Back to Journals » Cancer Management and Research » Volume 12
Identification of a Novel Prognostic Classification Model in Epithelial Ovarian Cancer by Cluster Analysis
Authors Chen K, Niu Y, Wang S, Fu Z, Lin H, Lu J, Meng X, Yang B, Zhang H, Wu Y, Xia D , Lu W
Received 29 February 2020
Accepted for publication 24 June 2020
Published 24 July 2020 Volume 2020:12 Pages 6251—6259
DOI https://doi.org/10.2147/CMAR.S251882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Kelie Chen,1,2,* Yuequn Niu,3,* Shengchao Wang,1,* Zhiqin Fu,4 Hui Lin,1,2 Jiaoying Lu,2 Xinyi Meng,2 Bowen Yang,2 Honghe Zhang,1 Yihua Wu,1,2 Dajing Xia,1,2 Weiguo Lu1
1Department of Gynecologic Oncology, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou 310058, People’s Republic of China; 2Department of Toxicology, School of Public Health, School of Medicine, Zhejiang University, Hangzhou 310058, People’s Republic of China; 3Department of Thoracic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, People’s Republic of China; 4Department of Gynecological Oncology, Zhejiang Cancer Hospital, Hangzhou 310022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dajing Xia
Department of Toxicology, School of Public Health, School of Medicine, Zhejiang University, 866 Yuhangtang Road, Hangzhou, Zhejiang 310058, People’s Republic of China
Tel/ Fax +86-0571-88208140
Email [email protected]
Weiguo Lu
Department of Gynecologic Oncology, Women’s Hospital, School of Medicine, Zhejiang University, 1 Xueshi Road, Hangzhou, Zhejiang 310006, People’s Republic of China
Tel +86-571-8706-1501
Fax +86-571-87061878
Email [email protected]
Background: Heterogeneity plays an essential role in ovarian cancer. Patients with different clinical features may manifest diverse patterns in diagnosis, treatment, and prognosis. The aim of the present study was to identify a novel ovarian cancer–classification model through cluster analysis and assess its significance in prognosis.
Methods: Among patients diagnosed with ovarian cancer in the Women’s Hospital School of Medicine, Zhejiang University between January 2014 and May 2019, 328 patients were included in a K-mean cluster analysis and 176 patients followed up. Major clinical indicators, overall survival, and recurrence-free survival in different subgroups were compared.
Results: Two clusters for ovarian cancer were identified and grouped as noninflammatory (n=247) and inflammatory subtypes (n=81). Compared with the noninflammatory subgroup, the inflammatory subgroup presented a statistically significantly higher level of median CRP (median (IQR) 20.4 [7.8– 47.3] vs 1.2 [0.4– 3.5], p< 0.001), neutrophil percentage (median (IQR) 76.9 [72.6– 81.3] vs 66.2 [61.0– 72.0], p< 0.001), leukocyte count (median (IQR) 8.9 [7.0– 10.0] vs 6.0 [5.1– 7.2], p< 0.001), fibrinogen (median (IQR) 5.0 [4.4– 6.0] vs 3.4 [2.9– 3.9], p< 0.001), and platelet count (median (IQR) 324 [270– 405] vs 229 [181.5– 269], p< 0.001). During a median follow-up of 52 months, 21 participants (16.3%) died in the noninflammatory group, while 14 (29.8%) died in the inflammatory group (HR 2.15, 95% CI 1.09– 4.23; p=0.024). Death/recurrence was observed in 38 (29.5%) patients from the noninflammatory group and 25 (53.2%) from the inflammatory group (HR 2.32, 95% CI 1.40– 3.85; p< 0.001).
Conclusion: Our study revealed a novel classification model of ovarian cancer that features inflammation. Inflammation predicts shorter survival and poorer prognosis, suggesting the significance of inflammation in the management of ovarian cancer.
