Back to Journals » Cancer Management and Research » Volume 12
Identification of Cholangiocarcinoma Associated with Hepatolithiasis via the Combination of miRNA and Ultrasound
Authors Jiang W, Deng X, Zhu T, Wei Y, Lei Z, Guo M, Yang J
Received 11 December 2019
Accepted for publication 2 March 2020
Published 12 March 2020 Volume 2020:12 Pages 1845—1853
DOI https://doi.org/10.2147/CMAR.S241870
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Wei Jiang,1 Xiaofei Deng,1 Ting Zhu,1 Yuya Wei,1 Zhen Lei,1 Meimei Guo,2 Jiong Yang2
1Department of Ultrasound, Huazhong University of Science and Technology Union Shenzhen Hospital, Shenzhen 518052, People’s Republic of China; 2Department of Gastroenterology, Huazhong University of Science and Technology Union Shenzhen Hospital, Shenzhen 518052, People’s Republic of China
Correspondence: Wei Jiang
Department of Ultrasound, Shenzhen Nanshan District People’s Hospital, No. 89, Taoyuan Road, Nanshan District, Shenzhen 518052, People’s Republic of China
Tel +86 13026628099
Email [email protected]
Background: Identification of cholangiocarcinoma (CCA) associated with hepatolithiasis (HL) is difficult. There is no effective method to discriminate CCA associated with HL (HL-CCA) from HL currently.
Objective: To explore the value of clinical data, ultrasonic characteristics and miRNA expression level in the identification of HL-CCA.
Methods: Thirty-one patients with HL-CCA in Huazhong University of Science and Technology Union Shenzhen Hospital were enrolled in the observation group, while 40 patients with HL alone were included in the control group. The clinical data, ultrasonic characteristics, and miRNA expression level of the two groups were recorded and analyzed to explore the potential indicators for the identification of HL-CCA.
Results: The accuracy of ultrasound in the diagnosis of HL-CCA was low (54.84%). Multivariate logistic regression analysis showed that liver abscess (P=0.021), indistinct border demarcation (P=0.015), non-homogenous echotexture (P=0.019), missed portal vein around lesion (P=0.032), miRNA-21 (P=0.018) and miRNA-221 (P=0.009) were the potential indicators for the identification of HL-CCA. The combined diagnosis based on logistic regression contained liver abscess, border demarcation, echotexture, portal vein around lesion, miRNA-21 and miRNA-221. The results showed that the accuracy of combined diagnosis identifying HL-CCA was the most accurate (AUC=0.911), which was significantly greater than the AUC of miRNA-21 or miRNA-221 individually (P< 0.05), with a sensitivity and specificity of 77.42% and 97.50%, respectively.
Conclusion: Patients with HL-CCA show high incidence of hepatic abscess and elevated miRNA-21 and miRNA-221 expression level. The ultrasonic features are more likely to show indistinct border demarcation, non-homogenous echotexture, and missed portal vein around lesion. The combined diagnosis is more accurate in the identification of HL-CCA.
