Back to Journals » OncoTargets and Therapy » Volume 15
Identification of a Rare BAIAP2-ROS1 Fusion and Clinical Benefit of Crizotinib in the Treatment of Advanced Lung Adenocarcinoma: A Case Report
Authors Lin Y, Lei Y, Li L, Su X, Tian Q, Wu W
Received 25 April 2022
Accepted for publication 21 July 2022
Published 28 July 2022 Volume 2022:15 Pages 831—836
DOI https://doi.org/10.2147/OTT.S372134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
YunYu Lin,1,* Yan Lei,2,* LinWei Li,2 Xiaoxing Su,2 Qiqi Tian,1 Wendy Wu2
1Respiratory Medicine, NingHai First Hospital, NingBo, 315600, People’s Republic of China; 2Berry Oncology Institutes, Berry Oncology Corporation, Fuzhou, 350200, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiqi Tian; Wendy Wu, Email [email protected]; [email protected]
Objective: Previous studies have shown that fusion partners have a potential role in influencing different tumorigenic abilities of ROS1 fusion variants, as well as potential differential responses to crizotinib. Therefore, it is important to accurately identify the type of ROS1 rearrangement in NSCLC for clinical treatment selection.
Materials and Methods: Deep-coverage targeting solid tumor 31 cancer-related genes panel was used to capture DNA-based NGS information to detect gene fusion. RNA fusion panel based on hybrid capture sequencing was performed to verify gene fusions from total RNA which isolated from formalin fixed paraffin-embedded (FFPE) tissue blocks.
Results: Using DNA-targeted NGS method, we identified a novel BAIAP2-ROS1 fusion in a 71-year-old non-smoking female patient with stage IVB lung adenocarcinoma. Rearrangement consisted of BAIAP2 in exon1-exon13 of chr17: q23 and ROS1 in exon35-exon43 of chr6: q22, which were further confirmed by RNA-based NGS methodology. A complete kinase domain in ROS1 fusion was preserved. The patient subsequently received crizotinib and showed significant tumor reduction until 17 months, who got benefit from targeted therapy.
Conclusion: This study discovered a novel BAIAP2-ROS1 rearrangement; it provides more knowledge of ROS1 fusion in clinical personalized treatment. The good response to crizotinib therapy emphasizes the importance of DNA-based and RNA-based NGS in rare fusion identification in clinical practice.
Keywords: BAIAP2-ROS1 fusion, advanced lung adenocarcinoma, sensitive to crizotinib, next-generation sequencing
Introduction
With the wide application of next-generation sequencing (NGS) technology in clinical practice, more and more rare fusion variant type has been found in non-small cell lung cancer (NSCLC). ROS1 fusion gene is detected in cancer patient with a certain mutation rate and incidence (1–2%).1 The incidence of ROS1 fusion is mainly concentrated in younger Asian patients with no smoking history and histological type of adenocarcinoma, and it rarely coexists with other driver gene mutations. ROS1 gene can rearrange with multiple genes, and the most frequent fusion partner is CD74.2 When ROS1 fuses with other genes, the kinase domain was usually retained and would activate signaling pathways that are critical to the occurrence and progression of cancer, such as PI3K/AKT/mTOR and JAK/STAT pathway.3,4
Currently, two targeted drugs, crizotinib and entrectinib, have been approved by FDA for the treatment of patients with ROS1 fusion, while the current development of ROS1 inhibitors is still relatively limited. The objective response rate (ORR) of this treatment can reach more than 70%, which brings clinical benefits to ROS1-positive advanced NSCLC.5 Although some new small molecule tyrosine kinase inhibitors (TKI) have been continuously developed after crizotinib, the median progression-free survival (PFS) of these TKIs was 9–12 months.6 Drug resistance has become the most common bottleneck to the clinical application of ROS1 inhibitors. At present, there is an urgent need for stronger and more selective ROS1 inhibitors to overcome these problems.7 Therefore, accurate identification of ROS1 rearrangement is the very first key step for clinical treatment selection.
