Back to Journals » Clinical Interventions in Aging » Volume 15
Identification of Predictors for Hemorrhagic Transformation in Patients with Acute Ischemic Stroke After Endovascular Therapy Using the Decision Tree Model
Authors Feng X, Ye G, Cao R, Qi P , Lu J, Chen J , Wang D
Received 12 April 2020
Accepted for publication 28 July 2020
Published 8 September 2020 Volume 2020:15 Pages 1611—1624
DOI https://doi.org/10.2147/CIA.S257931
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Xin Feng,1,* Gengfan Ye,1,* Ruoyao Cao,1 Peng Qi,2 Jun Lu,2 Juan Chen,2 Daming Wang1
1Department of Neurosurgery, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences; Graduate School of Peking Union Medical College, Beijing, 100730, People’s Republic of China; 2Department of Neurosurgery, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, 100730, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Daming Wang
Department of Neurosurgery, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences; Graduate School of Peking Union Medical College, No. 1 DaHua Road, Dong Dan, Beijing 100730, People’s Republic of China
Tel +86 10-85136281
Fax +86 10-85132621
Email [email protected]
Purpose: This study aimed to identify independent predictors for the risk of hemorrhagic transformation (HT) in arterial ischemic stroke (AIS) patients.
Methods: Consecutive patients with AIS due to large artery occlusion in the anterior circulation treated with mechanical thrombectomy (MT) were enrolled in a tertiary stroke center. Demographic and medical history data, admission lab results, and Circle of Willis (CoW) variations were collected from all patients.
Results: Altogether, 90 patients were included in this study; among them, 34 (37.8%) had HT after MT. The final pruned decision tree (DT) model consisted of collateral score and platelet to lymphocyte ratios (PLR) as predictors. Confusion matrix analysis showed that 82.2% (74/90) were correctly classified by the model (sensitivity, 79.4%; specificity, 83.9%). The area under the ROC curve (AUC) was 81.7%. The DT model demonstrated that participants with collateral scores of 2– 4 had a 75.0% probability of HT. For participants with collateral scores of 0– 1, if PLR at admission was < 302, participants had a 13.0% probability of HT; otherwise, participants had an 75.0% probability of HT. The final adjusted multivariate logistic regression analysis indicated that collateral score 0– 1 (OR, 10.186; 95% CI, 3.029– 34.248; p < 0.001), PLR (OR, 1.005; 95% CI, 1.001– 1.010; p = 0.040), and NIHSS at admission (OR, 1.106; 95% CI, 1.014– 1.205; p = 0.022) could be used to predict HT. The AUC for the model was 0.855, with 83.3% (75/90) were correctly classified (sensitivity, 79.4%; specificity, 87.3%). Less patients with HT achieved independent outcomes (mRS, 0– 2) in 90 days (20.6% vs. 64.3%, p < 0.001). Rate of poor outcomes (mRS, 4– 6) was significantly higher in patients with HT (73.5% vs. 19.6%; p < 0.001).
Conclusion: Both the DT model and multivariate logistic regression model confirmed that the lower collateral status and the higher PLR were significantly associated with an increased risk for HT in AIS patients after MT. PLR may be one of the cost-effective and practical predictors for HT. Further prospective multicenter studies are needed to validate our findings.
Keywords: acute ischemic stroke, mechanical thrombectomy; MT, hemorrhagic transformation; HT, decision tree model; DT
Introduction
Hemorrhagic transformation (HT) is a common and potentially catastrophic complication of acute ischemic stroke (AIS) in patients receiving mechanical thrombectomy (MT),1 and it results in worse outcomes and delays the initiation of antiplatelet or anticoagulation therapy.2,3 Therefore, to determine whether thrombolytic therapy is safe, it is essential to understand why HT can develop after AIS.4,5 Accumulating studies indicate that old age, hypertension, thrombolytic treatment, large infarct, long reperfusion time, high baseline National Institutes of Health Stroke Scale (NIHSS) score, high systolic blood pressure, pretreatment involvement of the basal ganglia, and the extent of ischemic injury prior to administration of therapy3,6,7 are risk factors for HT.
Inflammation response has been widely considered a critical factor participating in the pathophysiology process of AIS.8 Following AIS, infiltration of proinflammatory cytokines and the release of immune cells are involved in stroke-related brain damage, which contributes to exacerbated blood–brain barrier disruption, brain edema, and hemorrhagic complications.9 As a novel marker of baseline inflammatory response, platelet to lymphocyte ratios (PLR) gives an idea about both the aggregation and inflammation pathways, and it may be more valuable than either platelet or lymphocyte count alone in the prediction of atherosclerotic burden.10 Similarly, level of PLR on admission was found to be a potential diagnostic and prognostic markers of AIS.11 However, the critical role of PLR in HT in AIS patients treated by MT remains unclear.
