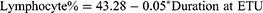Back to Journals » Journal of Blood Medicine » Volume 14
Identification of Laboratory Biomarkers for Early Detection and Clinical Management of Post-Acute Syndrome Among Survivors of the 2013–2016 West Africa Ebola Outbreak in Sierra Leone
Authors Guetiya Wadoum RE , Sevalie S, Baimba Kargbo M, Clarke A , Bangura S, Kargbo M, Sesay HM , Kamara AH, Bangura J, Kamara AF, Allieu S, Rogers H, Mattei M, Colizzi V, Montesano C, Momoh EJJ
Received 18 April 2022
Accepted for publication 7 February 2023
Published 11 February 2023 Volume 2023:14 Pages 119—132
DOI https://doi.org/10.2147/JBM.S371239
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Raoul Emeric Guetiya Wadoum,1,* Stephen Sevalie,2,* Maurice Baimba Kargbo,3 Andrew Clarke,4,5 Sherry Bangura,6 Mariatu Kargbo,6 Hawa Mariama Sesay,7 Abdul H Kamara,6 Jamil Bangura,6 Alie F Kamara,6 Sophie Allieu,1 Hassan Rogers,1 Maurizio Mattei,8 Vittorio Colizzi,9 Carla Montesano,8 Edwin JJ Momoh1,3
1Department of Public Health, Microbiology and Immunology, Ernest Bai Koroma University of Science and Technology, Makeni, Sierra Leone; 2 34th Military Hospital, The Republic of Sierra Leone Armed Forces, Freetown, Sierra Leone; 3Department of Agriculture and Food Security; Ernest Bai Koroma University of Science and Technology, Makeni, Sierra Leone; 4Global Programs Division, Save the Children UK, London, UK; 5Department/Division of Health Research, Lancaster University, Lancaster, UK; 6Sierra Leone Association of Ebola Survivors, Freetown, Sierra Leone; 7Department of Public Health, University of Makeni, Makeni, Sierra Leone; 8Department of Biology, University of Rome Tor Vergata, Rome, Italy; 9Department of Sciences and Technology, Evangelical University of Cameroon, Bandjoun, Cameroon
*These authors contributed equally to this work
Correspondence: Raoul Emeric Guetiya Wadoum; Edwin JJ Momoh, Email [email protected]; [email protected]; [email protected]
Background: The clinical management of persistent medical conditions affecting Ebola survivors, generally described as a post-Ebola syndrome, remains a public health concern. We aimed to analyze Ebola survivors’ laboratory biomarkers as compared to their non-infected household relatives to identify biomarkers that could guide the identification of survivors at increased risk of developing severe at odds with the non-severe post-Ebola syndrome.
Materials and Methods: Data were extracted from medical records of the Ebola survivors clinic, and we included only Ebola survivor’s parameters recorded during the first baseline follow-up visit 2 weeks interval after their second negative PCR result. Moreover, household non-infected family contacts of survivors visiting the clinic during the same period were recruited as community control.
Results: The mean age of survivors was 32.65 (IQR: 15.5, 38.25) years, and Ebola IgG immunoglobulin was detected in all, thus confirming their status. The statistical significance (all p < 0.05) observed in monocyte percentage (MONO%), cluster of differentiation 4 percentage (CD4%), alanine aminotransferase (ALT), creatinine (CREA), and creatinine kinase (C-kinase) proved to be clinically significant as compared to the household relatives’ group. Interestingly, the linear regression analysis indicated that the duration at ETU was negatively associated with lymphocyte percentage with a 5% lymphocyte decrease per day spent at ETU. Finally, there was a significant (p < 0.05) association between hematological (Hb, PCV, MCV, MCH), biochemical (ALT, CREA, C-kinase, T-cholesterol, triglycerides) parameters and the risk of developing severe complications.
Conclusion: We recommend clinicians closely monitor Hb, PCV, MCV, MCH, ALT, CREA, C-kinase, T-cholesterol, triglycerides and lymphocytes as clinically relevant laboratory biomarkers to identify survivors at higher risk of developing severe post-acute syndrome upon discharge from Ebola treatment unit including headache, abdominal pain, chest pain, ocular complication, arthralgia, hearing difficulty and erectile dysfunction which can impact health-related quality of life among Ebola survivors.
