Back to Journals » Clinical Epidemiology » Volume 14
Identification of Childhood-Onset Inflammatory Bowel Disease in Swedish Healthcare Registers: A Validation Study
Authors Mouratidou N , Malmborg P , Järås J, Sigurdsson V, Sandström O, Fagerberg UL, Bröms G, Ludvigsson JF , Olén O
Received 12 January 2022
Accepted for publication 5 April 2022
Published 29 April 2022 Volume 2022:14 Pages 591—600
DOI https://doi.org/10.2147/CLEP.S358031
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eyal Cohen
Natalia Mouratidou,1,2 Petter Malmborg,2,3 Jacob Järås,2 Vignir Sigurdsson,4 Olof Sandström,5 Ulrika L Fagerberg,6,7 Gabriella Bröms,2,8 Jonas F Ludvigsson,9,10 Ola Olén2,3 On behalf of the SWIBREG Study Group
1Astrid Lindgren Children’s Hospital, Karolinska University Hospital, Stockholm, Sweden; 2Division of Clinical Epidemiology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden; 3Sachs’ Children and Youth Hospital, Södersjukhuset, Stockholm, Sweden; 4Department of Pediatrics, Institute of Clinical Sciences, The Sahlgrenska Academy at University of Gothenburg and Queen Silvia’s Children’s Hospital, Gothenburg, Sweden; 5Department of Clinical Sciences, Pediatrics, Umeå University, Umeå, Sweden; 6Department of Women´s and Children´s Health, Karolinska Institutet, Stockholm, Sweden; 7Department of Pediatrics, Centre for Clinical Research, Västmanland Hospital, Västerås, Uppsala University, Västerås, Sweden; 8Gastroenterology, Danderyds Hospital, Stockholm, Sweden; 9Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Solna, Sweden; 10Department of Pediatrics, Örebro University Hospital, Örebro, Sweden
Correspondence: Natalia Mouratidou, Astrid Lindgren Children’s Hospital, Karolinska University Hospital, Stockholm, Sweden, Tel +46708108338, Fax +46812393251, Email [email protected]
Purpose: The Swedish National Patient Register (NPR) is often used in observational studies of childhood-onset inflammatory bowel disease (IBD) (< 18 years of age) and its subtypes, but the validity of previously used register-based algorithms for capturing childhood-onset IBD has never been examined.
Methods: We identified a random sample of 233 individuals with at least two first ever diagnostic listings of IBD in the NPR between 2002 and 2014. We calculated the test characteristics for different register-based definitions of IBD and its subtypes using the Copenhagen criteria and the revised Porto criteria as gold standard, both based on medical chart review. We made assumptions of the occurrence of undiagnosed IBD in the general child population based on available literature.
Results: Out of 233 individuals with at least two diagnostic listings of IBD, 216 had true IBD, resulting in a positive predictive value (PPV) = 93% (95% confidence interval (CI) 89– 96), sensitivity = 88% (95% CI 83– 92), specificity = 100% (95% CI 100– 100), and negative predictive value (NPV) = 100% (95% CI 100– 100). The PPV for the NPR-based definitions of IBD subtypes at time of first IBD diagnosis and at end of follow-up were 78% (95% CI 69– 86) and 88% (95% CI 80– 94), respectively, for Crohn’s disease and 74% (95% CI 63– 83) and 71% (95% CI 60– 80), respectively, for ulcerative colitis.
Conclusion: The validity of register-based definitions of childhood-onset IBD in the Swedish NPR is high and can be used to identify patients in observational research.
Keywords: health administrative data, Crohn’s disease, ulcerative colitis, disease progression, diagnostic delay
Plain Language Summary
What is new?
Validity of register-based definitions of childhood-onset IBD and its subtypes in the Swedish National Patient Register is excellent and acceptable, respectively.
What is adds to what was known?
Previous Swedish validation studies found high positive predictive value for register-based definitions of inflammatory bowel disease in adults. We can now confirm that the validity of the corresponding definitions in children is similar.
What is the implication?
Swedish registers can be used to perform high-quality observational research regarding pediatric IBD.