Keywords: ovarian cancer, classification, heterogeneity, cluster analysis, inflammation, prognosis
Introduction
Ovarian cancer is the fifth–most frequent cause of cancer death in women and the leading cause of death from gynecological cancer, accounting for 5% of estimated cancer deaths.1–3 With most patients diagnosed at an advanced stage, the prognosis remains unsatisfactory.1 In fact, ovarian cancer is a collective term for cancers of the ovaries, peritoneum, and fallopian tube. Ovarian cancer consists of heterogeneous subtypes classified by International Federation of Gynecology and Obstetrics (FIGO) stage, tissue of origin,4–6 pathological grade,7 clinical presentation,8 molecular profiling,9,10 and treatment.6,11 These subtypes can vary from each other in initial symptoms, diagnosis, treatment protocol, and prognosis.4,5 A practical classification of ovarian cancer is of great significance to differentiate specific subgroups, guide personalized treatment, and ultimately improve prognosis.
Several widely known models have been carried out to categorize ovarian cancers. The revised dualistic model, integrating histopathologic classification with molecular genetic features, classified ovarian cancers into two groups (type I and type II).7 Type I was further divided into three subtypes. In general, type I tumors usually derived from benign extraovarian lesions, while many type II carcinomas developed from intraepithelial carcinomas in the fallopian tube.7 Type I cancers were typically indolent, with a good prognosis. In contrast, type II cancers were more aggressive and accompanied worse outcomes.7 Emphasizing the dramatic differences between the two groups, the dualistic model was expected to guide ovarian cancer screening, prevention, and treatment. Another two studies focusing on genomic and/or proteomic data based on The Cancer Genome Atlas identified different subtypes and cast a new light on the understanding of ovarian cancer.9,10
The aforementioned classification models rely on histopathological and molecular features obtained after invasive procedures and operation. More accessible and convenient classification paradigms would be beneficial to have a better understanding of the condition of patients prior to invasive procedures and provide additional information for clinical decision-making. Therefore, the current study aimed to identify preoperative ovarian cancer subtypes through cluster analysis of easily accessible variables, such as demographic data and laboratory results, and to evaluate the feasibility of our novel classification paradigm in the survival prediction of ovarian cancer patients.
Methods
Study Population and Data Collection
This study included patients who had been histopathologically diagnosed with epithelial ovarian cancer at the Women’s Hospital School of Medicine, Zhejiang University between January 2014 and May 2019. Exclusion criteria were nonepithelial ovarian tumors, neoadjuvant chemotherapy before interval debulking surgery, suboptimal debulking, borderline tumors, metastatic ovarian cancer, previous surgery for ovarian cancer, recurrent ovarian cancer, history of malignant tumors, and scarce information to carry out our analysis. Results of laboratory tests taken prior to invasive operations were collected retrospectively from the electronic medical records. Age, BMI, histopathological subtype, FIGO stage, and grade were extracted as well. Patients diagnosed between January 2014 and December 2016 were followed up with telephone or hospital records until January 2020. The outcomes of interest were overall survival and recurrence-free survival. Overall survival was defined as the time between primary diagnosis and death. Recurrence-free survival was defined as the time from primary diagnosis to disease recurrence or progression or death from any cause, whichever occurred first. Disease progression was defined according to Response Evaluation Criteria in Solid Tumors version 1.1 or on the basis of CA125 level elevated from baseline. The present retrospective observational study was approved by the ethical committee of the Women’s Hospital School of Medicine, Zhejiang University (decision 20,180,196). Informed consent was waived, due to the retrospective nature of the study and anonymous analyses of the data. All analyses were carried out with confidentiality. This study was performed in compliance with the ethical standards of the Declaration of Helsinki.