In addition, Circle of Willis (CoW) plays an important role as the primary collateral in nature and might be essential in progresses of AIS patients.7 The SWIFT PRIME trial12 and the MR CLEAN trial13 found that pial collateral status according to computed tomography angiography (CTA) was positively related to favorable functional outcomes of AIS with anterior circulation large-vessel occlusion. However, it remains unclear whether the integrity of CoW predicts HT in patients with AIS treated with MT.
Decision tree (DT) are a data mining and classification tool and it overcomes the constraints of linear models, in which predictors are considered independent and additive and only predefined interactions are considered. Compared to other machine learning methods, DT offer a visual representation of prediction rules that can be more easily interpreted in clinical settings. In this study, we aimed to identify independent predictors for the risk of HT in AIS patients by using DT approach and multivariate logistics regression model. In addition to the risk factors reported in previous studies, we focused on the relationship between novel indicators (PLR and integrity of CoW) and HT.
Materials and Methods
Study Population
This study was approved by the Medical Ethics Committee of Beijing Hospital, and its protocol was in accordance with the principles of the Declaration of Helsinki. Patients with acute ischemic stroke who were treated by MT with or without thrombolysis between January 2015 and March 2019 were recruited in this study. All patients with complete occlusion of the internal carotid artery or the proximal segment of the middle cerebral artery (MCA) who were treated by MT were systematically included in this study, regardless of their age and stroke severity. For each patient, the decision to perform the endovascular treatment (EVT) was left at the discretion of the treating physician according to guidelines at the time of patient inclusion. All included patients provided written informed consent. If a patient was not competent to give consent (because of his or her cognitive state), his or her family members permitted by law provided written informed consent.
All patients underwent CT follow-up within 24 hours after procedure and immediately in case of a worsening condition. The occurrence of HT was diagnosed using follow-up CT scans. HT was classified as either hemorrhagic infarction (HI; including: HI1, scattered small petechiae, no mass effect; HI2, confluent petechiae, no mass effect) or parenchymal hematoma (PH; including: PH1 Hematoma within infarcted tissue, occupying <30%, no substantive mass effect; PH2 Hematoma occupying 30% or more of the infarcted tissue, with obvious mass effect) in accordance with the recommendations by the European Cooperative Acute Stroke Study.14 Two double-blinded clinicians reviewed the CT/MRI images to confirm the presence of HT and its subtype. Patients were divided into 2 groups according to the presence or absence of HT during hospitalization.
Collection of Clinical Characteristics
The following clinical data were collected prospectively: demographic feature (age and sex), comorbidities [atrial fibrillation, hypertension, diabetes mellitus, dyslipidemia, stroke or transient cerebral ischemia (TIA) history, smoking history, and coronary artery disease], current anticoagulant use, current antiplatelet use, whether received bridging thrombolysis, stroke etiology, and thrombus location (terminal ICA, first segment of MCA [M1], and second segment of MCA [M2)]. The neurological status was assessed by certified neurologists using the NIHSS before MT.
Measurement of Imaging Markers
All the patients underwent non-enhanced CT (NECT) (80 kV, 200 mAs, and 5-mm reconstructed slice thickness) and additionally underwent multiphase CT scanning (including NECT, CTA, and CT perfusion) (80 kV, 100 mAs, and 1-mm reconstructed slice thickness).
Absence or hypoplasia of arterial components in the CoW was assessed by an experienced neuroradiologist using axial reconstructions and volume-renderings of the multiple overlapping thin-slab-acquisition CTA data. Vertebral artery (VA) and A1 segments with diameters ≤ 50% compared with the contralateral side were defined as hypoplastic. Hypoplasia of the P1 segment of the posterior cerebral artery was defined as a diameter smaller than that of the feeding posterior communicating artery (PcomA).15,16 Configurations with hypoplastic P1 and/or A1 segments and/or VA were considered incomplete CoW.