Keywords: cholangiocarcinoma, hepatolithiasis, microRNA, differential diagnosis, combined diagnosis, ultrasound
Cholangiocarcinoma (CCA) associated with hepatolithiasis (HL) is a malignant tumor originating from the bile duct.1,2 The early symptoms of CCA associated with HL (HL-CCA) are not obvious, and they are easily confused with biliary inflammation caused by gallstones.3,4 Accurate diagnosis of HL-CCA is challenging and it is usually at an advanced stage when diagnosed, which indicates a worse prognosis and treatment outcomes. In addition, studies have reported an increased incidence of concurrent CCA in patients with HL.5–7 Hence early identification of HL-CCA is of great importance. Detection of HL-CCA is dependent on imaging modalities, such as ultrasound, CT, and MRI. However, it is difficult to differentiate CCA from fibrosis in HL since prolonged affected liver segments often become fibrotic and scarred. Ultrasound is the primary imaging modality for hepatobiliary diseases. But in the cases of HL-CCA, clinicians tend to rely on the characteristics of HL to attribute infiltration features to inflammation of the bile duct wall. Even if the tumor is developed at the middle and advanced stages, it is difficult to distinguish concomitant HL-CCA and HL only by ultrasound.8 Tumor markers such as CA19-9, CEA are commonly used indicators for the identification of benign and malignant liver tumors, but their roles in the identification of HL-CCA is controversial.9,10 To date, there is no effective method to differentiate concomitant CCA in HL. In recent researches, the changes in miRNA may be associated with the development of tumors, and their association with CCA has gradually been confirmed. Correa-Gallego et al11 analyzed the miRNA by deep sequencing technology and found that the expression levels of miRNA-21 and miRNA-221 in patients with CCA were significantly higher than in normal people. However, the role of microRNA in the identification of HL-CCA remains unclear. This study analyzed the clinical data, ultrasonic features and miRNA expression level in the patients with HL-CCA, and explored the value of different indicators in the differential diagnosis of HL alone and HL-CCA, aiming to find an appropriate identification method to further help the management of HL-CCA.
Materials and Methods
Participants
Seventy-one patients with HL who were admitted to Huazhong University of Science and Technology Union Shenzhen Hospital from January 2010 to June 2018 were recruited. Inclusion criteria were as follows: (1) Patients underwent surgical treatment and the pathological results were completely preserved. (2) Complete physical examinations, blood routines, tumor markers, and ultrasound examinations were performed and the results were preserved within 1 week before surgery. Patients combined with other malignant tumors or severe cardiovascular and cerebrovascular diseases were excluded in this study. According to the postoperative pathology, patients were divided into the observation group if HL-CCA was confirmed, while they were divided into the control group if HL alone was confirmed. The study was approved by the ethics committee of Huazhong University of Science and Technology Union Shenzhen Hospital (NO. 103004). All patients included in the study had a detailed understanding of the research content and signed informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Research Methods
Data Collection
Patients’ clinical data were collected in the study, including age, gender, family history of malignancy, liver or back pain, liver fibrosis, liver abscess, cirrhosis, portal hypertension, cholangitis, secondary bile duct stricture, and history of hepatitis B. The serological indicators, including serum alkaline phosphatase (ALP), alanine aminotransferase (AST), aspartate aminotransferase (ALT), glutamyl transpeptidase (GGT), total bilirubin (TBIL), carcinoembryonic antigen (CEA), carbohydrate antigen (CA19-9) were detected by Hitachi automatic biochemical analyzer 7060 (Hitachi, Yokohama, Japan).
Ultrasound Examination
In the fasting state, the patient was placed in the supine position and exposed to the upper abdomen. Ultrasound was performed using Resona 7 ultrasound diagnostic system (Mindary, Shenzhen, China) and the Acuson S2000 ultrasound system (Siemens, Erlangen, Germany). The ultrasonic characteristics of the lesion area were recorded in detail, including diameter, location, shape, border demarcation, echo density, echotexture and posterior attenuation. Meanwhile, the situation of intrahepatic bile duct dilatation and portal vein around lesion were observed.
miRNA Detection
Before surgery, 5 mL-fasting venous blood sample of each patient was collected. The sample was centrifuged at 4°C and temporarily stored in a refrigerator at −80°C. RNA was extracted from plasma samples and reversely transcripted to cDNA. The reverse transcription results were detected using a 7300-type real-time PCR instrument (Applied Biosystem, USA), and the relative concentrations of miRNA-21, miRNA-34c, miRNA-200b, and miRNA-221 were recorded.