Design and Procedures
Variables selected for all participants (Table 1) to enter the cluster analysis were age, BMI, and laboratory results. Histopathological characteristics were compared between groups after clustering. The dualistic model of epithelial ovarian carcinogenesis was adopted to divide tumors into type I and type II.7 Cluster analysis was validated by verifying its association with overall survival and recurrence-free survival. Patients diagnosed between January 2014 and December 2016 were included in the survival analysis.
 |
Table 1 Comparison of Baseline Characteristics Between Groups |
Statistical Analysis
Cluster analysis was performed using K-mean cluster analysis after data standardization. Baseline characteristics were compared according to the group after clustering. Continuous and categorical variables are expressed as medians (interquartile range, IQR) and number (percentage), respectively. Rank-sum, Kruskal–Wallis and ANOVA F tests were employed for comparing continuous variables between groups. Fisher’s exact or χ2 tests were employed to compare categorical variables. The Kaplan–Meier method with log-rank testing was used in survival analysis. The Cox regression model was used to calculate HR and 95% CI. Two-tailed p<0.05 was considered statistically significant. All statistical analyses were performed using R version 3.5.1.
Results
Among the 645 patients collected between January 2014 and May 2019, 23 duplicates were excluded and another 229 patients excluded according to the aforementioned exclusion criteria. A total of 65 patients were not able to be clustered because of missing data of at least one of the included variables. Finally, 328 patients were included in the present study. Characteristics of patients included were shown in Table 1. K-mean clustering identified two clusters of patients (Figure 1A): 247 patients in cluster 1 and 81 in cluster 2. Average silhouette width was 0.16 (Figure 1B).
 |
Figure 1 Results of kK-mean clustering. (A) Results from cluster analysis; (B) silhouette information from clustering. |
As shown in Table 2, cluster 2 featured a higher death/recurrence rate (p<0.001) and a higher proportion of advanced patients (p<0.001). Interestingly, cluster 2 presented higher levels of inflammation biomarkers, as shown in Table 1 and Figure 2. Median CRP levels were 20.4 (7.8–47.3) and 1.2 (0.4–3.5) in cluster 2 and cluster 1, respectively (p<0.001). Median leukocyte counts were 8.9 (7.0–10.0) and 6.0 (5.1–7.2) in cluster 2 and cluster 1, respectively (p<0.001). Median neutrophil percentages were 76.9 (72.6–81.3) and 66.2 (61.0–72.0) in cluster 2 and cluster 1, respectively (p<0.001). Cluster 2 was also characterized by higher fibrinogen levels (p<0.001), higher platelet counts (p<0.001), lower albumin levels (p<0.001) and higher CA125 levels (p<0.001).
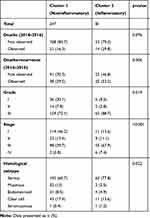 |
Table 2 Comparison of Death Rate and Histopathological Characteristics Between Groups |
Concerning higher proportion of advanced tumors and higher levels of inflammation biomarkers in cluster 2, Table 3 shows associations between laboratory findings and FIGO stages. Table 3 indicates that CRP levels (p<0.001), neutrophil percentage (p<0.001), fibrinogen (p<0.001), platelet counts (p<0.001), and CA125 levels (p<0.001) ascended from stage I to stage IV, while albumin levels (p<0.001) and lymphocyte percentage (p<0.001) descended.
 |
Table 3 Associations Between Laboratory Findings and FIGO Stages |
Survival Analysis
To further verify these results, survival analysis was carried out. Based on the elevated inflammation biomarkers, cluster 1 and cluster 2 were classed as noninflammatory and inflammatory subtypes of ovarian cancer, respectively. Median follow-up was 52 months, at which 21 (16.3%) patients in the noninflammatory group had died compared to 14 (29.8%) in the inflammatory group (p=0.076), as shown in Table 2. Cox regression analysis indicated an HR of 2.15 (95% CI 1.09–4.23, p=0.024 by log-rank test), as shown in Figure 3A. Death or recurrence was observed in 38 (29.5%) patients in the noninflammatory group and 25 (53.2%) in the inflammatory group (HR 2.32, 95% CI 1.40–3.85; p<0.001 by log-rank test) as shown in Figure 3B.