The collateral score was determined from MIP images according to the following rules:17 0, absent collaterals in > 50% of an M2 branch territory; 1, diminished collaterals in > 50% of an M2 branch territory; 2, diminished collaterals in < 50% of an M2 branch territory; 3, collaterals equal to the contralateral hemisphere. There are three different types of the aortic arch, which are delineated based on the vertical distance from the innominate artery and the top of the aortic arch.18 The arterial tortuosity (tortuosity, kinking, and coiling)19 and atherosclerotic stenosis (> 50%) of the proximal path vessels and contralateral or/and posterior main artery stenosis >50% (contralateral internal carotid artery/M1 artery, basilar artery, and dominant VA) were evaluated by head and neck CTA. The Alberta Stroke Program Early CT Score (ASPECTS) was assessed prospectively for each patient by a neuroradiologist, who was blind to the procedure, using the CT scan. The clot burden score is a quantified assessment of the intracranial thrombus burden within the anterior circulation.20
Blood samples were taken in all patients before the initiation of EVT. The levels of white blood cells, neutrophils, lymphocytes, and platelets were assessed, and neutrophil-to-lymphocyte ratio, PLR were calculated. In addition, the baseline coagulation test on admission was conducted, including international normalized ratios (INRs) or activated partial thromboplastin times (APTT), prothrombin time (PT), D-Dimer, and fibrinogen values.
Patients were treated in a dedicated neuroangiography suite with up-to-date equipment under conscious sedation or general anesthesia. The EVT procedure frontline strategy was chosen at the operator’s discretion and it included a stent retriever (SR), contract aspiration (CA), and a combination of stent retriever and aspiration (Solumbra). In addition, whether switched to other treatment strategies, number of maneuvers, revascularization outcomes (success eTICI2b-3 revascularization), and any hemorrhage events.
Clinical Outcomes
Clinical outcomes were assessed with the modified Rankin Scale (mRS) at 90 days during face-to-face interviews or via telephone conversations with the patient, the patient’s relatives, or the general practitioner. A poor clinical outcome was defined as an mRS score of 4–6, and a good clinical outcome was defined as an mRS score of 0–2.
Statistical Analysis
The differences in clinical data and histologic composition were examined between the HT and without HT groups. Continuous variables were expressed as mean (SD), and the Mann–Whitney U-test was used to detect the differences. Fisher test or chi-square test was used for categorical data. Additionally, logistic regression analysis was performed to detect the risk factors for HT. Considering the limited sample size, variables were selected for multivariate logistic regression based on the clinical importance and p-values < 0.2 in univariate analysis. A P-value of < 0.05 was considered significant. Logistic regression analyses were performed with SPSS software (version 25.0, IBM, USA).
In the current study, DT models were developed using the “rpart” and “caret” packages within.21 The “rpart” package implements the Classification and Regression Trees (CART) algorithm of Breiman and colleagues. We used a confusion matrix to determine the performance of the DT for HT. In this study, “HT” was defined as a positive event, and “without HT” was defined as a negative event. The confusion matrix for two classes was used to extract true positives, true negatives, false positives, and false negatives. A receiver operating characteristic (ROC) graph is a technique for visualizing, organizing, and selecting classifiers based on their performances. The area under the ROC curve (AUC) of the classifier can be described as the probability of the classifier to rank a randomly selected positive case higher than a randomly selected negative case.
Results
Characteristics of Patients
As shown in Table 1, 90 patients were included in this study (median age, 71.6 years; 49 males); among these patients, 34 (37.8%) had HT during MT, including 18 (20.0%) HI and 16 (17.8%) PH. Of 18 patients with HI, 8 (8.9%) were HI 1 and 10 (9.1%) were HI 2. Of 16 patients with PH, 12 (13.3%) were PH 1 and 4 (4.5%) patients were PH 2. Only one patient occurred remote hemorrhage. Consequently, 8 patients (8.9%) with HT were symptomatic and 26 (28.9%) were asymptomatic. The flowchart of this study was showed in Figure 1.
 |
Table 1 Baseline Characteristics of Patients with Hemorrhagic Transformation and Those with No Hemorrhagic Transformation |
 |
Figure 1 Flowchart of this study. Abbreviation: HT, hemorrhagic transformation. |
The stroke etiology was categorized as follows: cardiogenic embolism (46, 51.1%), large artery atherosclerosis (27, 30.0%), and cryptogenic stroke (17, 18.9%). Regarding thrombus location, 33 (336.7%) patients had ICA occlusion, 37 (41.1%) had M1 occlusion, 14 (15.6%) had M2 occlusion, and 6 (6.7%) had tandem occlusion. Twenty-five patients (27.8%) received bridging thrombolysis, and frontline MT strategies were classified as SR (30, 33.3%), CA (30, 33.3%), and Solumbra (30, 33.3%). Twenty-seven (30.0%) patients switched to other strategies. At the end of the procedure, 76 (84.4%) patients achieved eTICI2b-3 recanalization.