Keywords: Ebola virus disease, hematological, immunological, biochemical, biomarkers, post-acute syndrome
Introduction
The burden of the unprecedented West Africa Ebola epidemic was highest in Sierra Leone, with 14,124 reported cases and 3956 confirmed deaths.1 Despite the deathly nature of the disease with an average case fatality ratio of 50%, thousands survived and most often suffer from persistent clinical complications after hospital discharge described as post-Ebola sequelae.2,3 These complications often emanate from symptoms developed during acute infections with Ebola virus disease (EVD) exacerbated by the unavailable treatment for EVD. Clinical management during infection is mainly supportive, including administration of oral and intravenous fluids and electrolytes, co-infections, and symptom control. Moreover, EVD infection causes substantial biochemical, haematological and immunological abnormalities, which might be challenging to amend even after recovery.4 Therefore, the identification of laboratory predictors of the progression of complications towards severe or fatal form is urgently needed. These predictors will enable risk stratification, guide interventional follow-up to monitor survivors at enhanced risk of developing severe complications, and optimize limited technical and human resources allocation.
This study aimed to analyze Ebola survivors’ laboratory biomarkers as compared to their non-infected household relatives to determine biomarkers that could guide the identification of survivors at increased risk of developing severe at odds with the non-severe post-Ebola syndrome.
Materials and Methods
Ethical Consideration
The study was approved by the Sierra Leone Ethics and Scientific Review Committee and the Sierra Leone Ministry of Health and Sanitation. All study participants or guardians approve to be enrolled by signing or fingerprinting a written consent form following the 1964 Helsinki declaration and later amendments.
Description of 34th Regimental Military Hospital Ebola Survivor Clinic
Ebola survivor clinics were established nationwide by the government with support from local and international partners to manage sequelae as soon as the number of patients recovering from EVD started increasing. The MH34 clinic was one of the first established and started enrolment of survivors and their family relatives 2 weeks (~14 days) after the discharge of the first cohort from the MH34 Ebola Treatment Unit (ETU). Inclusion criteria for EVD survivors were confirmed EVD by polymerase chain reaction testing and/or IgM/IgG positive on serological testing plus verification of EVD discharge certificate. At the same time, household contacts were expected to be close family non-infected relatives living together during the outbreak and have never been admitted to an ETU as well as not enrolled in an EVD vaccine clinical trial.4 Moreover, survivors were recruited regardless of ETU initially treated, and medical care was provided even if survivors were not willing to be enrolled in the study.
Furthermore, no direct compensation was provided to them other than the potential benefits of clinical examination and blood analysis. All medical care, laboratory diagnosis and treatment were provided for free. Personal history was recorded during each medical consultation, and a medical examination was performed, including psychological assessment and counselling.
Data Collection
All the records of laboratory-confirmed EVD survivors and their household members were extracted for a detailed review. Data available included socio-demographic and clinical characteristics, sequelae, haematological, biochemical, and immune biomarkers.
Laboratory Methods
Ebola Virus RNA Detection by qRT-PCR and Anti-EBOV IgG Quantification Using ELISA
All samples were initially examined to detect the Ebola virus ribonucleic acid (RNA) using a real-time quantitative polymerase chain reaction (qRT-PCR) assay to ascertain the absence of the virus after the onset of symptoms in survivors. Total RNA was extracted from plasma manually using a QiaAmp viral RNA mini kit (Qiagen, Germany) according to the manufacturer’s protocol, and materials were eluted in 60 μL of AVE buffer and stored at −80 °C until needed. The assay was performed on a LightCycler 96 thermocycler (Roche) using the RealStar Filovirus Screen RT-PCR 1.0 kit from Altona Diagnostics and considered positive if cycle threshold (Ct) values were <40.
All qRT-PCR negative samples were subjected to human anti-glycoprotein (GP) IgG responses to Zaire Ebolavirus using an enzyme-linked immunosorbent assay (ELISA) commercial kit from Alpha Diagnostic International (ADI, Texas, USA) according to the manufacturer’s instructions. Positive control and calibrators provided with the kit were used in each test run, and optical density was read at 450 nm on a MultiScanGo microplate reader (ThermoFisher Scientific) with plasma diluted at 1:200. Antibody detection was further validated in oral fluid samples from identical participants by the use of a new IgG capture assay based on the EBOV Mayinga GP antigen (rGPδTM [catalogue 0501-016]; IBT Bioservices, Rockville, MD, USA) as described by Lambe et al5 and the presence of antibody was used as additional evidence for survivor status.