Case Presentation
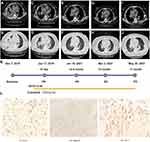
A 71-year-old female was admitted to the hospital on December 5, 2019 due to cough and chest tightness for 3 days. Chest CT (computed tomography) showed lung cancer was considered. Multiple nodules were found in both lungs, accompanied by enlarged right hilar and mediastinal lymph nodes, and right pleural effusion with adjacent lung parenchyma insufficiency (Figure 1A). The size of the target lesion in the lower right lobe is about 7.0×6.0cm (Figure 1B). The CEA value was 3.1ng/mL. After 18 days of the treatment with crizotinib, mediastinal lymph nodes are reduced (Figure 1C). Lung lesions are also reduced (Figure 1D). At the time of 12.5 months of crizotinib treatment, hilar lymph nodes decreased (Figure 1E), and the pleural effusion decreased (Figure 1F). After 14 months of crizotinib treatment, mediastinal lymph nodes changed (Figure 1G) and the right pleural effusion increased (Figure 1H). After 17 months of oral crizotinib, the mediastinal lymph nodes were enlarged (Figure 1I). The left pleural effusion was significantly increased (Figure 1J). The pathological results changed by the timeline of medication (Figure 1K). Subsequently, a lung biopsy was performed under the guidance of CT. Metastatic carcinoma of the right parietal pleura combined with the source of immunohistochemical lung adenocarcinoma was diagnosed (Figure 1L). Finally, through genetic testing, the diagnosis was revised: stage IV right lung adenocarcinoma, ROS1 positive.
With written informed consent, the formalin fixed paraffin embedded (FFPE) tissues in primary tumor tissues were detected by deep coverage NGS test targeting 31 tumor associated gene. Deep-coverage targeting solid tumor 31 cancer-related genes panel was used to capture DNA-based NGS information to detect gene fusion. RNA fusion panel based on hybrid capture sequencing was performed to verify gene fusions from total RNA which isolated from formalin fixed paraffin-embedded (FFPE) tissue blocks. The mutation profile showed a rearrangement between chromosome 17 and chromosome 6 with a mutant allele frequency of 69%. Sequences support of BAIAP2-ROS1 fusion was detected by DNA-Seq (Figure 2A) and RNA-Seq (Figure 2B) methods. Schematic of genome rearrangement of BAIAP2-ROS1 involving fusion breakpoints at mRNA and protein levels (Figure 2C). The fusion of BAIAP2-ROS1 in tissue was determined which was formed by exon1-exon13 fragment of BAIAP2 and exon35-exon43 fragment of ROS1 (Figure 2A and C). The rearrangement was further verified by RNA fusion plate hybrid capture sequencing (Berry Oncology Corporation) technology8 to confirm the existence of BAIAP2-ROS1 (B13: R36) fusion mutation (Figure 2B and C). As the complete ROS1 kinase domain was retained in this novel fusion, constitutive kinase activity and oncogenic transformation were achieved.
Based on the identification of this new ROS1 fusion, from December 30, 2019, the patient took crizotinib twice a day at a dose of 250 mg (Figure 1K). After 18 days of the treatment with crizotinib, the lesions in both lungs and mediastinum were reduced (Figure 1C and D). Partial response (PR) was achieved according to the RECIST guideline. The lesion size in the lower lobe of the right lung was about 3.9×2.7cm and the lymph nodes were reduced by 2.0cm. CEA value was 3.30 ng/mL. The right pleural effusion accompanied by insufficiency of the right lung was relived. The patient’s cough and chest tightness improved. PR was achieved after 12.5 months crizotinib treatment, the imaging map showed that the target lesion was a cord-like high-density shadow, the amount of pleural effusion was reduced, and the hilar lymph nodes were reduced (Figure 1E and F). CEA value was 4.2ng/mL. After 14 months of oral administration of crizotinib, the right pleural effusion increased and the lesion was evaluated as stable disease (SD) (Figure 1G and H). CEA value was 5.88 ng/mL. Finally, after 17 months of oral crizotinib, the target lesion was about 2.6 cm x 2.0 cm in the right lower hilum, and the left pleural effusion was significantly increased, and the lesion was PD (Figure 1I and J). CEA value was 5.54 ng/mL. The patient accidentally fell and injured the right femur and ribs at 15th month of medication because of severe osteoporosis. The patient had been taking crizotinib during her convalescence, the lesion was controlled again and assessed as PD at 17 months. But the patient’s physical condition was not so well and passed away one month later (July 2021). The results of clinical data showed that the novel BAIAP2-ROS1 fusion was sensitive to the first-generation tyrosine kinase inhibitor, crizotinib, and the patient had no obvious drug-related adverse reactions during the follow-up period of medication.