Univariate analysis for predicting HT was showed in Table 1, Compared with patients without HT, those with HT had more atrial fibrillation (61.8% vs. 37.5%, p = 0.025), relatively more coronary artery diseases (61.8% vs. 25.0%, p = 0.001), higher systolic blood pressure at admittance (1556 vs. 138% mmHg, p = 0.001), more ICA occlusion (58.8% vs. 23.2%, p = 0.001), higher ASPECT (6% vs. 7%, p = 0.019), relatively more hyperdense artery sign (61.8% vs. 32.1%, p = 0.006), relatively less platelet counts (222.8 vs. 183.2, 0.020), comparable neutrophil-to-lymphocyte ratios (11.95 vs. 6.40, p = 0.001), relatively higher PLR (260.92 vs. 170.25, p = 0.002), relatively lower lymphocyte-to-monocyte ratio (2 vs. 2, p = 0.064), and more contralateral or (and) posterior main artery stenosis > 50% (61.8% vs. 33.9%, p = 0.010). In addition, more patients with HT had collateral scores of 0–1 (61.8% vs. 10.9%, p = 0.001) and clot burden scores > 6 (61.8% vs. 19.6%, p = 0.001) than those without HT. There were no significant differences in demographics, other comorbidities, stroke etiology, or any hemorrhage events between these two groups.
Compared with patients without HI, those with PH had higher serum glucose (10.3 vs. 7.5, [mg/dl], p = 0.032), relatively higher NIHSS at admission (12.3 vs. 18.0, p = 0.008). However, differences in other variables between the HI and PH groups were not significant. (Table 2)
 |
Table 2 Univariate Analysis According to Patients with HI and PH |
Decision Tree
A DT was built with five nodes and three leaves, and the results are illustrated in Figure 2. The final model consisted of collateral score and PLR as predictors. PT, glucose was dropped as predictors during pruning. The model was further evaluated for its accuracy by applying a confusion matrix analysis, and the results showed that 82.2% (74/90) were correctly classified, with a sensitivity of 79.4% and specificity of 83.9%. The area under the ROC curve (AUC) for the model was 81.7% (Figure 3), demonstrating that this model has achieved higher accuracy in classifying the true positives rather than the false positives. The DT demonstrated that among those participants with collateral scores of 2–4, participants had a 75.0% probability of HT. For participants with collateral scores of 0–1, if PLR on admission was < 302, participants had a 13.0% probability of HT; otherwise, participants had an 75.0% probability of HT.
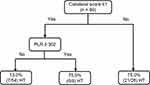 |
Figure 2 Decision tree path for 90 individuals with and without HT. Abbreviations: HT, hemorrhagic transformation; LMR, lymphocyte-to-monocyte ratio. |
 |
Figure 3 Performance of multivariate logistic regression model and decision tree model for predicting hemorrhagic transformation. |
Logistic Regression for Prediction of SE
The results of logistic regression are shown in Table 3. The multivariate model was created using variables with P values < 0.20 in the univariate analysis. In the final adjusted model, three factors were included in the multivariate analysis. The results indicated that collateral score 0–1 (OR, 10.186; 95% CI, 3.029–34.248; p < 0.001), lymphocyte-to-monocyte ratios (OR, 1.005; 95% CI, 1.001–1.010; p = 0.040), and NIHSS at admission (OR, 1.106; 95% CI, 1.014–1.205; p = 0.022) could be used to predict HT. The AUC for the model was 0.855, 95% confidence interval was 0.767–0.943 (Figure 3). The model was further evaluated for its accuracy by applying a confusion matrix analysis, the results showed that 83.3% (75/90) were correctly classified, with a sensitivity of 79.4% and specificity of 87.3%.
 |
Table 3 Multivariate Analysis of Independent Predictors for the Risk of Hemorrhagic Transformation in Arterial Ischemic Stroke Patients Treated by Mechanical Thrombectomy (MT) |
Clinical Outcomes
Less patients with HT achieved independent outcomes (mRS, 0–2) in 90 days (20.6% vs. 64.3%, p < 0.001). Poor outcomes (mRS, 4–6) were significantly higher in patients with HT (73.5% vs. 19.6%; p < 0.001). Both independent outcomes and Poor outcomes were not significantly associated with CoW variations, including variant-type with hypoplastic VA, P1, and A1 segments (Tables 1 and 4).
 |
Table 4 Univariate Analysis of According to 3-Month Clinical Outcome |
Discussion
The current study is the first to use the binary DT model to identify the independent predictors for the risk of HT in AIS patients. We found that poor collateral status and higher PLR were significantly associated with HT in both the DT model and the multivariate model. Confusion matrix analysis and AUC showed that both two model have good performance for predicting HT in AIS patients. However, no significant influence of CoW variations on HT in patients with AIS was found, and CoW variations, including variant-type with hypoplastic VA, P1, and A1 segments, were not associated with poor collateral status.
