Back to Journals » OncoTargets and Therapy » Volume 13
Identification of a Novel MPRIP-ROS1 Fusion and Clinical Efficacy of Crizotinib in an Advanced Lung Adenocarcinoma Patient: A Case Report
Authors Shu Y, Li H, Shang H, Chen J, Su X, Le W, Lei Y, Tao L, Zou C, Wu W
Received 10 July 2020
Accepted for publication 11 September 2020
Published 13 October 2020 Volume 2020:13 Pages 10387—10391
DOI https://doi.org/10.2147/OTT.S270961
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Yun Shu,1,* Hui Li,2,* Hongjuan Shang,1 Jun Chen,1 Xiaoxing Su,2 Wei Le,1 Yan Lei,2 Liming Tao,1 Cailiang Zou,1 Wendy Wu2
1Department of Medical Oncology, Third People’s Hospital of Jiujiang City, Jiujiang 332000, People’s Republic of China; 2Berry Oncology Corporation, Fuzhou 350200, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wendy Wu Email [email protected]
Yun Shu Email [email protected]
Objective: ROS1 fusions have been identified in 1– 2% of non-small-cell lung cancer (NSCLC) patients; they are validated as a driver of carcinogenesis and could be subjected to inhibition by crizotinib. However, previous studies suggested a variable progression-free survival (PFS) ranging from 9.1 to 20.0 months for crizotinib treatment in ROS1-rearranged NSCLC. Here, we reported a 45-year-old female diagnosed with stage IVB lung adenocarcinoma with multiple lymph nodes and bone metastasis carrying a novel MPRIP-ROS1 fusion, which was identified by RNA-based NGS (next-generation sequencing) and was sensitive to crizotinib treatment.
Materials and Methods: A targeted NGS panel was used to analyze genomic DNA and total RNA isolated from formalin-fixed paraffin-embedded (FFPE) tissue block of the patient. An RNA fusion panel based on amplicon sequencing was designed for detection fusion variation. Fusion results were validated using reverse transcriptase polymerase chain reaction and Sanger sequencing.
Results: We reported a novel MPRIP-ROS1 fusion identified in this advanced lung adenocarcinoma case. The rearrangement was generated by exons 1– 21 of MPRIP at chr17: p11.2 joined to exons 35– 43 of ROS1 at chr6: q22.1, which retained an intact kinase domain of ROS1. The primary tumor and metastatic lymph nodes were eliminated on computed tomographic (CT) scan imaging after 2 months’ crizotinib treatment, and the multiple bone metastatic lesions were significantly relieved according to bone scintigraphy images. To date, the treatment has lasted 16 months, and the patient is still in follow-up showing sustained response to crizotinib.
Conclusion: The study identified a novel MPRIP-ROS1 fusion that was sensitive to crizotinib, which provided a new driver of lung adenocarcinoma and potential therapeutic target for crizotinib. It also expanded NSCLC treatment of ROS1 rearrangement and highlighted the importance of genetic testing for precise treatment decision-making.
Keywords: MPRIP-ROS1 fusion, advanced lung adenocarcinoma, sensitive to crizotinib, next-generation sequencing
Introduction
Lung cancer remains the leading malignancy worldwide, and non-small-cell lung cancer (NSCLC) accounts for approximately 80% of cases.1,2 Chromosome rearrangements involving the ROS proto-oncogene 1 receptor tyrosine kinase (ROS1) gene generally define a unique molecular subset and have been identified in 1–2% of NSCLC patients.3 The morbidity is slightly higher in East Asian populations, which reports a frequency of 2.4%.4 In terms of an estimated 1.5 million new cases of NSCLC worldwide each year,5 approximately 15,000–30,000 may be driven by oncogenic ROS1 fusions. To date, more than 20 different fusion partners for ROS1 have been reported in lung cancer, such as CD74, SLC34A2, EZR, SDC4 and TPM3.6 CD74-ROS1 fusion occurs most frequently in NSCLC, accounting for 40–45%. With the development of next-generation sequencing (NGS), numerous unknown gene fusions have been detected in parallel to other oncogenic mutations, and novel ROS1 fusion partner genes have been identified in lung cancer. Especially, RNA-based NGS technology is currently the most efficient strategy for exact fusion transcripts detection, which has been widely used in the molecular diagnosis of gene rearrangement.
All of the fusion genes involving ROS1 5ʹ deletion are identified retaining the tyrosine kinase domain. It is a key feature for ROS1 rearrangement to stimulate multiple downstream signaling pathways such as MAPK/ERK, PI3K/AKT and JAK/STAT to drive uncontrolled cellular proliferation, survival and migration.7 And the tyrosine kinase domain could also be the target region of the small molecule tyrosine kinase inhibitor, crizotinib, which had been found to be valid to ROS1/ALK/MET fusion. The efficacy data from the phase I PROFILE 1001 study demonstrated that advanced ROS1-rearranged metastatic NSCLC patients with crizotinib obtained an objective response rate (ORR) of 72% and median progression-free survival (PFS) of 19.2 months, and crizotinib consequently had been approved by US Food and Drug Administration (FDA) on March 11, 2016.5 However, subsequent studies have suggested a shorter PFS ranging from 9.1 to 15.9 months for crizotinib treatment in ROS1-rearranged NSCLC.8–12 More evidence is warranted for verifying whether different fusion partners can affect the therapeutic efficacy of ROS1 inhibitors, especially novel and rare ROS1 fusion.