Statistical Methods
All data were processed using Statistical Product and Service Solutions (Chicago, IL, USA) software (version 22.0) and plotted by R package version 3.6.2 and MedCalc version 12 (MedCalc Software, Ostend, Belgium). The categorical variables were expressed in number (percentage), and the chi-square test and Fisher’s exact test were used for comparison. The numerical data conforming to the normal distribution were expressed as, and independent sample t-tests were used for comparison. The numerical data that did not meet the normal distribution were expressed as median (interquartile range), and Mann–Whitney U-tests were used for comparison. The association of potential variables with the risk of HL-CCA was performed using multivariate logistic regression analysis. ROC curves were established to evaluate the accuracy of potential indicators for identifying HL-CCA. Statistical significance was defined as 2-tailed P<0.05 for all tests.
Results
Ultrasonic and Pathological Features of HL-CCA
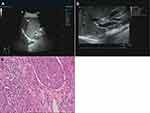
In this study, 40 patients with HL (Control Group) were accurately diagnosed by ultrasound. The ultrasound images of HL were mainly characterized by fine-like, spot-like round or clump-like hyperechoic mass with irregular shape in the liver. The gallstones were mostly located in the left lobe of the liver. The hyperechoic mass caused by gallstones in HL were distributed along the intrahepatic bile duct. It often merged with a dendritic expansion of intrahepatic bile duct, and was located in the dilated bile duct. The diagnostic accuracy of ultrasound for HL-CCA was low. In this study, only 17 of 31 patients with HL-CCA (Observation group) were correctly diagnosed (54.84%). The ultrasonic features of HL-CCA were mainly characterized by irregular clump-like hyperechoic mass in the liver, and the border demarcation between the masses and the bile duct wall were indistinct. The mass was displayed in isoechogenicity or mixed echogenicity, and it often surrounded the gallstone which showed a hyperechoic mass. At the bile duct truncation, the mass often protruded into the lumen. The portal vein around lesion of HL-CCA were hazy or missed. Pathological examination showed that there were 15 cases of papillary carcinoma, 14 cases of tubular adenocarcinoma, 2 cases of mucinous adenocarcinoma (Figure 1).
Comparison of the Clinical Data Between the Observation Group and Control Group
Compared with the clinical data between the observation group and control group, the proportions of liver abscess and cirrhosis in the observation group were close to 20%, which were greater than those in the control group (P<0.05). The remaining clinical data were similar in the two groups (P>0.05, Table 1).
 |
Table 1 Comparison of Clinical Data Between the Observation Group and Control Group |

Comparison of the Ultrasonic Characteristics Between the Observation Group and Control Group
Compared with the ultrasonic characteristics between the observation group and control group, the proportions of indistinct border demarcation, non-homogenous echo texture, missed or hazy portal vein around lesion in the observation group were higher than those in the control group (P<0.05). The remaining ultrasonic characteristics were similar between the two groups (P>0.05, Table 2).
 |
Table 2 Comparison of Ultrasound Characteristics Between the Observation Group and Control Group |
Comparison of the Laboratory Indicators Between the Observation Group and Control Group
The expression of miRNA-21 and miRNA-221 in the observation group was higher than those in the control group (P<0.05), while the remaining indicators were similar in the two groups (P>0.05, Table 3).
 |
Table 3 Comparison of the Laboratory Indicators Between the Observation Group and Control Group |
Associations of Differentiated Indicators with the Risk of HL-CCA
Multivariate logistic regression analysis was performed to further analyze the differentiated indicators between the two groups to explore the potential identification value for HL-CCA. It revealed that except the cirrhosis, the liver abscess (P=0.021), indistinct border demarcation (P=0.015), non-homogenous echotexture (P=0.019), missed portal vein around lesion (P=0.032), miRNA-21 (P=0.018) and miRNA-221 (P=0.009) were the potential indicators for the identification of HL-CCA (Figure 2).