Inflammation response has been widely considered a critical factor in the pathophysiology process of AIS.22,23 Following AIS, the infiltration of immune cells and the release of proinflammatory cytokines are involved in the secondary injury, including blood–brain barrier disruption, brain edema, and HT. Recent studies have found that in response to proximal occlusion, early accumulation of platelets, neutrophil, and fibrinogen occurs mostly in the venous compartments of downstream microcirculation, a phenomenon known as downstream microvascular thromboinflammation,23–25 which might worsen ischemic damage and precipitate blood–brain barrier disruption and subsequent HT.25 Therefore, it is significant to identify the inflammatory markers that can be used to predict HT and prognosis of AIS patients, especially for those who have been successfully recanalized. Recent studies have demonstrated a significant decrease in lymphocytes in patients with poor outcomes, indicating that low lymphocyte counts may be an important predictor for poor long-term functional outcomes after AIS. As a necessary factor against HT following ischemia/reperfusion because of its hemostatic function, previous study found that lower level of platelets was associated with early HT in patients with IVT.26 Except for its hemostatic function, platelet also plays an important role in the inflammatory response.27 As a novel marker, PLR gives an idea about both the inflammatory response and hemostatic function, and it may be more valuable than either platelet or lymphocyte count alone in the prediction of HT risk in AIS patients. In the final DT model and multivariate model, higher PLR was found to be a significant predictor for HT in patients with AIS after MT. This novel biomarker is easier to obtain before MT for AIS patients, so its clinical application value deserves attention and needs further prospective study.
Previous studies reported that the incidence of HT decreased with a low baseline NIHSS score.28,29 After adjustment of other confounding factors, a higher baseline NIHSS score was an independent risk factor of HT. The results of this study were consistent with previous findings. The NIHSS score reflects the degree of neurological deficits. A higher NIHSS score at onset indicates a larger infarct or poorer collateral circulation. As a key imaging indicator of AIS severity, ASPECTS was found to be of value to predict HT risk after acute cardioembolic stroke and may be a quick risk assessment approach before reperfusion therapy.30 This finding is consistent with the result of our univariate analysis. However, no significant association was found in the final DT model and multivariate model. This result may be caused by the adjusted effect of other factors and the limited sample size of our study.
CoW variations or configurations, which are not completely symmetric, are found in up to 40% of the population.31 The CoW is a primary collateral pathway that compensates quickly for a drop in cerebral blood flow under certain pathological conditions.32,33 Notably, Furukawa et al described a rapid resolution of symptoms after an acute ischemic attack in patients with complete CoW.34 Complete CoW also indicates the integrity of the proximal MCA and distal MCA occlusion with collateral flows. It has been shown that secondary and tertiary MCA branch occlusions are more likely to undergo early recanalization than occlusions of more proximal large vessels.35 CoW intracranial vessel occlusion has been demonstrated to be associated with poorer outcomes.36,37 Such anatomic variations have been shown to be associated with physiologic changes in blood flow, and the cerebral blood flow distribution in different configurations of the CoW was assessed previously.38 For instance, Katarina Millesi et al found that the CoW and anterior temporal artery contributed to leptomeningeal collaterals, although they were dependent on the outcomes of AIS patients after MT; Chuang et al39 demonstrated that incomplete CoW was associated with a 3-fold increase in the rate of symptomatic intracerebral hemorrhage in ischemic stroke patients treated with intravenous thrombolysis; Liang et al revealed that complete CoW decreased the risk of cerebral reperfusion injury and subsequent hemorrhage after revascularization.40 However, studies evaluating the CoW in AIS patients treated with MT are rare. In this study, no significant influence of CoW variations on HT in AIS patients was found, and CoW variations, including variant-type with hypoplastic VA, P1, and A1 segments, were not associated with the HT risk. In addition, poor collateral status was significantly associated with the higher risk of HT in both the DT model and multivariate model, although configurations of the CoW were not associated with collateral status (Table 3). The findings are in line with previously published results.41
Ischemic penumbra after AIS can remain viable because blood flow is somewhat sustained through arterial communications between the extracranial and intracranial circulation, the CoW (an equalizing distributor), and the leptomeningeal anastomoses.42 Therefore, severe stenosis of the contralateral internal carotid artery, M1 artery, basilar artery, and dominant VA may affect the compensatory blood supply of the collateral circulation. This study is the first to investigate the impact of the contralateral main artery or basilar artery/dominant VA stenosis ≥ 50% on HT in AIS patients treated by MT. Our results showed that the contralateral main artery, basilar artery, and dominant VA stenosis ≥ 50% was an important predictor for the risk of HT in AIS patients after MT. Indeed, the blood flow in these patients is greater after recanalization, thereby increasing the risk of hypoperfusion after ischemia.43
Lymphocyte-to-monocyte ratio, neutrophil to lymphocyte ratio at admission, high blood glucose, atrial fibrillation were are considered important predictors of development of HT in AIS patients after MT.44–46 However, these factors had no added value for the prediction of HT when other risk factors were accounted for. This finding does not mean that these factors are not important risk factors for HT in isolation, but, instead, that these factors were not significantly associated with HT beyond the three predictors included in our final DT model and multivariate model.