As one of the most important parameters of inflammation, CRP was selected to verify the association between inflammation and prognosis. CRP was dichotomized into two groups according to the median. The results in Table 4 indicate that CRP was an independent risk factor of overall survival (HR 3.79, 95% CI 1.54–9.33; p=0.001) and recurrence-free survival (HR 1.99; 95% CI 1.13–3.50; p=0.014). Subgroup analysis indicated that higher CRP was associated with poorer survival at both stage I–IIA (p=0.0094) and stage IIIB–IVB (p=0.026), as shown in Figure 4.
 |
Table 4 Univariate and Multivariate Analyses for overall Survival and Recurrence-free Survival |
 |
Figure 4 Subgroup survival analysis. Kaplan–Meier curves showed higher CRP was associated with poorer overall survival in (A) stage I–IIA and (B) stage IIIB–IVB. |
Discussion
Via K-mean clustering with preoperative laboratory results, we divided ovarian cancer patients into noninflammatory and inflammatory subtypes. The inflammatory subtype showed higher CRP, leukocyte counts, neutrophil percentage, fibrinogen, platelet counts, CA125, a higher proportion of patients in advanced stages, and lower levels of albumin. The inflammatory subtype was significantly associated with poor overall survival and recurrence-free survival.
Elevated fibrinogen and platelet counts were observed along with elevated inflammatory markers in the inflammatory subtype. Inflammation is well recognized as a regulator of coagulation and fibrinolytic activity, which are context-dependent.12 In inflammatory conditions, hemostatic balance may be shifted toward prothrombotic and antifibrinolytic states, with an increase in circulating serum factors.12 Meanwhile, the proinflammatory effect of fibrinogen has been reported, either by stimulating macrophages to release inflammatory cytokine or leading the cascade of tissue repair and inflammatory responses.13,14 In addition to hemostatic mechanisms, platelet-derived molecules have been found to mediate inflammation.15––17 This evidence justifies the classification model in the present study, which emphasizes inflammation and interacting networks with various physiological processes.
The present study demonstrated that the ovarian cancer inflammatory subtype was linked to poor prognosis through cluster analysis. Similar results have been identified in previous studies. Neutrophil:lymphocyte ratio and platelet:lymphocyte ratio have been well investigated as inflammatory parameters closely related to ovarian cancer prognosis.18––26 A meta-analysis verified the prognostic value of inflammatory markers in ovarian cancer patients.27 With a novel method, K-mean cluster analysis, our study showed results consistent with previous research. Clustering is an unsupervised technique to classify a group of objects without prespecified labels into subgroups based on the similarity measure between objects.28 In the present study, leukocyte count, neutrophil percentage, and platelet count were higher in the inflammatory subgroup, which was related to poorer prognosis. In contrast, lymphocyte percentage was lower. Under the circumstances, both neutrophil:lymphocyte and platelet:lymphocyte ratios in the inflammatory subgroup were higher, and thus expected to be related to worse outcomes.
In our study, the inflammatory subgroup presented a higher proportion of advanced ovarian cancer patients. Inflammation and advanced stages are likely to be closely related. However, no causal relationship can be inferred from the results. Inflammation is well recognized as a hallmark of cancer.29,30 Cross-talk between inflammation and cancer is quite complicated. Chronic inflammation predisposes tumor formation, while tumors elicit inflammation by reshaping the tumor microenvironment.31 On one hand, inflammation takes part in tumor progression and metastasis.31 Several signaling pathways involved in the inflammatory response play important roles in ovarian cancer progression and metastasis. As a widely studied proinflammation cytokine, IL6 has been found to enhance expression of MMP9, which promoted tumor expansion and tumor-cell invasion in SKOV3 cells.32 IL6 produced by M2 macrophages also stimulates the proliferation of SKOV3 cells via STAT3 activation.33 In addition, it has been reported that IL6 trans-signaling on endothelial cells contributed to ovarian cancer progression by activating ERK.34 These findings suggest the close relationship between inflammation and progression of ovarian cancer. On the other hand, tumors reshape the tumor microenvironment.31 One pathway is that tumors promote inflammation in a hypoxia-dependent manner,35,36 especially in advanced cancers.37 Compared with the normal nondiseased controls, ROS concentration has been reported at 96% higher in malignant ovarian tissue.38 Although more studies are expected to elucidate the complexity, promising cancer treatment through inflammation manipulation is on the way.39,40 The findings of the present study validated the impact of preoperative inflammation on ovarian cancer survival, which would provide more possibilities in ovarian cancer management.