 |
Figure 2 Forest plot of the logistic regression analysis for the influence of potential variables on HL-CCA. |
Accuracy Analysis of the Diagnosis for HL-CCA
The accuracy of the potential indicators identifying HL-CCA independently was not high. The specificity of liver abscess was high (97.50%) but the sensitivity was very low (22.58%), indicating that it would cause a large number of missed diagnosis. The specificity of the border demarcation, echotexture and the sensitivity of the portal vein around lesion were less than 60%, suggesting that the border demarcation, echotexture and portal vein around lesion were not suitable for the identification of HL-CCA independently. The AUC of miRNA-221 identifying HL-CCA was below 0.8 (greater than miRNA-21), suggesting that it was also not suitable for identification. These results revealed that the identification of HL-CCA was very difficult, and the individual identification of potential indicators could not achieve an ideal accuracy. This study combined liver abscess, border demarcation, echotexture, portal vein around lesion, miRNA-21 and miRNA-221 based on logistic regression model in order to improve the identification accuracy. It found that the accuracy of the combined diagnosis was the highest (AUC=0.911), which was significantly greater than the AUC of miRNA-21 and miRNA-221 (P<0.05). The best diagnostic point was 0.48, and the sensitivity and specificity was 77.42% and 97.50%, respectively (Table 4 and Figure 3).
 |
Table 4 ROC Analysis of Potential Indicators for Differential Diagnosis of HL-CCA |
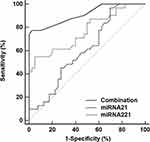 |
Figure 3 ROC analysis of miRNA-21, miRNA-221 and their combination in the differential diagnosis of HL-CCA. |
Discussion
The incidence of CCA was about 5–13%.12 HL-CCA can be detected at any stage, including the evaluation, treatment, or follow up of HL. HL is a known risk factor for CCA, which has been well documented. In cases of HL-CCA, there are no specific symptoms other than the clinical manifestation of HL. Hence, the diagnosed accuracy of HL-CCA is low.13 Currently, early diagnosis of HL-CCA in clinical setting is still challenging even though there have been advances in diagnostic modalities and various efforts to identify it in early stages.14
It has been known that miRNAs are involved in almost all life activities of cells including cell proliferation, differentiation and apoptosis.15,16 Recent studies have shown that the miRNA expression level may be related to cancers.17,18 Therefore miRNAs are very promising diagnostic, prognostic biomarkers and therapeutic targets.19 Profiling of miRNA has been explored as an invasive procedure for the detection of cancer. Wang et al20 found that the miRNA profile differentiated patients with pancreatic adenocarcinoma from healthy people. Moreover, the combination of miRNAs and CA19-9 was more effective in discriminating carcinoma.21 Because miRNAs are involved in the tumorigenesis processes, the up-regulation of onco-miRNAs or the down-regulation of tumor suppressor miRNA can be utilized as prognostic indicators. Studies have revealed a significant correlation between elevated miRNA expression and OS.22 The up-regulation of onco-miRNA leads to anti-apoptosis, proliferation and etastasization while the downregulation of tumor suppressor miRNA leads to cancer spreading, which may lead to therapeutic possibilities.
However, despite this interesting perspective, critical obstacles that often involve the delivery of miRNA-targeting agents must still be overcome before transition to clinical applications. There are numerous preclinical data but few clinical trials on the use of miRNAs nowadays.23 This study compared the clinical data, ultrasonic features, and miRNAs expression level of patients with HL alone and HL-CCA in order to find an accurate method for identifying HL-CCA.
Clinical and Laboratory Indicators of HL and HL-CCA
The present study revealed that patients with HL-CCA had differences in the incidence of liver abscess and cirrhosis compared to the patients with HL alone. Multivariate regression analysis revealed that liver abscess was an independent risk factor for HL-CCA. It may be because that the gallstones block the bile ducts and cause cholestasis. The local inflammation promotes necrosis and liquefaction of the liver lobes to form liver abscess.24 When associated with CCA, the incidence and severity of bile duct obstruction are worsened. Compared with HL alone, the possibility of liver abscess in HL-CCA is increased. However, not all patients with HL-CCA accompanied liver abscesses, and the ROC analysis of liver abscess in identifying HL-CCA indicated a low sensitivity.