Clinical Laboratory Assays
Anticoagulated whole blood was used for assessment of complete blood count with a five-part differential automated haematology analyzer (Beckman Coulter, CA, USA), including haemoglobin (Hb), packed cell volume/hematocrit (PCV/HCT), leukocyte (WBC), mean corpuscular volume (MCV), red blood cell count (RBC), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), platelets (PLT), basophile (BASO), eosinophils (EOSIN), neutrophils (NEUT), monocytes (MONO), and lymphocytes (LYM).
Another blood portion was used for a cluster of differentiation 4 (CD4) cell count and CD4 percentage analysis with a FACSCalibur flow cytometer (Becton Dickinson Immunochemistry, California, USA). Serum chemistries were performed on cryopreserved samples on a Roche Reflotron Plus clinical chemistry analyzer with manufacturer reagent strips specific to each of the following parameters: alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), bilirubin (BIL), creatinine (CREA), urea, creatine kinase (C-kinase), pancreatic amylase (P-amylase), total cholesterol (T-cholesterol), and triglycerides.
All biomarkers were compared with clinical reference values established by the World Health Organization to identify clinical differences.6
Statistical Analysis
Data were transformed into a Microsoft Excel spreadsheet and analysed using Statistical Package for Social Sciences (SPSS) Version 20.0. Descriptive statistics, mean, standard deviations and interquartile range (IQR) were used to present the findings. The analysis of biomarkers levels in Ebola survivors against household relatives was performed using Mann–Whitney U-tests. Multivariate correlation for biomarkers level and survivor duration at the ETU were analysed with the nonparametric Spearman’s test (alpha 0.05) running on a JMP Software, v13.1.0 (SAS Institute, Cary, NC, USA). A colour map matrix represented correlations (Spearman r). A point-biserial correlation was further performed to ascertain whether the post–Ebola syndrome was associated with various biomarkers. A linear regression model was used to establish whether the biomarkers significantly higher in survivors than relatives were predicted by the duration at the ETU.
Results
A total of 213 participants were enrolled in the present study, including 83 EVD survivors and 133 household relatives of survivors (Figure 1). Their characteristics are outlined in Table 1.
 |
Table 1 Demographic and Clinical Characteristics of Survivors and Their Household Relatives |
The mean age of survivors was 32.65 years (IQR: 15.5, 38.25). In addition to the clinical report and discharge certificate review, EVD survivor status was confirmed using combined serological assays to detect Ebola virus IgG response (Figure 2).
 |
Figure 2 Ebola IgG antibody level in survivors and their household non-infected relatives. Abbreviation: IgG, immunoglobulin G. |
Both serological methods proved effective in distinguishing survivors from their household non-infected relatives, as evidenced by the positive correlation (r = 0.476) between the IgG antibody activity in the serum and saliva of survivors (Figure 3).
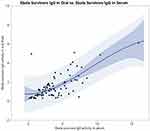 |
Figure 3 Quadratic correlation representation between Ebola IgG antibody level in serum and oral fluid of survivors. Abbreviation: IgG, immunoglobulin G. |
The analysis of haematological biomarkers showed that haemoglobin concentration and red blood cell count were significantly different in the survivor group than in household relatives. A similar pattern was observed with the mean corpuscular volume, mean corpuscular haemoglobin and mean corpuscular haemoglobin concentration (p < 0.05), as shown in Table 2. However, there is no significant difference in white blood cells, haematocrit and platelet count between the survivors and household relative’s cohort (all p > 0.05). Regarding haemoglobin and mean corpuscular volume estimation, the survivors’ cohort significantly reported a higher level for both than the household relative’s cohort (p < 0.05).
 |
Table 2 Comparison of Clinical Biomarkers of Survivors and Their Household Relatives |
As displayed in Table 2, the immunological profile of biomarkers shows that the monocyte count and CD4 percentage were significantly higher in the survivor’s group than in the household relatives’ group with p < 0.001 and 0.04, respectively. Nevertheless, there was no significant difference in basophil, eosinophil, neutrophil, lymphocyte and CD4 count in both cohorts (p > 0.05).
Analysis of the biochemical profile showed that the proportion of these biomarkers significantly higher was more relevant in the survivor cohort than the household relative cohort. The findings indicated that the survivor cohort had a significantly higher (p < 0.05) concentration of alanine transaminase, aspartate transaminase, bilirubin, creatinine and creatine kinase than the household relative cohort. Besides, the level of alkaline phosphatase, urea, and triglyceride was not significantly different (all p > 0.05) in both cohorts. Nonetheless, gamma-glutamyl transferase and total cholesterol levels were significantly higher in the household relative cohort than in the survivor cohort (p<0.05).