Introduction
Inflammatory bowel disease (IBD) is a chronic disease characterized by inflammation in the gastrointestinal tract with diverse clinical presentation and prognosis. Approximately 10% of IBD patients are diagnosed in childhood.1–3 Globally rising rates of childhood-onset IBD have been demonstrated, and the highest incidence rates have been reported from Scandinavia and Canada.3–7
Healthcare registers enable researchers to conduct large population-based observational studies in real-world data (collected prospectively in routine medical practice) for research questions where randomized controlled trials are challenging for practical reasons.8–10 In a recent validation study by Jakobsson et al, the validity of the most commonly used IBD definition in the Swedish National Patient Register (NPR) was found to be high (a positive predictive value [PPV] of 93% (95% CI 87–97)).11 When data from the NPR (≥1 record) were combined with data from the Swedish national quality register for IBD (SWIBREG) (≥1 record), the PPV of any IBD listing in both registers was 99% (95% 97–100) for any IBD. However, this validation study included almost exclusively adult-onset IBD patients.
Algorithms developed in adults are not necessarily optimal for the identification of children with the same disease.12 In the IBD context, it is plausible that the validity of the register-based definition of IBD in NPR differs between children and adults, since childhood-onset IBD patients more often have extensive colitis and more aggressive disease compared to adult-onset IBD patients, fewer differential diagnoses are plausible in childhood and children are generally seen more often by their physician.3,13,14
We therefore applied a commonly used register-based algorithm to identify a random sample of potential childhood-onset IBD patients in the NPR and performed a review of their medical records with the main objective to validate the aforementioned algorithm. Our secondary objective was to assess the validity of register-based definitions of IBD subtypes.
Methods
Study Design
We calculated test characteristics of previously used register-based definitions of IBD and IBD subtypes9,15,16 by comparing them with definitions based on medical charts (all of which are described comprehensively below).
Setting and Data Sources
In Sweden, patients with childhood-onset IBD are diagnosed and treated by pediatric gastroenterologists until the age of 18 years.17 Sweden is a high-income country with publicly funded health care almost exclusively provided by physicians employed by the state or region. In Sweden, a large number of mandatory healthcare registers with close to complete coverage are available for medical research. It is possible to use the personal identity number, assigned to all Swedish residents, to link data from different data sources.18 Detailed information about healthcare registries used in this study can be found in the Supplementary text S1.19–22
Study Population
We checked the NPR for suspected incident cases of childhood-onset IBD (defined by relevant ICD-codes [Table S1A]) between 2002 and 2014 and found 6158 cases with at least one first ever diagnostic listing of IBD (4963 of which had ≥2 diagnostic listings). According to Statistics Sweden, 3,243,845 children without any IBD diagnostic listings resided in Sweden during the study period (Figure 1). The National Board of Health and Welfare provided personal identity numbers for a random sample of individuals with at least one diagnostic listing of IBD. We were able to acquire 288/289 medical records, of which 8 were excluded because of incomplete records. The included 280 individuals represented both inpatients and outpatients as well as regional and university hospitals in Sweden (Karolinska University Hospital and Södersjukhuset, Stockholm, Akademiska sjukhuset, Uppsala, Sahlgrenska University Hospital Göteborg, Umeå University Hospital, Skåne University Hospital in Lund and Malmö, Västmanland Hospital Västerås, Eskilstuna Hospital, and Sundsvall Hospital).
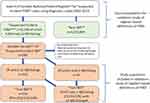 |
Figure 1 Flow chart. Abbreviation: PIBD, Pediatric Inflammatory Bowel Disease. Notes: ^The Swedish National Patient Register includes all hospitalizations and outpatient visits in non-primary care in Sweden. *All children living in Sweden 2002–2014 (according to Statistics Sweden [www.scb.se]) and who had never had a diagnostic listing of IBD. In this group, the prevalence of IBD can be expected to be extremely low. Certainly, below the prevalence of diagnosed PIBD (ie, 0.08% in Sweden [Ludvigsson et al, BMC Gastroenterology 2017]). **“Suspected incident PIBD” defined as at least one (1) first ever diagnostic code of IBD (K50, K51, K52.3) before the 18th birthday, at any point 2002–2014. ^^Random sample of “Suspected incident PIBD”. The sampling is done within the Swedish National Patient Register. ¤Fulfilled the Copenhagen criteria, based on a combination of clinical, laboratory, endoscopic and histologic criteria. #Proportion of non-IBD patients despite having 2 diagnostic codes. |
Register-Based Definitions
Patients needed to have ≥2 first ever diagnostic listings of IBD with the first listing before the 18th birthday recorded in the NPR by a physician on two separate occasions during the study period to fulfil the register-based definition that we aimed to validate.11 Patients were further categorized as ulcerative colitis (UC), Crohn's disease (CD), or IBD-unclassified (IBD-U) either according to the first two diagnostic codes ([incident definition] Table S1A) or according to all information available at end of follow-up ([prevalent definition] Table S1B).16 As supplementary information, we have also described the transition of IBD subtypes according to ICD-10 codes in NPR and based on Porto criteria in medical notes from first diagnosis of IBD to end of follow-up (last visit in pediatric clinic).
