant, is widely prescribed for the treatment of major depressive disorder. The potential predictive factors of the efficacy of mirtazapine and the tolerability based on the incidence of oversedation and jitteriness/anxiety syndrome were evaluated.
Patients and methods: Patients with major depressive disorder were retrospectively investigated. Study subjects comprised 68 patients with depression who received mirtazapine as an initial antidepressant at the Department of Psychiatry of the Tokyo Women’s Medical University Hospital from September 2009 to March 2013. The efficacy of mirtazapine monotherapy was evaluated based on the Clinical Global Impression Improvement score. Clinical characteristics were compared between remission and nonremission groups to determine the factors predicting the efficacy. Moreover, discontinuation rates due to adverse effects, including oversedation and jitteriness/anxiety syndrome, were examined, and the effects of confounding factors were evaluated.
Results: The remission rate of mirtazapine monotherapy was 36.8% among the 68 enrolled subjects. The mean final doses in the remission and nonremission groups were 27.6±13.5 mg and 26.0±14.1 mg, respectively, and there was no significant difference between them. Multiple logistic analyses revealed that the absence of guilt (odds ratio [OR] =0.15; 95% CI [1.66–37.24], P=0.006) and the presence of psychomotor retardation (OR =4.30; 95% CI [1.30–16.60], P=0.016) were significantly related to the efficacy of mirtazapine monotherapy. The discontinuation rates due to oversedation and jitteriness/anxiety syndrome were 13.2% and 11.8%, respectively. Age did not differ significantly between patients with or without oversedation or jitteriness/anxiety syndrome (P=0.078 and P=0.579, respectively).
Conclusion: The absence of guilt and the presence of psychomotor retardation may predict the efficacy of mirtazapine, and mirtazapine may be tolerable for all ages.
Keywords: feelings of guilt, psychomotor retardation, oversedation, jitteriness, anxiety syndrome
Introduction
Several new-generation antidepressants have been introduced over the past 20 years to overcome the disadvantages of the first-generation antidepressants, such as tricyclic and tetracyclic antidepressants, and to enhance therapy. Mirtazapine, a new-generation antidepressant, is classified as a noradrenergic and specific serotonergic antidepressant. The antidepressant effect of mirtazapine is believed to be mediated by its combined noradrenergic and serotonergic effects. It enhances both noradrenergic and serotonergic transmission via blockade of α2-adrenergic autoreceptors and heteroreceptors.1,2 Some meta-analyses reported that the onset of action of mirtazapine may be faster than that of selective serotonin reuptake inhibitors (SSRIs),3–5 and mirtazapine has thus become widely prescribed as the initial treatment for major depressive disorders (MDDs). Approximately two-thirds of patients receiving initial antidepressant therapy do not achieve remission,6 and clinical predictors of the efficacy of mirtazapine monotherapy for MDD have not been well studied. Moreover, a recent study reported that early improvements in insight, early-morning awakening, and general somatic symptoms as well as overall depressive symptoms were associated with remission in patients with MDD receiving mirtazapine;7 however, pretreatment depressive symptoms were not evaluated.
Mirtazapine also acts as an antagonist at postsynaptic 5-hydroxytryptamine (5-HT)2 and 5-HT3 receptors, which is believed to contribute to its both therapeutic effects and tolerability, and the 5-HT2 blocking effect benefits sleep.8 Mirtazapine also blocks histamine receptors, which may contribute to its sedative effects. Somnolence is one of the most common side effects of mirtazapine,9 and oversedation increases the risk of fall, especially in the elderly. Moreover, an increased risk of suicidality, especially in the young population, is a side effect of all antidepressants.10 We earlier reported the incidence of neuropsychiatric symptoms emerging after the introduction of antidepressants referred to together as “jitteriness/anxiety syndrome”,11 but the incidence of this syndrome in association with mirtazapine has not been fully investigated.
In this article, we retrospectively investigated the efficacy of mirtazapine for MDD to determine the clinical predictors of its efficacy and also examined the discontinuation rates due to adverse effects of oversedation and jitteriness/anxiety syndrome to evaluate tolerability.