Limitation
The retrospective and single-center design was one of the major limitations of our study. Secondly, the relatively small sample size also increased the probability of selection bias. Thirdly, we could not measure the amount or direction of blood flowing through collateral vessels or correctly demonstrate the patency of the PCoA or P1 on CTA images because high-quality lateral projection images were lacking for some patients. Fourthly, previous studies used different definitions for hypoplasia of vascular segments, and the conclusions in this study were only based on our definition. Finally, our conclusions would be more convincing if we could record dynamic changes in the monocyte and lymphocyte counts; therefore, prospective studies with monocyte and lymphocyte count calculation at more time points are needed. Moreover, we did not take into account the possible role of arterial stiffness that may be an important predictor for HT after (intravenous, mechanical or both).47,48 Finally, this study did not exclude patients without recanalization success because this study aimed to explore the impact of anatomical factors on HT and identify biomarkers of blood samples in AIS patients treated by MT.
Conclusion
Both the DT model and multivariate logistic regression model confirmed that the poor lower collateral status and the higher PLR were significantly associated with an increased risk for HT in AIS patients after MT. As a clinically available biomarker, PLR may be one of the cost-effective and practical predictors for HT. Further prospective multicenter studies are needed to validate our findings.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Álvarez-Sabín J, Maisterra O, Santamarina E, et al. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol. 2013;12:689–705. doi:10.1016/S1474-4422(13)70055-3
2. García-Culebras A, Palma-Tortosa S, Moraga A, et al. Toll-like receptor 4 mediates hemorrhagic transformation after delayed tissue plasminogen activator administration in in situ thromboembolic stroke. Stroke. 2017;48:1695–1699. doi:10.1161/STROKEAHA.116.015956
3. Huang G-Q, Zeng Y-Y, Cheng Q-Q, et al. Low triiodothyronine syndrome is associated with hemorrhagic transformation in patients with acute ischaemic stroke. Aging. 2019;11:6385–6397. doi:10.18632/aging.102195
4. Wang T, Duan Y-M, Fu Q, et al. IM-12 activates the Wnt–β-catenin signaling pathway and attenuates rtPA-induced hemorrhagic transformation in rats after acute ischemic stroke. Biochem Cell Biol. 2019;97:702–708. doi:10.1139/bcb-2018-0384
5. Jin R, Xiao AY, Li J, et al. PI3Kγ (Phosphoinositide 3-Kinase-γ) inhibition attenuates tissue-type plasminogen activator-induced brain hemorrhage and improves microvascular patency after embolic stroke. Hypertension. 2019;73:206–216. doi:10.1161/HYPERTENSIONAHA.118.12001
6. Li F, Ren Y, Cui X, et al. Postoperative hyperglycemia predicts symptomatic intracranial hemorrhage after endovascular treatment in patients with acute anterior circulation large artery occlusion. J Neurol Sci. 2019;409:116588. doi:10.1016/j.jns.2019.116588
7. Semerano A, Laredo C, Zhao Y, et al. Leukocytes, collateral circulation, and reperfusion in ischemic stroke patients treated with mechanical thrombectomy. Stroke. 2019;50:3456–3464. doi:10.1161/STROKEAHA.119.026743
8. Yu J, Zhu H, Taheri S, et al. Serum amyloid A-mediated inflammasome activation of microglial cells in cerebral ischemia. J Neurosci. 2019;39:9465–9476. doi:10.1523/JNEUROSCI.0801-19.2019
9. Tsygan NV, Trashkov AP, Litvinenko IV, et al. Autoimmunity in acute ischemic stroke and the role of blood-brain barrier: the dark side or the light one? Front Med. 2019;13:420–426. doi:10.1007/s11684-019-0688-6
10. Serrano CV, de Mattos Fernando R, Pitta FG, et al. Association between neutrophil-lymphocyte and platelet-lymphocyte ratios and coronary artery calcification score among asymptomatic patients: data from a cross-sectional study. Mediators Inflamm. 2019;2019:6513847. doi:10.1155/2019/6513847
11. Zhang Y, Yang P, Wang J. Peripheral blood platelet to lymphocyte ratio as potential diagnostic and prognostic markers of acute cerebral infarction and its clinical significance. Clin Lab. 2019;65.