Medical Record-Based Definitions
IBD was defined according to the Copenhagen criteria,23,24 and IBD subtypes were categorized according to the revised Porto criteria, using the IBD classes smartphone app (https://apps.apple.com/se/app/ibd-classes/id1248829991) which guides clinicians to make objective diagnostic decisions (Table S3, Figure S1).25 The Copenhagen criteria consider clinical picture, radiology, macroscopic and microscopic findings to establish the IBD diagnosis (Table S2).26 Data collection was performed by four experienced pediatric gastroenterologists (NM, ULF, VS, OS) who entered information needed to fulfill Copenhagen and Porto criteria into a pre-specified REDCap database that was possible to link to the register-based data. They reviewed medical notes including information about radiology, endoscopy, pathology, and surgical notes. The same notes were not reviewed by more than one person, and thus kappa statistic could not be calculated. The readers of the reference standard were not blinded to the subtype classification in the medical records. As supplementary information, both information on diagnostic delay and progression of IBD subtype was extracted from medical records. We defined diagnostic delay as the time between the first symptoms suggestive of IBD (eg, abdominal pain, weight loss, diarrhea as described in medical records) to the date of diagnostic endoscopy.27,28
Statistical Methods
Given the previously reported PPV of 85–95% in the earlier validation study of the same definition in Swedish (predominantly) adult patients11 and thus expecting similar PPV in our study, we needed approximately 230 individuals in order to estimate the PPV with a confidence interval less than ±5%. Since our experience from earlier validation studies is that some 20% of medical records can be very hard or even impossible to acquire (because the original medical record has disappeared [mainly in older calendar periods that had to rely on paper] or because of lack of willingness or time to cooperate at the hospital in question), we identified a random sample of 289 individuals from seven Swedish healthcare regions with at least one diagnostic listing of IBD in the NPR before the 18th birthday between 1 January 2002 and 1 January 2014 using relevant ICD-codes (Table S1A). The National Board of Health and Welfare provided personal identity numbers for sampled individuals.
We estimated the proportion of patients who fulfilled the register-based definitions of IBD and IBD subtypes who also fulfilled the corresponding criteria on medical chart review. We calculated the PPV for definitions of IBD diagnosis and IBD subtype both in NPR and Swibreg. Since we only performed medical chart review of individuals with at least one diagnostic IBD listing (ie, medical records of children without IBD listings were not reviewed), other test characteristics than PPV must be based on assumptions. Earlier literature has reported a prevalence of childhood-onset IBD in Sweden of approximately 0.08%.29 In order not to overestimate test characteristics, we assumed that the prevalence of undiagnosed IBD among individuals with no IBD diagnostic listings was at least not higher than a quarter of the prevalence of diagnosed IBD, thus 0.02%. Please see Tables S4 and S5 for a detailed review of the literature that formed the basis for the assumptions made.
Diagnostic delay was presented as median (IQR). Transition of IBD subtypes from diagnosis to end of follow-up (defined as last visit to a pediatric clinic) was estimated through medical records in all patients with a post-diagnostic endoscopy. If the information about investigations was missing, we regarded them as not having undergone the investigations. Statistical analyses were performed using R statistical software (version 4.0.4, R Foundation for Statistical Computing, Vienna, Austria) and Stata/IC 16.1.
This project was approved by the Stockholm Ethics Review Board (2007/785-31/5, 2014/1288-31/4, 2017/1959-32, and 2018/1865-32). The data accessed complied with relevant data protection and privacy regulations.
Results
Validity of Our Previously Used Register-Based Definition of IBD
We identified 280 patients with ≥1 diagnostic listing of IBD in NPR and whose medical charts contained relevant information (Figure 1, Table 1). Among 280 patients with ≥1 diagnostic listing of IBD in NPR, 47 (17%) had only 1 diagnostic listing, of which none had IBD according to medical records.
 |
Table 1 Validity of Different Register-Based Definitions of Inflammatory Bowel Disease. N (%) if Not Otherwise Stated |
Out of the remaining 233 patients with at least two diagnostic listings of IBD, 216 fulfilled the Copenhagen criteria for IBD on medical chart review, resulting in a PPV of 93% (95% confidence interval [CI] 89–96), sensitivity (88% [95% confidence interval [CI] 59–70]), specificity (100% [95% CI 100–100], and NPV (100% [95% CI 100–100]).