Moreover, a Spearman correlation was conducted to examine the relationship between haematological, immunological and biochemical biomarkers with the Ebola survivor duration at the ETU (Table 3).
 |
Table 3 Biomarkers’ Correlation with Survivor Duration at ETU and Presence or Absence of Some Post-Acute Sequelae |
Overall, weak non-significant correlations were observed for several biomarkers and the duration at ETU. However, we observed a weak positive significant correlation between the duration at ETU and the mean corpuscular haemoglobin (Spearman r = 0.23; N=83; p= 0.037) as well as with alkaline phosphatase (Spearman r = 0.25; N=83; p= 0.023); while a weak negative significant correlation was observed between the duration at ETU and lymphocyte (Spearman r = - 0.24; N=83; p= 0.029) same as with the platelet level (Spearman r = - 0.29; N=83; p= 0.008). All results were represented in a colour scatterplot matrix, where statistically supported associations (p<0.05) between duration at ETU and various biomarkers were highlighted (Figures 4–6).
Interestingly, there was a significant (p < 0.05) association between haematological (Hb, PCV, MCV, MCH), biochemical (ALT, CREA, C-kinase, T-cholesterol, triglycerides), and immune (lymphocytes%) parameters and the risk of developing severe complications (Table 3).
A simple linear regression model was calculated to predict the impact of the admission period at the Ebola Treatment Unit on survivors’ haematological, immunological and biochemical biomarkers taken individually. The overall regression analysis demonstrated no significant prediction between the duration at the ETU and various biomarkers of survivors. However, a significant negative regression model was found between the duration at ETU and the lymphocyte percentage of survivors (F (1, 81) = 4.29, p=0.0415), with an R2 of 0.05 (Figure 7).
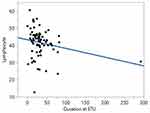 |
Figure 7 Linear regression model of the duration at the ETU and lymphocytes % of survivors. Abbreviation: ETU, Ebola Treatment Unit. |
Considering the lymphocyte percentage as y and the duration at ETU as x, the regression equation will be equal to y= a + bx; thus,
Discussion
With continuous medical complications reported by survivors, essential clinical care should be provided continuously following the WHO reference document on clinical care for survivors of EVD.4 However, good clinical decision-making requires appropriate medical laboratory investigations that are often expensive in sub-Saharan Africa, which hosts more than 90% of EVD survivors. Therefore, identifying key laboratory biomarkers with a clinical signature could rapidly guide risk stratification and monitoring of survivors at an enhanced level of developing severe complications. Unfortunately, little is known regarding the long-term effects of EVD on the haematological, immunological, and biochemical profile of patients after discharge from ETU, even though some preliminary studies provided relevant insights during acute infection.7
The study showed that post-Ebola syndrome remains a severe health concern affecting survivors with the most reported sequelae being arthralgia, myalgia, and persistent headache. These findings concur with previous studies reporting clinical sequelae of survivors after release from ETU.3,8
A comparison of haematological biomarkers showed that the survivor cohort had higher Hb, MCV and platelet levels, but mostly still within normal ranges with a positive correlation observed between the duration at ETU and the mean corpuscular haemoglobin level. In contrast, this correlation was negative with platelet count. These findings align with related studies on viral haemorrhagic fever diseases.9
We also observed a significant difference among monocytes and CD4 percentage between survivors and their household relatives, similar to earlier studies.10 Even though there was no difference in lymphocytes between both cohorts, regression analysis indicated that duration at ETU during acute infection was negatively associated with lymphocytes percentage, with the equation indicating 5% lymphocytes decrease per day spent at ETU. This conclusion agrees with studies indicating that EVD is associated with pronounced lymphopenia that highly correlates with fatalities.10
In the present study, many biochemical biomarkers showed a significant difference between survivors and their household relatives, including AST, ALT, ALP, BIL, CREA, C-kinase, and triglycerides, with a correlation observed between duration at ETU and ALP. These abnormalities in these biomarkers suggested that EVD may damage hepatic, myocardial, renal, and other survivor’s organs. These findings are consistent with earlier research describing complications associated with EVD.7 However, Clark et al did not observe any clinically relevant abnormalities in biochemistry biomarkers among 70 EVD survivors 3 years after discharge from ETU.11
Generally, there were statistically significant differences between the two groups for several biomarkers. However, that is not always the same as a clinically significant difference. Some biomarkers in both groups were still within normal ranges; therefore, individual biomarkers alone might be difficult to predict for clinicians. However, describing a more concrete group of biomarkers from our findings will help typify and identify those most at risk.