Studies have analyzed the laboratory indicators of patients with CCA and found that hepatobiliary injury caused by CCA can cause the rise of laboratory indicators such as ALP, GGT and TBIL. However these indicators are also increased in HL.25 In this study, the TBIL, ALP, and GGT in patients with HL-CCA were only slightly higher than in patients with HL (P>0.05). It is worth noting that we did not find any difference in serum CEA and CA19-9 between the two groups. It maybe indicated that the laboratory indicators and the common tumor markers such as CEA and CA19-9 were limited in the differentiation of HL-CCA and HL alone.
Comparison of Ultrasonic Characteristics Between HL and HL-CCA
In this study, compared with HL alone, the ultrasonic features of patients with HL-CCA showed an indistinct border demarcation, non-homogenous echotexture, and a high proportion of missed portal vein around lesion. These ultrasonic features are independent risk factors for HL-CCA. However, the ROC analysis revealed that the sensitivity and specificity of the border demarcation, echotexture and portal vein around lesion are not high, suggesting that it is difficult to diagnose HL-CCA merely rely on ultrasonic features.
Identification of CCA by miRNAs
It is well known that miRNAs can participate in cell proliferation, differentiation, etc., and directly regulate the expression of protooncogene and tumor suppressor gene. miRNA-21 has been shown to be overexpressed in many types of tumors (lung, stomach, liver, breast, etc.).26–29 It has become a tumor marker for tumor staging, treatment and prognosis. The study by Huang et al30 has found that miRNA-21 can enhance the invasion and metastasis of CCA cells, suggesting that it may play an important role in the invasion and metastasis of CCA. Volinia et al31 reported that miRNA-21 is significantly overexpressed in human CCA cells and plays the role of an oncogene. Meng et al32 found that miRNA21 was overexpressed in CCA even at an early stage. Inhibition of miRNA-21 can reduce the proliferation and invasion of CCA cells.
miRNA-221 has also been widely reported in various tumors. It has been found that miRNA-221 detected in tumor tissue or serum may be used as a diagnostic marker for malignant tumors and used to predict tumor aggressiveness and prognosis. Hence, the changes of miR-221 may indicate the presence of malignant tumors.33,34 According to the study of Correa-Gallego et al,12 miR-221 may be a potential marker for the diagnosis of CCA. And miRNA-221 silencing can inhibit the increase of tumor value or promote its apoptosis, which provides the basis for the study of miRNA-221 as a therapeutic target.
The results of the present study revealed that the levels of miR-21 and miR-221 in patients with HL-CCA were significantly higher than those in patients with HL alone. Both of them could be used as independent risk factors for HL-CCA. However, the ROC analyses revealed that when miR-21 or miR-221 was used as a diagnostic indicator individually. Their specificity or sensitivity was limited to identify HL-CCA.
Combined Diagnosis Can Improve the Diagnostic Efficacy of HL-CCA
ROC analysis of the risk factors for HL-CCA revealed that the clinical symptoms, ultrasonic features, and miRNA expression level had different degrees of deficiencies in diagnosing HL-CCA. In this study, a logistic regression model was used to establish a combined diagnosis model. The accuracy of the combined diagnosis was significantly increased (AUC=0.911), which was significantly higher than the AUCs of each indicator. It indicated that when combined with liver abscess, miR-21 & miR-221 levels and ultrasonic features, the diagnosis of HL-CCA is the most accurate. The best diagnostic point for the combined diagnosis was 0.48, with a sensitivity and specificity of 77.42% and 97.50%, respectively. It indicated that the SPSS software could used to establish the combination model to determine the probability of patients with HL-CCA after recording the data of liver abscess, miR-21, miR221 levels and ultrasonic features. The patient with HL is more likely to develop CCA if the probability is >0.480.