Seventeen (17/233=7%) of the patients with ≥2 diagnostic listings did not have IBD (Table 1). Most of these non-IBD patients had received their ICD codes by mistake or turned out to have functional gastrointestinal disorders (eg, irritable bowel syndrome, functional abdominal pain, constipation, etc.), food allergies or eosinophilic disease.
All patients registered in SWIBREG (n = 58) were confirmed to have IBD, resulting in a PPV of 100% (95% CI 94–100) for this subpopulation (Table 1). A combination of ≥1 IBD listing of IBD with a pathology code for IBD (which has been used in several previous papers including childhood-onset IBD data30,31) resulted in a PPV of 95% (95% CI 91–98).31 Furthermore, combining ≥2 IBD listings and ≥1 pathology code suggestive of IBD increased the PPV to 97% (95% CI 94–99).
Median diagnostic delay was longer in CD than in UC (5 versus 3 months p = 0.03) (Figure S2).
Validity of IBD Subtypes
Compared to the revised Porto criteria (based on medical chart review and the app “IBD classes”), the PPV for the NPR-based “incident” definitions of IBD subtypes at first diagnosis were 78% (95% CI 69–86) for CD, 74% (95% CI 63–83) for UC and 23% for IBD-U (95% CI 10–40) (Table 2). Corresponding figures for subtypes according to Swibreg at start of follow-up were 75% (95% CI 57–89) for CD and 85% for UC (95% CI 62–97). At end of follow-up, the “prevalent” definitions of IBD subtypes resulted in PPVs of 88% (95% CI 80–94) for CD, 71% (95% CI 60–80) for UC and 21% for IBD-U (95% CI 10–35) but individuals with IBD-U were few.
Change of IBD Subtypes During Follow-Up
In total, 147 patients also performed additional endoscopies before the end of follow-up (Table S6). Change in IBD subtypes of these patients based on medical notes and NPR during follow-up are demonstrated in Figures S3 and S4.
Discussion
Main Findings
We found the PPV of a commonly used register-based definition of childhood-onset IBD in the National Patient Register (≥2 diagnostic listings of IBD) to be high (PPV = 93%) when using the Copenhagen criteria based on medical chart review as gold standard.23,24 The PPV for a definition also using pathology reports suggestive of IBD was even higher (95–97%) and 100% for patients that were also registered in SWIBREG. When using the revised Porto criteria as gold standard (albeit published after the study period),25 the PPV for different IBD subtypes based on ICD-codes in the NPR at the start of follow-up (incident definition) was 78% for CD and 74% for UC and somewhat higher for CD at the end of follow-up (prevalent definition: 88% for CD and 71% for UC). PPV for IBD-U was low both at diagnosis and at end of follow-up (23% [95% CI 10–40] and 21% [95% CI 10–35] respectively).
Findings Compared to Earlier Studies
A definition of IBD as ≥2 diagnostic listings of IBD in NPR resulted in a high PPV for an IBD diagnosis in general (according to the Copenhagen criteria) and also reasonably high PPV for subtypes (according to the Porto criteria or medical notes). Our findings in the studied pediatric population are almost identical to earlier Swedish and Danish studies, assessing PPVs of the same register-based IBD definition in mostly or exclusively adult populations.11,32 Two previous studies from Sweden and Denmark found markedly higher PPVs for IBD subtypes in adult patients (PPV = 89–97%), but these studies only included patients with confirmed IBD.33,34
Two earlier studies from Canada and Israel validating register-based IBD definitions in childhood-onset IBD have chosen not to test an already established register-based definition of IBD (as we did) but instead tested a wide range of combinations to identify register-based definitions optimal for different scenarios.35,36 Both studies found considerably lower PPVs for incident IBD compared to our study.
In the Canadian study, the algorithm was developed using the PIBD database (183 children <15 years) at Toronto SickKids’ as the positive controls. All other children residing in Toronto at the time were negative controls (936,514 children <15 years). Billing claims for physician services provided by the Ontario Health Insurance Plan (including both specialised and primary care) were used as variables in the algorithm.35 The most accurate algorithm required either an endoscopy followed by four physician contacts or two hospitalizations with an IBD diagnosis within three years (or, in case of no endoscopy, seven physician contacts or three hospitalizations) which resulted in a PPV of 59% (95% CI 53–65). The algorithm was then validated in another dataset generated from 12 medical practices (six paediatric gastroenterology, five adult gastroenterology, and one general paediatrics) consisting of 593 PIBD patients and 1241 controls, confirming the high sensitivity (91% [95% CI 88–93]) and specificity (100% [99–100]) from the algorithm development phase.