Finally, the impact of the haematological, immunological, and biochemical variations described is still unclear regarding the cause and consequence of the persistence of sequelae and the vulnerability of survivors to other communicable and non-communicable diseases. In addition, the variations observed may be due to confounding factors such as co-infection with other infectious diseases and underlying unreported medical status. Therefore, further research is needed on a significant survivor population across multiple sites to reach a specific conclusion.
Limitations
This retrospective cross-sectional cohort study is limited to one EVD survivor’s clinic and does not provide cause-and-effect relationships. These studied biomarkers require further investigation. By studying many survivors, we will surely gain more precise information about the long-term effects of the Ebola virus infection on haematological, immunological, and biochemical parameters in survivors and their potential clinical applications, which will further help improve their care.
Conclusion
This study provides new insights into the vital role of hematologic, immunological and biochemical laboratory biomarkers in managing clinical features in survivors of Ebola virus disease. We recommend clinicians closely monitor Hb, PCV, MCV, MCH, ALT, CREA, C-kinase, T-cholesterol, triglycerides and lymphocytes as clinically relevant biomarkers to identify survivors at higher risk of developing severe post-acute syndrome upon discharge from Ebola treatment unit including headache, abdominal pain, chest pain, ocular complication, arthralgia, hearing difficulty and erectile dysfunction, which can impact health-related quality of life among Ebola survivors. However, these biomarkers need further evaluation in a more significant number of survivors.
Data Sharing Statement
The authors declare that all data supporting the findings of this study are available within the article. The datasets generated, used, and analyzed during this study are available from the corresponding author Dr Raoul Emeric Guetiya Wadoum upon reasonable request.
Acknowledgments
We want to extend our thanks and appreciation to the Ebola survivors and their household relatives for their participation in this study. We also want to thank the Ministry of Health and Sanitation, the Ministry of Social Welfare, Gender and Children’s Affairs, and the Sierra Leone Association of Ebola Survivors for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. World Health Organization (WHO). Ebola data and statistics: situation summary data; 2016. Available from: http://apps.who.int/gho/data/view.ebola-sitrep.ebola-summary-20160511?lang=en.
2. Tiffany A, Vetter P, Mattia J, et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis. 2016;62(11):1360–1366. doi:10.1093/cid/ciw158
3. Guetiya Wadoum RE, Samin A, Mafopa NG, et al. Mobile health clinic for the medical management of clinical sequelae experienced by survivors of the 2013–2016 Ebola virus disease outbreak in Sierra Leone, West Africa. Eur J Clin Microbiol Infect Dis. 2017;36(11):2193–2200. PMID: 28695354. doi:10.1007/s10096-017-3045-1
4. WHO. Clinical care for survivors of Ebola virus disease: interim guidance. Available from: http://apps.who.int/iris/bitstream/10665/204235/1/WHO_EVD_OHE_PED_16.1_eng.pdf?ua=1.
5. Lambe T, Rampling T, Samuel D, et al. Detection of vaccine-induced antibodies to Ebola virus in oral fluid. Open Forum Infect Dis. 2016;3(1):ofw031. doi:10.1093/ofid/ofw031
6. El-Nagel M, Heuck C, Appel W, Vandepitte J, Engbaek K, Gibbs W. Basics of quality assurance for intermediate and peripheral laboratories. WHO Regional Office for the Eastern Mediterranean; 1992. Available from: https://apps.who.int/iris/bitstream/handle/10665/120053/dsa90.pdf.
7. Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015;15(11):1292–1299. doi:10.1016/S1473-3099(15)00144-9
8. Scott JT, Sesay FR, Massaquoi TA, Idriss BR, Sahr F, Semple MG. Post-Ebola syndrome, Sierra Leone. Emerg Infect Dis. 2016;22(4):641–646. PMID: 26983037; PMCID: PMC4806950. doi:10.3201/eid2204.151302
9. Clark D, Jahrling P, Lawler J. Clinical management of filovirus-infected patients. Viruses. 2012;4(9):1668–1686. doi:10.3390/v4091668
10. Qureshi A. Clinical manifestations and laboratory diagnosis of Ebola virus infection. Ebola Virus Dis. 2016;117–138. doi:10.1016/B978-0-12-804230-4.00009-1
11. Clark DV, Kibuuka H, Millard M, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis. 2015;15(8):905–912. doi:10.1016/S1473-3099(15)70152-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.