Limitation and Prospective
Although many target genes directly regulated by miRNA-21 and miRNA-221 have now been predicted, few have been confirmed in clinical setting. The application value of miRNA-21 and miRNA-221 in the diagnosis, treatment and prognosis of HL-CCA needs to be further explored. In addition, there are some differences in the results of different methods for detecting miRNA. This study plan to establish a CCA database based on the data from multi-center hospitals to further explore the combined model in clinical setting.
Conclusion
This study compared the clinical data, ultrasonic characteristics and miRNA expression level in patients with HL alone and HL-CCA. Patients with HL-CCA have high incidence of hepatic abscess and elevated miR-21 and miR-221 expression levels. The ultrasonic features are more likely to show indistinct border demarcation, non-homogenous echotexture, and missed portal vein around lesion. The combination of these indicators can more accurately discriminate HL-CCA from HL.
Acknowledgment
This study was supported by Nanshan District Science and Technology Plan Project (NO. 2016038).
Funding
Supported by Nanshan District Science and Technology Plan Project (NO. 2016038).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sempoux C, Jibara G, Ward SC, et al. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis. 2011;31(1):49–60. doi:10.1055/s-0031-1272839
2. Tao LY, He XD, Qu Q, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int. 2010;30(2):215–221.
3. Su CH, Shyr YM, Lui WY, et al. Hepatolithiasis associated with cholangiocarcinoma. Br J Surg. 1997;84(7):969–973. doi:10.1002/bjs.1800840717
4. Xiao J, Zhu J, Liu Z, et al. Role of surgical treatment for hepatolithiasis-associated intrahepatic cholangiocarcinoma: a retrospective study in a single institution. J Cancer Res Ther. 2017;13(5):756–760. doi:10.4103/jcrt.JCRT_356_17
5. Sheen-Chen SM, Chou FF, Eng HL. Intrahepatic cholangiocarcinoma in hepatolithiasis: a frequently overlooked disease. J Surg Oncol. 1991;47(2):131–135. doi:10.1002/jso.2930470213
6. Zhu Y, Zhu Y, Cai F, et al. Prognostic risk factors associated with recurrence and metastasis after radical resection in patients with hepatolithiasis complicated by intrahepatic cholangiocarcinoma. Cell Biochem Biophys. 2015;73(2):455–460. doi:10.1007/s12013-015-0665-x
7. Zhu QD, Zhou MT, Zhou QQ, et al. Diagnosis and surgical treatment of intrahepatic hepatolithiasis combined with cholangiocarcinoma. World J Surg. 2014;38(8):2097–2104. doi:10.1007/s00268-014-2476-4
8. Ye J, Xie X, Lin Y, et al. Imaging features of combined hepatocellular-cholangiocarcinoma on contrast-enhanced ultrasound: correlation with clinicopathological findings. Clin Radiol. 2018;73(3):237–243. doi:10.1016/j.crad.2017.10.003
9. Ince AT, Yildiz K, Baysal B, et al. Roles of serum and biliary CEA, CA19-9, VEGFR3, and TAC in differentiating between malignant and benign biliary obstructions. Turk J Gastroenterol. 2014;25(2):162–169. doi:10.5152/tjg
10. Tang X, Zhang J, Chen Y, et al. Correlation between clinicopathological features and CA19-9/CEA in patients with extrahepatic cholangiocarcinoma. Zhonghua Zhong Liu Za Zhi. 2014;36(9):662–666.