Patients and methods
Study design
In this retrospective study, the subjects comprised the patients with depression who received mirtazapine as an initial antidepressant monotherapy for the depressive episode at the Department of Psychiatry of the Tokyo Women’s Medical University Hospital from September 2009 to March 2013. Eligible patients met the Text Revision of the Diagnostic and Statistical Manual of Mental Disorders IV criteria for MDD. Patients who had taken other antidepressants, antipsychotics, and mood stabilizers combined with mirtazapine were excluded, and patients with concomitant use of benzodiazepine were included in the subjects. All data were extracted from the patients’ medical records, that is, all depressive symptoms simply relied on notations in the records which indicated the presence of them. The treatment efficacy of mirtazapine monotherapy was evaluated based on the Clinical Global Impression Improvement (CGI-I) score at least 1 week after initiating mirtazapine treatment. Subjects maintaining steady states with a CGI-I score of 1 (very much improved) or 2 (much improved) for more than 4 weeks were defined as the remission group, and those who dropped out within 4 weeks after achieving the CGI-I score of 1 or 2 were excluded from the analysis because we could not define their remission. On the other hand, subjects with scores ranging from 3 (minimally improved) to 7 (very much worse) were defined as the nonremission group, which included the subjects discontinued mirtazapine before achieving CGI-I score of 1 or 2 due to the adverse effects. We compared the two groups with regard to demographic variables (age and sex), clinical variables (onset age, number of recurrent major depressive episodes, family history, and severity), pharmacologic variables (duration of mirtazapine monotherapy and dose of mirtazapine), and depressive symptoms (somatic symptoms, appetite loss, insomnia, feelings of guilt, suicidal ideation, psychotic symptoms, anxiety, psychomotor retardation, and agitation). Illness severity was evaluated based on the CGI Severity (CGI-S) score at the initiation of mirtazapine administration: subjects with scores of 2 (borderline mentally ill) or 3 (mildly ill) were classified into the mild group, subjects with scores of 4 (moderately ill) or 5 (markedly ill) were classified into the moderate group, and subjects with scores of 6 (severely ill) or 7 (among the most extremely ill) were classified into the severe group. None of the subjects scored 1 (normal, not at all ill).
We also evaluated the discontinuation rates due to adverse effects of oversedation and jitteriness/anxiety syndrome. Mirtazapine-induced jitteriness/anxiety syndrome was defined according to our earlier study, that is, patients who experienced any of ten symptoms (anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia, hypomania, and mania) during the first month after initiating mirtazapine administration were classified as having jitteriness/anxiety syndrome.11 As confounding factors of these adverse effects, we examined demographic variables (age and sex) and pharmacologic variables (dose of mirtazapine and combined use of benzodiazepine).
In the current study, the patients discontinued mirtazapine before achieving remission due to the adverse effects were included in the nonremission group to determine the clinical predictors of the efficacy, because it is impossible to predict the adverse effects of mirtazapine and exclude these patients before the treatment in clinical practice.
We informed the patients about the study using bulletin board posting and obtained oral agreement. The study was conducted in accordance with the Declaration of Helsinki as revised in 1989, and the ethics committee of the Department of Psychiatry of the Tokyo Women’s Medical University Hospital approved the study. The ethics committee waived the requirement for written consent, as the study was retrospective.
Statistical analyses
A chi-square test or Fisher’s exact test for categorical variables and a t-test for continuous variables were performed to compare differences between the two groups. Multiple logistic regression analysis was performed between the two groups as a dependent variable selected the independent variables for the model using the stepwise method based on Akaike’s information criterion12 using JMP statistical software (Version 11; SAS Institute Inc., Cary, NC, USA). A P-value of <0.05 was considered statistically significant.