In a similar Israeli study, an IBD defining algorithm was developed and validated in datasets derived from the compulsory Israeli Health Insurance System.36 The algorithm development cohort included 216 children with confirmed childhood-onset IBD and 170 confirmed non-IBD patients from gastroenterology clinics, but test characteristics were not reported for childhood-onset IBD separately. The algorithm found to perform best was based on at least five diagnostic IBD codes or at least one diagnostic IBD code in combination with purchases of IBD-related medications. They reported a pooled PPV for a prevalent IBD diagnosis of 92% (95% CI 90–94), a sensitivity of 89% (95% CI 87–92), a specificity 99% (95% CI 99–100), and an NPV of 99% (95% CI 99–100), but considerably lower values for the incident algorithm (PPV 82%). When using the three most recent codes, or the most recent code when only one or two codes were available, PPV for IBD subtypes (CD/UC) was 97% (IBD-U was coded as UC or CD) though only true IBD cases were included.
Why Algorithms and Test Characteristics Might Differ Between Countries
The need for much more complex algorithms to identify IBD in Canada and Israel35,36 compared to the corresponding Danish and Swedish algorithms11,32,34 is likely due to several differences between the healthcare systems and databases used. The Canadian algorithms were created based on billing claims in systems where diagnostic codes for IBD seem to have been used also before a definitive diagnosis had been confirmed. In Ontario, there was incentive to code for IBD during the study period, since a fee-for-service physician would get a chronic disease premium for seeing these patients (rather than using unspecific codes such as diarrhoea during work-up) (personal communication, Dr Eric Benchimol, October 18, 2021). Swedish physicians have monthly salaries, which are not affected by diagnostic codes used. Swedish physicians also seem to use codes for gastrointestinal symptoms during work-up of IBD, and the actual codes for IBD only after the diagnosis have been confirmed by say endoscopy (Figure S5). Moreover, the Canadian and Israeli algorithms were developed in databases including both primary care and specialised care while in Sweden IBD patients are seen exclusively by specialists in medical and/or surgical gastroenterology (adults) or pediatric gastroenterologists/paediatricians, not in the primary care, likely resulting in a need for less complex algorithms.
The PPV for prevalent subtype of Crohn’s disease was relatively high in our study similarly to both the Canadian and the Israeli studies, whereas the PPV for our incident definition of subtypes was lower. This was expected since we know that subtypes can be hard to determine at diagnosis and that subtypes change during follow-up in as much as 18% of cases.16 Additionally, unique features of Crohn’s disease could make it easier to assign the patient to subtype. The lower PPV for subtype definitions in our study can also be partly explained by the fact that the gold standard was based on the updated Porto criteria (which was not even published when the most recent patient in this study was diagnosed with IBD).
Strengths and Limitations
Our study has several strengths: In this validation study, pediatric patients were sampled from the National Patient Register covering all Swedish children and including both regional hospitals and university hospitals, inpatients and outpatients, regardless of disease severity and residence. Very few patients had to be excluded due to missing information, thus ensuring high generalizability. We used well-accepted international definitions to define IBD and its subtypes during meticulous medical chart review by paediatric gastroenterologists, which should reduce the risk of misclassification. We analyzed both prevalent and incident IBD subtype definitions, since both have been previously used in the literature.16 We followed STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) as well as RECORD statement (REporting of studies Conducted using Observational Routinely collected health Data) checklists which expand STROBE to report our findings.37,38 We also followed guidelines for validation studies of health administrative data.12
This study also has some limitations that need to be mentioned. Due to retrospective study design, some of the information could be not found in the notes, though it applied to very few patients. The same medical records were not reviewed by more than one pediatric gastroenterologist, and thus kappa statistic could not be cited. The readers reviewing the notes were also not blinded to the subtype classification in the medical records. However, only experienced pediatric gastroenterologists evaluated the notes and they used standardized Porto criteria and the IBD classes smartphone app to classify the patients.
We cannot know for sure how many individuals in our source population without IBD codes who actually had IBD (false negatives). Of course, it was impossible to perform endoscopies on all these children, but knowing that prevalence of pediatric IBD is low and based on a previous Swedish adult study39 we could make reasonable assumptions of the proportion of false negatives in the source population. When a disease is rare, the NPV is expected to be close to 100%, which is in accordance with our assumptions.