Herein we reported a first MPRIP-ROS1 fusion advanced lung adenocarcinoma case identified by RNA-based NGS, which achieved a superior response to crizotinib, and expanded the therapeutic approaches for NSCLC patients carrying ROS1 rearrangement.
Case Presentation
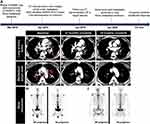
A 45-year-old Chinese female was admitted to the hospital in March 2019 with a 2-month history of cough and white sticky phlegm (Figure 1A). The patient had a history of no smoking, special medical history and cancer family history. The chest computed tomography (CT) scan showed an irregular mass in the left upper lung lobe with multiple mediastinal lymph nodes enlargement (Figure 1B and E). 99mTc-Methylene Diphosphate (MDP) bone scintigraphy images revealed that osseous destruction in the patient was characterized by multiple osteoblastic metastases, including the head of the right humerus, multiple ribs, multiple vertebras, pelvis and femurs (Figure 1H). No brain metastatic lesions were identified by cranial magnetic resonance imaging (figures not shown). The lung mass biopsy established the definitive pathologic diagnosis of invasive lung adenocarcinoma, and the clinical analysis indicated a stage IVB (cT4N3M1c).
With written informed consent, the formalin-fixed paraffin-embedded (FFPE) tissue acquired from the primary tumor biopsy was detected by targeted NGS method. A novel ROS1 rearrangement involving myosin phosphatase Rho interacting protein (MPRIP) gene was identified by RNA fusion panel based on amplicon sequencing (Berry Oncology Corporation), which revealed the harboring of MPRIP exon 21-ROS1 exon 35 rearrangement (Figure 2A and B). The breakpoints and fusion sequence were further confirmed using reverse transcriptase polymerase chain reaction (RT-PCR) followed by Sanger sequencing (Figure 2C and D). The novel MPRIP-ROS1 fusion retained the coiled-coil domains of MPRIP and the intact kinase domain of ROS1, which could result in constitutive kinase activity and oncogenic transformation.
Based on the identification of this novel ROS1 fusion, crizotinib was given to the patient at a dose of 250 mg twice daily from April 10, 2019. Two months later (June 2019), a complete response in the primary lung tumor was achieved according to chest CT scan imaging, showing that the left upper lobe mass and metastatic lymph nodes were eliminated (Figure 1C and F). After 9 months of crizotinib treatment (January 2020), no tumor and no metastasis were found in lung (Figure 1D and G), and bone scintigraphy images demonstrated that multiple bone metastatic lesions were significantly relieved (Figure 1I). No recurrence was observed until now (August 2020), yielding a progression-free survival of at least 16 months, and the patient is still in follow-up for progression.
Discussion
In this case study, we reported a novel MPRIP-ROS1 fusion in a young female non-smoker with advanced lung adenocarcinoma. MPRIP (myosin phosphatase Rho interacting protein) includes 25 exons and harbors three coiled-coil domains, which makes a promising feature of 5′ fusion gene partners to activate tyrosine kinase domain in 3′ partners. To our knowledge, MPRIP has been described as fusion partner of ALK, RET and neurotrophic receptor tyrosine kinase 1 (NTRK1) in lung cancer.13–15 This is the first case report of MPRIP joined with ROS1 in lung adenocarcinoma. The novel rearrangement retained the coiled-coil domains of MPRIP and the intact kinase domain of ROS1, presumably leading to dimerization or high-level expression and subsequent activation of ROS1 kinase domain.