12. Jadhav AP, Diener H-C, Bonafe A, et al. Correlation between clinical outcomes and baseline CT and CT angiographic findings in the SWIFT PRIME trial. AJNR Am J Neuroradiol. 2017;38:2270–2276. doi:10.3174/ajnr.A5406
13. Guglielmi V, LeCouffe Natalie E, Zinkstok SM, et al. Collateral circulation and outcome in atherosclerotic versus cardioembolic cerebral large vessel occlusion. Stroke. 2019;50:3360–3368. doi:10.1161/STROKEAHA.119.026299
14. Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian acute stroke study (ECASS II). Stroke. 2001;32:438–441. doi:10.1161/01.STR.32.2.438
15. Cornelissen BMW, Schneiders JJ, Sprengers ME, et al. Aneurysmal parent artery-specific inflow conditions for complete and incomplete circle of willis configurations. AJNR Am J Neuroradiol. 2018;39:910–915. doi:10.3174/ajnr.A5602
16. Rinaldo L, McCutcheon BA, Murphy ME, et al. Relationship of A1 segment hypoplasia to anterior communicating artery aneurysm morphology and risk factors for aneurysm formation. J Neurosurg. 2017;127:89–95. doi:10.3171/2016.7.JNS16736
17. Tan IYL, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–531. doi:10.3174/ajnr.A1408
18. Madhwal S, Rajagopal V, Bhatt DL, Bajzer CT, Whitlow P, Kapadia SR. Predictors of difficult carotid stenting as determined by aortic arch angiography. J Invasive Cardiol. 2008;20:200–204.
19. Zhou R, Liu D, Yu K, et al. Carotid and vertebral arterial variations in Alzheimer’s disease. Curr Alzheimer Res. 2015;12:368–376. doi:10.2174/1567205012666150325183903
20. Puetz V, Dzialowski I, Hill MD, et al. Calgary CTA study group. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: the clot burden score. Int J Stroke. 2008;3:230–236. doi:10.1111/j.1747-4949.2008.00221.x
21. Kuhn M. Building predictive models in R using the caret package. J Stat Software. 2008;28(5):1–26. doi:10.18637/jss.v028.i05
22. Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21:2076–2097. doi:10.2174/0929867321666131228205146
23. Desilles JP, Loyau S, Syvannarath V, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. 2015;46:3241–3248. doi:10.1161/STROKEAHA.115.010721
24. Desilles JP, Syvannarath V, Ollivier V, et al. Exacerbation of thromboinflammation by hyperglycemia precipitates cerebral infarct growth and hemorrhagic transformation. Stroke. 2017;48:1932–1940. doi:10.1161/STROKEAHA.117.017080
25. Desilles JP, Syvannarath V, Di Meglio L, et al. Downstream microvascular thrombosis in cortical venules is an early response to proximal cerebral arterial occlusion. J Am Heart Assoc. 2018;7:e0078804. doi:10.1161/JAHA.117.007804
26. He W, Ruan Y, Yuan C, et al. High neutrophil-to-platelet ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. Front Neurol. 2019;10:1310. doi:10.3389/fneur.2019.01310
27. Garcia-Culebras A, Duran-Laforet V, Pena-Martinez C, et al. Myeloid cells as therapeutic targets in neuroinflammation after stroke: specific roles of neutrophils and neutrophil-platelet interactions. J Cereb Blood Flow Metab. 2018;38:2150–2164. doi:10.1177/0271678X18795789
28. Cao R, Ye G, Wang R, et al. Collateral vessels on 4D CTA as a predictor of hemorrhage transformation after endovascular treatments in patients with acute ischemic stroke: a single-center study. Front Neurol. 2020;11:60. doi:10.3389/fneur.2020.00060
29. Wen L, Zhang S, Wan K, Zhang H, Zhang X. Risk factors of haemorrhagic transformation for acute ischaemic stroke in Chinese patients receiving intravenous thrombolysis: a meta-analysis. Medicine. 2020;99:e18995. doi:10.1097/MD.0000000000018995
30. Liu L, Wu B, Zhao J, et al. Computed tomography perfusion alberta stroke program early computed tomography score is associated with hemorrhagic transformation after acute cardioembolic stroke. Front Neurol. 2017;8:591. doi:10.3389/fneur.2017.00591
31. Kapoor K, Singh B, Dewan LI. Variations in the configuration of the circle of Willis. Anat Sci Int. 2008;83:96e106. doi:10.1111/j.1447-073X.2007.00216.x
32. Ross MR, Pelc NJ, Enzmann DR. Qualitative phase contrast MRA in the normal and abnormal circle of Willis. Am J Neuroradiol. 1993;14:19–25.