Results
Mirtazapine monotherapy was performed in 72 depressive patients; however, four patients were excluded from the study because they dropped out before the definition of remission. Among the subjects comprised the remaining 68 patients, 25 were classified into the remission group and 43 were classified into the nonremission group (Figure 1 and Table 1), that is, the efficacy rate of mirtazapine monotherapy was 36.8% (25/68). There were no differences in the demographic and clinical variables between the two groups. There was a significant difference of the duration of mirtazapine monotherapy by the time of the definition of the treatment efficacy between the remission (10.2±6.3 weeks) and nonremission groups (4.8±6.6 weeks), because the patients discontinued mirtazapine due to the adverse effects were included in the nonremission group (P=0.002; Table 1). The mean final dose in the remission and nonremission groups was 27.6±13.5 mg and 26.0±14.1 mg, respectively, and there was no significant difference between them (P=0.642; Table 1). Among the depressive symptoms, mirtazapine monotherapy tended to be more effective for depressive patients with psychomotor retardation (P=0.065; Table 1), and patients without feelings of guilt responded significantly better to mirtazapine monotherapy than those with feelings of guilt (P=0.029; Table 1). In multiple logistic regression analysis, feelings of guilt, psychomotor retardation, and severity of depression were selected as independent variables from the other variables, using the minimum Akaike’s information criterion by the stepwise method. Feelings of guilt (odds ratio [OR] =0.15; 95% CI [1.66–37.24], P=0.006) and psychomotor retardation (OR =4.30; 95% CI [1.30–16.60], P=0.016) were significantly related to the efficacy of mirtazapine monotherapy (Table 2).
  | Table 1 Demographic and clinical characteristics of subjects |
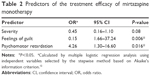  | Table 2 Predictors of the treatment efficacy of mirtazapine monotherapy |
Regarding adverse effects, the discontinuation rates due to oversedation and jitteriness/anxiety syndrome were 13.2% and 11.8%, respectively (Figure 1). Age (P=0.078 and P=0.579, respectively), sex (P=0.280 and P=1.000, respectively), and the combined use of benzodiazepine (P=0.156 and P=1.000, respectively) did not significantly differ between patients with and without oversedation or jitteriness/anxiety syndrome. There was no significant difference in the mean final dose of mirtazapine between patients with and without jitteriness/anxiety syndrome (P=0.121), but the mean final dose of mirtazapine was significantly lower in the patients with oversedation than in those without oversedation (14.2±7.0 and 28.5±13.6, respectively, P=0.0005; Table 3). In the remission group, jitteriness/anxiety syndrome occurred in one patient; however, no one discontinued mirtazapine due to the adverse effects.
In the comparison between the remission and nonremission groups without the patients discontinuing mirtazapine monotherapy due to adverse effects, feelings of guilt remained significant in the multiple logistic regression analysis (P=0.011, data not shown).
Discussion
In the current study, the efficacy of mirtazapine was 36.8%, which is consistent with findings in earlier studies.3,13,14 There was no significant sex difference of mirtazapine efficacy (efficacy rate in male: 36.7%, female: 36.8%), as well as the recent study.7 Our findings suggest that the absence of feelings of guilt and the presence of psychomotor retardation are predictive of the treatment efficacy of mirtazapine. Both feelings of guilt and psychomotor retardation are characteristics of melancholia, but one may be considered a negative predictor, and the other a positive predictor, of mirtazapine monotherapy. Earlier studies reported that serotonin–norepinephrine reuptake inhibitors are highly effective for depressive patients with psychomotor retardation,15 whereas psychomotor retardation predicts a negative response to SSRIs.16 Furthermore, Martinot et al17 reported that dopamine hypofunction is related to depressive patients with psychomotor retardation. Mirtazapine, which enhances both noradrenergic and serotonergic transmission, was effective for depressive patients with psychomotor retardation in the current study, suggesting that actions on both monoamine systems are important for treating depressive patients with psychomotor retardation. Feelings of guilt were a negative predictor of mirtazapine monotherapy in the current study, and Gerra et al18 reported that patients with high negative affectivity, including guilt and anxiety, respond preferentially to antidepressants that selectively enhance serotonin neurotransmission. This finding suggests that antidepressants with high selectivity for serotonin transmission are applicable for treating depressive patients with feelings of guilt. On the other hand, anxiety was not related to mirtazapine efficacy. Further studies are needed to compare the efficacy for anxiety, as well as other clinical variables, between mirtazapine and other types of antidepressants, such as SSRIs and serotonin–norepinephrine reuptake inhibitors.