ROS1 rearrangements are a well-known driver mutation of NSCLC and are often highly susceptible to small-molecule tyrosine kinase inhibitors (TKIs); they typically occur in young, never or light smokers with histologic features of pulmonary adenocarcinoma.3 Three prospective trials demonstrated that crizotinib was effective in ROS1-rearranged advanced NSCLC patients across differing ethnicities. The phase I PROFILE 1001 study in the United States reported a median PFS of 19.2 months in ROS1-rearranged metastatic NSCLC.5 Prospective phase II studies in European and East Asian patients with advanced ROS1-positive NSCLC confirmed the activity of crizotinib, with a median PFS of 20.0 and 15.9 months, respectively.9,16 However, retrospective data of patients with ROS1-rearranged NSCLC showed lower responses to crizotinib than the prospective studies. The EUROS1 Cohort study reported that ROS1 fusion patients treated with crizotinib had a median PFS of 9.1 months.8 A Korean study showed a median PFS of 12.7 months for TKIs.11 Two retrospective studies consisting entirely of Chinese NSCLC patients with ROS1 rearrangement revealed a median PFS of 9.8–12 months for crizotinib.10,12
The patient in this present case had a significant and sustained response to crizotinib, with at least 16 months' PFS and is still in follow-up. The clinical outcome was comparable with published efficacy of crizotinib for canonical ROS1 rearrangements and showed a more prominent response than pemetrexed-based chemotherapy with a median PFS of 6–8 months.10,11 Our report revealed that MPRIP-ROS1 fusion represented a potential therapeutic target for crizotinib, which highlighted the significance of NGS methodology to discover new drug-sensitive genetic variations and expanded the comprehensive molecular profiling of ROS1 fusion to optimize clinical treatment and improve survival.
In summary, a 45-year-old female non-smoker patient had been diagnosed with advanced lung adenocarcinoma with bone metastasis. A novel MPRIP-ROS1 fusion was identified through NGS method, and the consequent crizotinib treatment achieved superior response. The primary tumor and metastatic lymph nodes were eliminated on CT scan imaging, and the multiple bone metastatic lesions were significantly relieved according to bone scintigraphy images. This report identified a novel MPRIP-ROS1 fusion, which makes a potential therapeutic target for crizotinib. It provided new insight into NSCLC treatment of ROS1 rearrangement and highlighted the importance of genetic testing for precise therapy aiming at the maximum clinical benefit to NSCLC patients.
Ethics Statement
The study was approved by the Ethical Committee of Medical Research, Third People’s Hospital of Jiujiang City.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Hui Li, Xiaoxing Su, Yan Lei and Wendy Wu are employees of Berry Oncology Corporation. The other authors declare no conflicts of interest.
References
1. Liao Y, Wang Y, Cheng M, Huang C. Weighted gene coexpression network analysis of features that control cancer stem cells reveals prognostic biomarkers in lung adenocarcinoma. Front Genet. 2020;11:311. doi:10.3389/fgene.2020.00311
2. Liao Y, Xiao H, Cheng M. Bioinformatics analysis reveals biomarkers with cancer stem cell characteristics in lung squamous cell carcinoma. Front Genet. 2020;11:427. doi:10.3389/fgene.2020.00427
3. Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. doi:10.1200/JCO.2011.35.6345
4. Chen YF, Hsieh MS, Wu SG, et al. Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. J Thorac Oncol. 2014;9(8):1171–1179. doi:10.1097/JTO.0000000000000232
5. Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. doi:10.1056/NEJMoa1406766
6. Lin JJ. Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol. 2017;12(11):1611–1625. doi:10.1016/j.jtho.2017.08.002
7. Davies KD. Doebele RC: molecular pathways: ROS1 fusion proteins in cancer. Clin Cancer Res. 2013;19(15):4040–4045. doi:10.1158/1078-0432.CCR-12-2851
8. Mazieres J, Zalcman G, Crino L, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33(9):992–999. doi:10.1200/JCO.2014.58.3302
9. Wu YL, Yang JC, Kim DW, et al. Phase ii study of crizotinib in east asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–1411. doi:10.1200/JCO.2017.75.5587
10. Zhang L, Jiang T, Zhao C, et al. Efficacy of crizotinib and pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 rearrangement. Oncotarget. 2016;7(46):75145–75154. doi:10.18632/oncotarget.12612
11. Park S, Ahn BC, Lim SW, et al. Characteristics and outcome of ROS1-positive non-small cell lung cancer patients in routine clinical practice. J Thorac Oncol. 2018;13(9):1373–1382. doi:10.1016/j.jtho.2018.05.026
12. He Y, Sheng W, Hu W, et al. Different types of ROS1 fusion partners yield comparable efficacy to crizotinib. Oncol Res. 2019;27(8):901–910. doi:10.3727/096504019X15509372008132
13. Fang W, Gan J, Hong S, Lu F. MPRIP-ALK, a novel ALK rearrangement that responds to alk inhibition in NSCLC. J Thorac Oncol. 2019;14(7):e148e151. doi:10.1016/j.jtho.2019.02.030
14. Fang P, Yan Z, Liu W, et al. Detection of a novel RET gene fusion in a non-small cell lung cancer patient using AMP chemistry. J Thoracic Oncol. 2016;11(2):S21S22. doi:10.1016/j.jtho.2015.12.035
15. Vaishnavi A, Capelletti M, Le AT, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med. 2013;19(11):1469–1472.
16. Michels S, Massuti B, Schildhaus HU, et al. Safety and efficacy of crizotinib in patients with advanced or metastatic ros1-rearranged lung cancer (EUCROSS): A European phase ii clinical trial. J Thorac Oncol. 2019;14(7):1266–1276. doi:10.1016/j.jtho.2019.03.020
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.