There were major limitations of our study. Only some of the included patients were followed, and the follow-up might not have been long enough. This might have resulted in underestimated prognosis differences; however, according to the significantly different prognoses in the results, our follow-up duration might be sufficient to demonstrate prognosis differences. With further analysis of patient data and longer follow-up in future, it will be intriguing to explore more potential effects of our novel classification model in the clinical management of ovarian cancer. Another potential limitation is subjectivity in selection of variables to include and determination of the optimal number of clusters. Although the clustering was validated with survival analysis, the possibility still exists that other variables and other number of clusters might lead to a better classification paradigm.
Conclusion
Our cluster analysis identified two subtypes of ovarian cancer: noninflammatory and inflammatory. The inflammatory group featured higher CRP, leukocyte counts, neutrophil percentage, fibrinogen, platelet counts, CA125, and proportion of patients in advanced stages, with lower albumin levels and lymphocyte percentage. The inflammatory group was associated with poorer prognosis. The results of the present study should lead to further investigation into the cross-talk between inflammation and cancer.
Abbreviations
CA125, cancer antigen 125; CRP, C-reactive protein; BMI, body-mass index; GGT, γ-glutamyl transpeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBil, total bilirubin; DBil, direct bilirubin; IBil, indirect bilirubin; BUN, blood urea nitrogen; UA, uric acid; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; HR hazard ratio; CI, confidence interval; IQR, interquartile range; FIGO, International Federation of Gynecology and Obstetrics.
Acknowledgments
The authors would like to thank Jiang Huang and Xin Zhang for the help in data collection. The authors would like to thank Xiaodong Cheng for the advice during manuscript revision. Yihua Wu, Dajing Xia, and Weiguo Lu contributed equally to this work in conceptualization and supervision.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi:10.3322/caac.21456
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
4. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet (London, England). 2019;393(10177):1240–1253. doi:10.1016/S0140-6736(18)32552-2
5. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet (London, England). 2014;384(9951):1376–1388. doi:10.1016/S0140-6736(13)62146-7
6. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compreh Cancer Net. 2019;17(8):896–909. doi:10.6004/jnccn.2019.0039
7. Kurman RJ, Shih IM. The Dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186(4):733–747. doi:10.1016/j.ajpath.2015.11.011
8. Lisio MA, Fu L, Goyeneche A, Gao ZH, Telleria C. High-grade serous ovarian cancer: basic sciences, clinical and therapeutic standpoints. Int J Mo Sci. 2019;20:4. doi:10.3390/ijms20040952
9. Zhang H, Liu T, Zhang Z, et al. Integrated proteogenomic characterization of human high-grade serous ovarian cancer. Cell. 2016;166(3):755–765. doi:10.1016/j.cell.2016.05.069