The discontinuation rate due to oversedation in the current study was 13.2%, and this result is consistent with the incidence reported in an earlier study.19 Neither patient age nor sex differed significantly between those with oversedation and those without oversedation, suggesting that mirtazapine may be tolerable for both male and female patients of all ages. There are some reports, however, of mirtazapine-induced delirium in older patients or patients with organic brain disorders,20,21 and thus attention should be paid to the incidence of delirium when using mirtazapine in older patients. The mean final dose of mirtazapine was significantly lower in patients with oversedation than in those without oversedation. An earlier study reported that adverse events were no more frequent or severe during the treatment that began at high dose of mirtazapine than at low dose, and the treatment that began at high dose had greater beneficial effects on sleep initiation and duration.19 In the current study, it is possible that some patients had lower dosage of mirtazapine in response to experiencing severe sedation at high dose.
The discontinuation rate due to jitteriness/anxiety syndrome was 11.8% in the current study, and this finding was within the wide range of the incident rate (from 4% to 65%) reported in earlier studies.11,22 There was no significant difference of patient age and sex between those with jitteriness/anxiety syndrome and those without jitteriness/anxiety syndrome, as well as oversedation. Hammad et al23 reported that the use of antidepressants in pediatric patients is associated with a modest increase in the suicide risk in their review of 24 placebo-controlled trials. Moreover, a recent study reported that the rate of suicide, attempted suicide, and self-harm associated with mirtazapine was higher than that with citalopram.24 In the current study, age was not associated with the incidence of mirtazapine-induced jitteriness/anxiety syndrome, and there was no incidence of suicide, attempted suicide, and self-harm. The wide range of the incident rate of jitteriness/anxiety syndrome would depend on sample size of each study, and additional prospective studies with large sample size are needed to examine the suicidality risk of mirtazapine considering the effects of age and sex.
Limitations
This study was retrospective, and the data were extracted from patients’ medical records. Therefore, we could not use clinical scales such as Hamilton Depression Rating Scale and Montgomery Asberg Depression Rating Scale for assessment, and the evaluation of remission and severity was based on the CGI-I and CGI Severity. We might have overlooked important clinical information because of the retrospective nature of our study. We carefully extracted each depressive symptom from the medical records exactly, and senior psychiatrists followed up all the patients, and therefore the information described in their medical records is considered accurate, which may minimize the effects of these limitations. The other limitations are as follows: the small sample size of the current study would have an effect on the results, and there is no placebo-controlled study to support our results.
Conclusion
The absence of guilt and the presence of psychomotor retardation may predict the treatment efficacy of mirtazapine monotherapy, and mirtazapine may be tolerable for all ages. Further prospective placebo-controlled studies comprising a larger number of subjects are needed to validate the results of the current study.
Acknowledgments
This study was supported by the Toshi Miyahara Research Grant for Support Center for Women Health Care Professionals and Researchers, Tokyo Women’s Medical University.