Clinical Significance
The markedly different need for complexity in algorithms used to identify IBD in registers in, eg, Denmark, Sweden, Canada, and Israel makes it obvious that the healthcare system and types of registers need to be considered when developing accurate definitions. In accordance with previous studies, our study confirmed that having only one diagnostic listing of IBD would result in a very high proportion of false positives IBD cases and should not be used. The NPR-based definitions of IBD (two or more diagnostic listings) have hitherto been used in many observational register-based studies that have the potential to inform clinical decision-making and guidelines. It is reassuring that even with a PPV on par with the lower limit of the 95% CI that we found in the current study, reported relative risks in observational studies based on this definition of IBD would not underestimate the true relative risk in IBD more than marginally (Figure S5). In studies where it is of utmost importance not to have any false-positive IBD cases, the register-based definitions combining SWIBREG data or at least one or two diagnostic IBD listings with a pathology code suggestive of IBD may be used instead.
Conclusion
Our study showed that the validity of ICD-based definitions of childhood-onset IBD and its subtypes in the NPR is excellent and acceptable, respectively, with the exception of IBD-U.
Study Report Guideline
The study is presented according to the recommendation in the STROBE statement on how to report observational studies in epidemiology. STROBE checklist for cohort-studies was used.
Abbreviations
IBD, Inflammatory bowel disease; UC, Ulcerative Colitis; CD, Crohn’s disease; IBDU, IBD unclassified; PPV, Positive predictive value; CI, Confidence interval.
Data Sharing Statement
No additional data are available because of Swedish regulations.
Acknowledgments
The SWIBREG study group consists of the following researchers:
Malin Olsson1, Pär Myrelid1, Henrik Hjortswang2, Jonas Bengtsson3, Hans Strid4, Marie Andersson4, Susanna Jäghult5, Michael Eberhardson2 Caroline Nordenvall7,8, Jan Björk9,10, Martin Rejler14,15, Olof Grip16, Pontus Karling17, and Jonas Halfvarson18. 1Department of Surgery, County Council of Östergötland and Department of Clinical and Experimental Medicine, Linköping University, Linköping Sweden; 2Department of Gastroenterology, County Council of Östergötland and Department of Clinical and Experimental Medicine, Linköping University, Linköping, Sweden; 3Department of Surgery, Sahlgrenska University Hospital/Östra, Gothenburg, Sweden; 4Department of Internal Medicine, Södra Älvsborgs Hospital, Borås, Sweden; 5Stockholm Gastro Center, Karolinska Institutet, Stockholm, Sweden; 6Department of Medicine, Karolinska Institutet, Stockholm, Sweden; 7Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; 8Department of Colorectal Cancer Karolinska University Hospital, Stockholm, Sweden; 9 Unit of Internal Medicine, Institute Medicine Solna, Karolinska Institutet, Stockholm, Sweden; 10 Patient Area Gastroenterology, Dermatovenerology and Rheumatology, Inflammation and Infection Theme Karolinska University Hospital, Stockholm, Sweden; 14Department of Medicine, Höglandssjukhuset Eksjö, Region Jönköping County Council, Jönköping, Sweden; 15Jönköping Academy for Improvement of Health and Welfare, Jönköping University, Jönköping, Sweden; 16Department of Gastroenterology, Skåne University Hospital, Malmö, Sweden; 17Department of Public Health and Clinical Medicine, Umeå University, Umeå, Sweden; 18 Department of Gastroenterology, Faculty of Medicine and Health, Örebro University, Örebro, Sweden.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Mouratidou was supported by the Crown Princess Louisa’s memory fund. Malmborg was supported by grants from Karolinska Institutet foundations, the Swedish Medical Society (Bengt Ihres fond), Mag-tarmfonden and the Bengt Ihre foundation. Bröms was supported by Stockholm Region (postdoctoral appointment). Olén was supported by grants from the Swedish Research Council (Dnr 2020-02002) and the Regional Agreement on Medical Training and Clinical Research between Stockholm County Council and Karolinska Institutet (ALF; Dnr 201 90638). None of the funding organizations has had any role in the design and conduct of the study; in the collection, management, and analysis of the data; or in the preparation, review, and approval of the manuscript.
Disclosure
Olén has been PI on projects at Karolinska Institutet partly financed by investigator-initiated grants from Janssen, Ferring, Takeda, AbbVie, and Pfizer. None of those studies have any relation to the present study. Karolinska Institutet has received fees for Olen’s lectures and participation on advisory boards from Janssen, Ferring, Takeda, and Pfizer regarding topics not related to the present study. Ludvigsson coordinates a study unrelated to the present study on behalf of the Swedish IBD Quality Register (SWIBREG). That study has received funding from Janssen. Fagerberg has received consulting fees to the institution from Ferring Läkemedel AB, Adacyte Therapeutics S.L. and Index Pharmaceuticals AB and payment for lectures to the institution from Abigo Medical. The authors report no other conflicts of interest in this work.