33. Henninger H, Fisher M. Stimulating circle of Willis nerve fiber preserves the diffusion-perfusion mismatch in experimental stroke. Stroke. 2007;38:2779–2786. doi:10.1161/STROKEAHA.107.485581
34. Furukawa S, Takaya A, Nakagawa T, Sakaguchi I, Nishi K. Rapid resolution of symptoms after transient ischemic attack and the circle of Willis. Int J Emerg Med. 2011;6(2).
35. Saito I, Segawa H, Shikawa Y, Taniguchi M, Tsutsumi K. Middle cerebral artery occlusion: correlation of computed tomography and angiography with clinical outcome. Stroke. 1987;18:863–868. doi:10.1161/01.STR.18.5.863
36. Coutts SB, O’Reilly C, Hill MD, et al. Computed tomography and computed tomography angiography findings predict functional impairment in patients with minor stroke and transient ischaemic attack. Int J Stroke. 2009;4:448–453. doi:10.1111/j.1747-4949.2009.00346.x
37. Schomer DF, Marks MP, Steinberg GK, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med. 1994;330:1565–1570. doi:10.1056/NEJM199406023302204
38. Hendrikse J, van Raamt AF, van der Graaf Y, et al. Distribution of cerebral blood flow in the circle of Willis. Radiology. 2005;235:184–189. doi:10.1148/radiol.2351031799
39. Chuang Y-M, Chan L, Lai Y-J, et al. Configuration of the circle of Willis is associated with less symptomatic intracerebral hemorrhage in ischemic stroke patients treated with intravenous thrombolysis. J Crit Care. 2013;28:166–172. doi:10.1016/j.jcrc.2012.08.018
40. Liang F, Fukasaku K, Liu H, Takagi S. A computational model study of the influence of the anatomy of the circle of Willis on cerebral hyperperfusion following carotid artery surgery. Biomed Eng Online. 2011;10:84. doi:10.1186/1475-925X-10-84
41. Nawabi J, Kniep H, Broocks G, et al. Clinical relevance of asymptomatic intracerebral hemorrhage post thrombectomy depends on angiographic collateral score. J Cereb Blood Flow Metab. 2019;0271678X19871253.
42. Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi:10.1016/S1474-4422(11)70195-8
43. Kneihsl M, Niederkorn K, Deutschmann H, et al. Increased middle cerebral artery mean blood flow velocity index after stroke thrombectomy indicates increased risk for intracranial hemorrhage. J Neurointerv Surg. 2018;10:882–887. doi:10.1136/neurintsurg-2017-013617
44. Pikija S, Sztriha LK, Killer-Oberpfalzer M, et al. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J Neuroinflammation. 2018;15:319. doi:10.1186/s12974-018-1359-2
45. Song Q, Pan R, Jin Y, et al. Lymphocyte-to-monocyte ratio and risk of hemorrhagic transformation in patients with acute ischemic stroke. Neurol Sci. 2020;1–10.
46. Zhang W-B, Zeng Y-Y, Wang F, Cheng L, Tang W-J, Wang X-Q. A high neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation of large atherosclerotic infarction in patients with acute ischemic stroke. Aging. 2020;12(3):2428–2439. doi:10.18632/aging.102752
47. Acampa M, Camarri S, P E. L, et al. Increased arterial stiffness is an independent risk factor for hemorrhagic transformation in ischemic stroke undergoing thrombolysis. Int J Cardiol. 2017;243:466–470. doi:10.1016/j.ijcard.2017.03.129
48. Acampa M, Romano DG, P E. L, et al. Increased arterial stiffness is associated with poor collaterals in acute ischemic stroke from large vessel occlusion. Curr Neurovasc Res. 2018;15(1):34–38. doi:10.2174/1567202615666180326100347
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