Author contributions
All the authors contributed to the conception and design of the study. TT, HS, RI, and MA contributed to data collection. SS provided advice regarding the statistical analysis methods. TT and HS performed the statistical analyses and wrote the manuscript. All the authors read and approved the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
During the past 3 years, JI received speakers’ bureau honoraria from Dainihon Sumitomo, Mochida, Shionogi, and Otsuka Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
de Boer T. The effects of mirtazapine on central noradrenergic and serotonergic neurotransmission. Int Clin Psychopharmacol. 1995;10(suppl 4):19–23. | ||
Haddjeri N, Blier P, de Montigny C. Noradrenergic modulation of central serotonergic neurotransmission: acute and long-term actions of mirtazapine. Int Clin Psychopharmacol. 1995;10(suppl 4):11–17. | ||
Thase ME, Nierenberg AA, Vrijland P, van Oers HJ, Schutte AJ, Simmons JH. Remission with mirtazapine and selective serotonin reuptake inhibitors: a meta-analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. Int Clin Psychopharmacol. 2010;25(4):189–198. | ||
Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;12:CD006528. | ||
Watanabe N, Omori IM, Nakagawa A, et al. Mirtazapine versus other antidepressants in the acute-phase treatment of adults with major depression: systematic review and meta-analysis. J Clin Psychiatry. 2008;69(9):1404–1415. | ||
Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. | ||
Funaki K, Nakajima S, Suzuki T, Mimura M, Uchida H. Early improvements in individual symptoms to predict later remission in major depressive disorder treated with mirtazapine. J Clin Pharmacol. Epub 2016 Jan 27. | ||
Nutt DJ. Tolerability and safety aspects of mirtazapine. Hum Psychopharmacol. 2002;17(suppl 1):S37–S41. | ||
Montgomery SA. Safety of mirtazapine: a review. Int Clin Psychopharmacol. 1995;10(suppl 4):37–45. | ||
Friedman RA, Leon AC. Expanding the black box – depression, antidepressants, and the risk of suicide. N Engl J Med. 2007;356(23):2343–2346. | ||
Harada T, Inada K, Yamada K, Sakamoto K, Ishigooka J. A prospective naturalistic study of antidepressant-induced jitteriness/anxiety syndrome. Neuropsychiatr Dis Treat. 2014;10:2115–2121. | ||
Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6(2):165–175. | ||
Fang Y, Yuan C, Xu Y, et al. Comparisons of the efficacy and tolerability of extended-release venlafaxine, mirtazapine, and paroxetine in treatment-resistant depression: a double-blind, randomized pilot study in a Chinese population. J Clin Psychopharmacol. 2010;30(4):357–364. | ||
Nagao K, Kishi T, Moriwaki M, et al. Comparative clinical profile of mirtazapine and duloxetine in practical clinical settings in Japan: a 4-week open-label, parallel-group study of major depressive disorder. Neuropsychiatr Dis Treat. 2013;9:781–786. | ||
Sechter D, Vandel P, Weiller E, et al; study co-coordinators. A comparative study of milnacipran and paroxetine in outpatients with major depression. J Affect Disord. 2004;83(2–3):233–236. | ||
Higuchi H, Sato K, Yoshida K, et al. No predictors of antidepressant patient response to milnacipran were obtained using the three-factor structures of the Montgomery and Asberg Depression Rating Scale in Japanese patients with major depressive disorders. Psychiatry Clin Neurosci. 2008;62(2):197–202. | ||
Martinot M, Bragulat V, Artiges E, et al. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry. 2001;158(2):314–316. | ||
Gerra ML, Marchesi C, Amat JA, Blier P, Hellerstein DJ, Stewart JW. Does negative affectivity predict differential response to an SSRI versus a non-SSRI antidepressant? J Clin Psychiatry. 2014;75(9):e939–e944. | ||
Radhakishun FS, van den Bos J, van der Heijden BC, Roes KC, O’Hanlon JF. Mirtazapine effects on alertness and sleep in patients as recorded by interactive telecommunication during treatment with different dosing regimens. J Clin Psychopharmacol. 2000;20(5):531–537. | ||
Bailer U, Fischer P, Kufferle B, Stastny J, Kasper S. Occurrence of mirtazapine-induced delirium in organic brain disorder. Int Clin Psychopharmacol. 2000;15(4):239–243. | ||
Ladino M, Guardiola VD, Paniagua M. Mirtazapine-induced hyponatremia in an elderly hospice patient. J Palliat Med. 2006;9(2):258–260. | ||
Sinclair LI, Christmas DM, Hood SD, et al. Antidepressant-induced jitteriness/anxiety syndrome: systematic review. Br J Psychiatry. 2009;194(6):483–490. | ||
Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332–339. | ||
Coupland C, Hill T, Morriss R, Arthur A, Moore M, Hippisley-Cox J. Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database. BMJ. 2015;350:h517. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