11. Correa-Gallego C, Maddalo D, Doussot A, et al. Circulating plasma levels of MicroRNA-21 and MicroRNA-221 are potential diagnostic markers for primary intrahepatic cholangiocarcinoma. PLoS One. 2016;11(9):e0163699. doi:10.1371/journal.pone.0163699
12. Khan SA, Toledano MB, Taylor-robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford). 2008;10(2):77–82. doi:10.1080/13651820801992641
13. Kim HJ, Kim JS, Joo MK, et al. Hepatolithiasis and intrahepatic cholangiocarcinoma: a review. World J Gastroenterol. 2015;21(48):13418–13431. doi:10.3748/wjg.v21.i48.13418
14. Chinchilla-Lopez P, Aguilar-Olivos NE, Garcia-Gomez J, et al. Prevalence, risk factors, and survival of patients with intrahepatic cholangiocarcinoma. Ann Hepatol. 2017;16(4):565–568. doi:10.5604/01.3001.0010.0293
15. Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell. 2009;136(4):586–591. doi:10.1016/j.cell.2009.02.005
16. Tuna M, Machado AS, Calin GA. Genetic and epigenetic alterations of microRNAs and implications for human cancers and other diseases. Genes Chromosomes Cancer. 2016;55(3):193–214. doi:10.1002/gcc.v55.3
17. Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi:10.1038/cr.2008.282
18. Clancy C, Joyce MR, Kerin MJ. The use of circulating microRNAs as diagnostic biomarkers in colorectal cancer. Cancer Biomark. 2015;15(2):103–113. doi:10.3233/CBM-140456
19. Yahya SM, Elsayed GH. A summary for molecular regulations of miRNAs in breast cancer. Clin Biochem. 2015;48(6):388–396. doi:10.1016/j.clinbiochem.2014.12.013
20. Wang J, Chen J, Chang P, et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila). 2009;2(9):807–813. doi:10.1158/1940-6207.CAPR-09-0094
21. Liu J, Gao J, Du Y, et al. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int J Cancer. 2012;131(3):683–691. doi:10.1002/ijc.v131.3
22. Greither T, Grochola LF, Udelnow A, et al. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer. 2010;126(1):73–80. doi:10.1002/ijc.24687
23. Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3(120). doi:10.3389/fgene.2012.00120
24. Shah V, Arora A, Tyagi P, et al. Intrahepatic cholangiocarcinoma masquerading as liver abscess. J Clin Exp Hepatol. 2015;5(1):89–92. doi:10.1016/j.jceh.2014.12.006
25. Miwa M, You G, Tanaka H, et al. Analysis of new biomarkers for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21(6):397–398. doi:10.1002/jhbp.2014.21.issue-6
26. Komatsu S, Ichikawa D, Tsujiura M, et al. Prognostic impact of circulating miR-21 in the plasma of patients with gastric carcinoma. Anticancer Res. 2013;33(1):271–276.
27. Zheng J, Xue H, Wang T, et al. miR-21 downregulates the tumor suppressor P12 CDK2AP1 and stimulates cell proliferation and invasion. J Cell Biochem. 2011;112(3):872–880. doi:10.1002/jcb.22995
28. Asaga S, Kuo C, Nguyen T, et al. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57(1):84–91. doi:10.1373/clinchem.2010.151845
29. Tsujiura M, Ichikawa D, Komatsu S, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102(7):1174–1179. doi:10.1038/sj.bjc.6605608
30. Huang Q, Liu L, Liu CH, et al. MicroRNA-21 regulates the invasion and metastasis in cholangiocarcinoma and may be a potential biomarker for cancer prognosis. Asian Pac J Cancer Prev. 2013;14(2):829–834. doi:10.7314/APJCP.2013.14.2.829
31. Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103(7):2257–2261. doi:10.1073/pnas.0510565103
32. Meng F, Henson R, Lang M, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130(7):2113–2129. doi:10.1053/j.gastro.2006.02.057
33. Karakatsanis A, Papaconstantinou I, Gazouli M, et al. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52(4):297–303. doi:10.1002/mc.v52.4
34. Rong M, Chen G, Dang Y. Increased miR-221 expression in hepatocellular carcinoma tissues and its role in enhancing cell growth and inhibiting apoptosis in vitro. BMC Cancer. 2013;13:21. doi:10.1186/1471-2407-13-21
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.