Discussion
In recent years, DNA-based next-generation sequencing (NGS) has been widely used as an important diagnostic method for detecting ROS1 rearrangements, besides fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC). DNA-based NGS fusion detection method has advantage on detecting the exons and introns of genes, detecting multiple gene fusions in one test, and determining the fusion partners and breakpoints,9 which could eliminate false positives for ROS1 fusion detected by IHC. RNA-based NGS technology could exactly detect gene rearrangement performed in the transcription process, and many studies have proved that the combination of DNA and RNA-based NGS technology can comprehensively inspect gene fusion/rearrangement and significantly improve the detection rate of rare fusion.1,10 Simultaneous determination of targets and rare and effective fusion mutations through NGS methodology can improve possible missed detections and unclear fusion genes in traditional detection methods, effectively improve the detection rate of fusion genes, and offer help to doctors in clinical diagnosis and treatment.11
This is the first reported case of BAIAP2-ROS1 (B13:R35) fusion in advanced NSCLC patient. This study found a novel ROS1 rearrangement fused with a new gene partner, BAIAP2, which was sensitive to crizotinib in a female advanced lung adenocarcinoma patient. The BAIAP2-ROS1 fusion was determined by targeted DNA 31 panel next-generation sequencing methodology and verified by RNA fusion panel based on hybrid capture sequencing (Berry Oncology Corporation). Subsequent crizotinib treatment yielded a favorable 17-month response, superior to many ROS1-fusion crizotinib treatments, with the majority of cases developing resistance within 9–12 months.12,13 This report provided the clinical evidence on this novel ROS1 rearrangement and offered drug-sensitive support for targeted therapy in lung adenocarcinoma with this new-found gene fusion. It also expands NSCLC’s treatment of ROS1 rearrangement and emphasizes the importance of genetic testing for precise treatment decision.
Ethics Statement
The study was approved by the Ethical Committee of Medical Research, NingHai First Hospital of NingBo City.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Wendy Wu, Yan Lei, Linwei Li and Xiaoxing Su are the employees of Berry Oncology Corporation. The other authors declare no conflict of interest.
References
1. Shu Y, Li H, Shang H, et al. Identification of a novel MPRIP-ROS1 fusion and clinical efficacy of crizotinib in an advanced lung adenocarcinoma patient: a case report. Onco Targets Ther. 2020;13:10387–10391.
2. Lin JJ, Shaw AT. Recent advances in targeting ROS1 in lung cancer. J Thoracic Oncol. 2017;12(11):1611. doi:10.1016/j.jtho.2017.08.002
3. Drilon A, Jenkins C, Iyer S, et al. ROS1-dependent cancers biology, diagnostics and therapeutics. Nat Rev Clin Oncol. 2020;18:35–55.
4. Ou SI, Nagasaka MA. Catalog of 5’ fusion partners in ROS1-positive NSCLC circa 2020. JTO Clin Res Rep. 2020;1(3):100048. PMID: 34589944. doi:10.1016/j.jtocrr.2020.100048
5. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: non-small cell lung cancer. Version 1; 2021.
6. Drilon A, Jenkins C, Iyer S, Schoenfeld A, Keddy C, Davare MA. ROS1-dependent cancers - biology, diagnostics and therapeutics. Nat Rev Clin Oncol. 2021;18(1):35–55. doi:10.1038/s41571-020-0408-9
7. D’Angelo A, Sobhani N, Chapman R, et al. Focus on ROS1-positive non-small cell lung cancer (NSCLC): crizotinib, resistance mechanisms and the newer generation of targeted therapies. Cancers. 2020;12(11):3293. doi:10.3390/cancers12113293
8. Xia P, Zhang L, Li P, et al. Molecular characteristics and clinical outcomes of complex ALK rearrangements identified by next-generation sequencing in non-small cell lung cancers. J Transl Med. 2021;19:308. doi:10.1186/s12967-021-02982-4
9. Li W, Guo L, Liu Y, et al. Potential unreliability of uncommon ALK, ROS1, and RET genomic breakpoints in predicting the efficacy of targeted therapy in NSCLC. J Thoracic Oncol. 2020;16:404–418.
10. Zhang X, Feng J, Su X, et al. Next generation sequencing reveals a synchronous trilateral lung adenocarcinoma case with distinct driver alterations of EGFR 19 deletion or EGFR 20 Insertion or EZR-ROS1 fusion. Onco Targets Ther. 2020;13(2020):12667–12671. doi:10.2147/OTT.S283617
11. Jurkiewicz M, Saqi A, Mansukhani, MM, et al. Efficacy of DNA versus RNA NGS-based Methods in MET Exon 14 skipping mutation detection. J Clin Oncol. 2020;38(15_suppl):9036. doi:10.1200/JCO.2020.38.15_suppl.9036
12. Shaw AT, Ou S-HI, Bang Y-J, et al. Crizotinib in ROS1 -rearranged non–small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. doi:10.1056/NEJMoa1406766
13. Wu Y-L, Yang JC-H, Kim D-W, et al. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:1405–1411. doi:10.1200/JCO.2017.75.5587
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.