References
1. Ludvigsson JF, Mahl M, Sachs MC, et al. Fracture risk in patients with inflammatory bowel disease: a nationwide population-based cohort study from 1964 to 2014. Am J Gastroenterol. 2019;114(2):291–304. doi:10.14309/ajg.0000000000000062
2. Burgess CJ, Henderson P, Jones GR, Lees CW, Wilson DC. Lothian IBDRG. paediatric patients (less than age of 17 years) account for less than 1.5% of all prevalent inflammatory bowel disease cases. J Pediatr Gastroenterol Nutr. 2020;71(4):521–523. doi:10.1097/MPG.0000000000002842
3. Everhov AH, Halfvarson J, Myrelid P, et al. Incidence and treatment of patients diagnosed with inflammatory bowel diseases at 60 years or older in Sweden. Gastroenterology. 2018;154(3):518–528. doi:10.1053/j.gastro.2017.10.034
4. Benchimol EI, Bernstein CN, Bitton A, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. 2017;112(7):1120–1134. doi:10.1038/ajg.2017.97
5. Ghione S, Sarter H, Fumery M, et al. Dramatic increase in incidence of ulcerative colitis and crohn’s disease (1988–2011): a population-based study of French adolescents. Am J Gastroenterol. 2018;113(2):265–272. doi:10.1038/ajg.2017.228
6. Roberts SE, Thorne K, Thapar N, et al. A systematic review and meta-analysis of paediatric inflammatory bowel disease incidence and prevalence across Europe. J Crohns Colitis. 2020;14(8):1119–1148. doi:10.1093/ecco-jcc/jjaa037
7. Everhov AH, Olen O, Ludvigsson JF. Editorial: importance of definition of inflammatory bowel disease and an increased incidence in children. Aliment Pharmacol Ther. 2017;45(10):1369–1370. doi:10.1111/apt.14035
8. Olen O, Askling J, Sachs MC, et al. Increased mortality of patients with childhood-onset inflammatory bowel diseases, compared with the general population. Gastroenterology. 2019;156(3):614–622. doi:10.1053/j.gastro.2018.10.028
9. Olen O, Askling J, Sachs MC, et al. Childhood onset inflammatory bowel disease and risk of cancer: a Swedish nationwide cohort study 1964–2014. BMJ. 2017;358:j3951. doi:10.1136/bmj.j3951
10. Malmborg P, Mouratidou N, Sachs MC, et al. Effects of childhood-onset inflammatory bowel disease on school performance: a nationwide population-based cohort study using Swedish Health and Educational Registers. Inflamm Bowel Dis. 2019;25(10):1663–1673. doi:10.1093/ibd/izz040
11. Jakobsson GL, Sternegard E, Olen O, et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish Quality Register for IBD (SWIBREG). Scand J Gastroenterol. 2017;52(2):216–221. doi:10.1080/00365521.2016.1246605
12. Benchimol EI, Manuel DG, To T, Griffiths AM, Rabeneck L, Guttmann A. Development and use of reporting guidelines for assessing the quality of validation studies of health administrative data. J Clin Epidemiol. 2011;64(8):821–829. doi:10.1016/j.jclinepi.2010.10.006
13. Chaparro M, Garre A, Ricart E, et al. Differences between childhood- and adulthood-onset inflammatory bowel disease: the CAROUSEL study from GETECCU. Aliment Pharmacol Ther. 2019;49(4):419–428. doi:10.1111/apt.15114
14. Malham M, Jakobsen C, Vester-Andersen MK, et al. Paediatric onset inflammatory bowel disease is a distinct and aggressive phenotype—a comparative population-based study. GastroHep. 2019;1(6):266–273. doi:10.1002/ygh2.368
15. Mouratidou N, Malmborg P, Sachs MC, et al. Adult height in patients with childhood-onset inflammatory bowel disease: a nationwide population-based cohort study. Aliment Pharmacol Ther. 2020;51(8):789–800. doi:10.1111/apt.15667
16. Everhov AH, Sachs MC, Malmborg P, et al. Changes in inflammatory bowel disease subtype during follow-up and over time in 44,302 patients. Scand J Gastroenterol. 2019;54(1):55–63. doi:10.1080/00365521.2018.1564361
17. Wettergren B, Blennow M, Hjern A, Soder O, Ludvigsson JF. Child health systems in Sweden. J Pediatr. 2016;177:S187–S202. doi:10.1016/j.jpeds.2016.04.055
18. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. doi:10.1007/s10654-009-9350-y
19. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. doi:10.1186/1471-2458-11-450
20. Forss A, Myrelid P, Olen O, et al. Validating surgical procedure codes for inflammatory bowel disease in the Swedish National patient register. BMC Med Inform Decis Mak. 2019;19(1):217. doi:10.1186/s12911-019-0948-z
21. Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology reports in Sweden). Clin Epidemiol. 2019;11:101–114. doi:10.2147/CLEP.S191914
22. Ludvigsson JF, Andersson M, Bengtsson J, et al. Swedish inflammatory bowel disease register (SWIBREG) - a nationwide quality register. Scand J Gastroenterol. 2019;54(9):1089–1101. doi:10.1080/00365521.2019.1660799
23. Munkholm P. Crohn’s disease - Occurrence, course and prognosis - An epidemiologic cohort-study. Dan Med Bull. 1997;44:287–302.