10. Network TCGAR. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615.
11. Colombo N, Sessa C, Du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann Oncol. 2019;30(5):672–705. doi:10.1093/annonc/mdz062
12. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–520. doi:10.1182/blood-2018-07-818211
13. Hsieh JY, Smith TD, Meli VS, Tran TN, Botvinick EL, Liu WF. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta biomaterialia. 2017;47:14–24. doi:10.1016/j.actbio.2016.09.024
14. Gobel K, Eichler S, Wiendl H, Chavakis T, Kleinschnitz C, Meuth SG. The coagulation factors fibrinogen, thrombin, and factor XII in inflammatory disorders-a systematic review. Front Immunol. 2018;9:1731. doi:10.3389/fimmu.2018.01731
15. Srivastava K, Cockburn IA, Swaim A, et al. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4(2):179–187. doi:10.1016/j.chom.2008.07.003
16. Otterdal K, Smith C, Oie E, et al. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood. 2006;108(3):928–935. doi:10.1182/blood-2005-09-010629
17. Mantovani A, Garlanda C. Platelet-macrophage partnership in innate immunity and inflammation. Nature Immunol. 2013;14(8):768–770. doi:10.1038/ni.2666
18. Ceran MU, Tasdemir U, Colak E, Gungor T. Can complete blood count inflammatory parameters in epithelial ovarian cancer contribute to prognosis? - a survival analysis. J Ovarian Res. 2019;12(1):16. doi:10.1186/s13048-019-0491-7
19. Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011;13(7):499–503. doi:10.1007/s12094-011-0687-9
20. Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. doi:10.1186/s12885-016-2352-8
21. Li Z, Hong N, Robertson M, Wang C, Jiang G. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep. 2017;7:43001. doi:10.1038/srep43001
22. Miao Y, Yan Q, Li S, Li B, Feng Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomark. 2016;17(1):33–40. doi:10.3233/CBM-160614
23. Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012;23(4):265–273. doi:10.3802/jgo.2012.23.4.265
24. Wang Y, Liu P, Xu Y, et al. Preoperative neutrophil-to-lymphocyte ratio predicts response to first-line platinum-based chemotherapy and prognosis in serous ovarian cancer. Cancer Chemother Pharmacol. 2015;75(2):255–262. doi:10.1007/s00280-014-2622-6
25. Wang YQ, Jin C, Zheng HM, et al. A novel prognostic inflammation score predicts outcomes in patients with ovarian cancer. Clinica Chimica Acta. 2016;456:163–169. doi:10.1016/j.cca.2016.03.013
26. Williams KA, Labidi-Galy SI, Terry KL, et al. Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol. 2014;132(3):542–550. doi:10.1016/j.ygyno.2014.01.026
27. Zhu Y, Zhou S, Liu Y, Zhai L, Sun X. Prognostic value of systemic inflammatory markers in ovarian Cancer: a PRISMA-compliant meta-analysis and systematic review. BMC Cancer. 2018;18(1):443. doi:10.1186/s12885-018-4318-5
28. Karthik Ramasubramanian AS. Machine Learning Using R. 2017.
29. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
30. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi:10.1016/S1470-2045(14)70263-3
31. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi:10.1016/j.immuni.2019.06.025
32. Rabinovich A, Medina L, Piura B, Segal S, Huleihel M. Regulation of ovarian carcinoma SKOV-3 cell proliferation and secretion of MMPs by autocrine IL-6. Anticancer Res. 2007;27(1a):267–272.
33. Takaishi K, Komohara Y, Tashiro H, et al. Involvement of M2-polarized macrophages in the ascites from advanced epithelial ovarian carcinoma in tumor progression via Stat3 activation. Cancer Sci. 2010;101(10):2128–2136. doi:10.1111/j.1349-7006.2010.01652.x
34. Lo CW, Chen MW, Hsiao M, et al. IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res. 2011;71(2):424–434. doi:10.1158/0008-5472.CAN-10-1496
35. Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138(5):1058–1066. doi:10.1002/ijc.29519
36. Triner D, Shah YM. Hypoxia-inducible factors: a central link between inflammation and cancer. J Clin Invest. 2016;126(10):3689–3698. doi:10.1172/JCI84430
37. Chan CY, Yuen VW, Wong CC. Hypoxia and the Metastatic Niche. Advan Exp Med Biol. 2019;1136:97–112.
38. Cohen S, Mehrabi S, Yao X, Millingen S, Aikhionbare FO. Reactive oxygen species and serous epithelial ovarian adenocarcinoma. Cancer Res J. 2016;4(6):106–114. doi:10.11648/j.crj.20160406.13
39. Ritter B, Greten FR. Modulating inflammation for cancer therapy. J Exp Med. 2019;216(6):1234–1243. doi:10.1084/jem.20181739
40. Shalapour S, Karin M. Pas de Deux: control of anti-tumor immunity by cancer-associated inflammation. Immunity. 2019;51(1):15–26. doi:10.1016/j.immuni.2019.06.021
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