24. Langholz E. Ulcerative colitis - An epidemiological study based on a regional inception cohort, with special reference to disease course and prognosis. Dan Med Bull. 1999;46:400–415.
25. Birimberg-Schwartz L, Zucker DM, Akriv A, et al. Development and validation of diagnostic criteria for IBD subtypes including IBD-unclassified in children: a multicentre study from the pediatric IBD porto group of ESPGHAN. J Crohns Colitis. 2017;11(9):1078–1084. doi:10.1093/ecco-jcc/jjx053
26. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17(6):1314–1321. doi:10.1002/ibd.21493
27. Ricciuto A, Fish JR, Tomalty DE, et al. Diagnostic delay in Canadian children with inflammatory bowel disease is more common in Crohn’s disease and associated with decreased height. Arch Dis Child. 2018;103(4):319–326. doi:10.1136/archdischild-2017-313060
28. Jimenez Trevino S, Pujol Muncunill G, Martin-Masot R, et al. Spanish pediatric inflammatory bowel disease diagnostic delay registry: SPIDER study from sociedad espanola de gastroenterologia, hepatologia y nutricion pediatrica. Front Pediatr. 2020;8:584278. doi:10.3389/fped.2020.584278
29. Ludvigsson JF, Busch K, Olen O, et al. Prevalence of paediatric inflammatory bowel disease in Sweden: a nationwide population-based register study. BMC Gastroenterol. 2017;17(1):23. doi:10.1186/s12876-017-0578-9
30. Olen O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395(10218):123–131. doi:10.1016/S0140-6736(19)32545-0
31. Nguyen LH, Örtqvist AK, Cao Y, et al. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol. 2020;5(11):986–995. doi:10.1016/S2468-1253(20)30267-3
32. Rye C, Rubin KH, Moller FT, Julsgaard M, Jess T, Andersen V. Positive predictive value of diagnostic codes for inflammatory bowel disease in the Danish National Patient Registry among individuals 50+ years, using patient records as reference standard. Clin Epidemiol. 2021;13:335–344. doi:10.2147/CLEP.S298770
33. Shrestha S, Olen O, Eriksson C, et al. The use of ICD codes to identify IBD subtypes and phenotypes of the Montreal classification in the Swedish National Patient Register. Scand J Gastroenterol. 2020;55(4):430–435. doi:10.1080/00365521.2020.1740778
34. Lo B, Vind I, Vester-Andersen MK, Burisch J. Validation of ulcerative colitis and Crohn’s disease and their phenotypes in the Danish National Patient Registry using a population-based cohort. Scand J Gastroenterol. 2020;55(10):1171–1175. doi:10.1080/00365521.2020.1807598
35. Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58(11):1490–1497. doi:10.1136/gut.2009.188383
36. Friedman MY, Leventer-Roberts M, Rosenblum J, et al. Development and validation of novel algorithms to identify patients with inflammatory bowel diseases in Israel: an epi-IIRN group study. Clin Epidemiol. 2018;10:671–681. doi:10.2147/CLEP.S151339
37. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi:10.1371/journal.pmed.0040297
38. Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi:10.1371/journal.pmed.1001885
39. Kjellstrom L, Molinder H, Agreus L, Nyhlin H, Talley NJ, Andreasson A. A randomly selected population sample undergoing colonoscopy: prevalence of the irritable bowel syndrome and the impact of selection factors. Eur J Gastroenterol Hepatol. 2014;26(3):268–275.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

