Back to Journals » Cancer Management and Research » Volume 13
Identification and Functional Validation of Differentially Expressed microRNAs in Ascites-Derived Ovarian Cancer Cells Compared with Primary Tumour Tissue
Authors Jiang Y, Shi Y, Lyu T, Liu H, Shen L, Zhou T, Feng W
Received 19 May 2021
Accepted for publication 5 August 2021
Published 21 August 2021 Volume 2021:13 Pages 6585—6597
DOI https://doi.org/10.2147/CMAR.S320834
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Yahui Jiang,* Yiwen Shi,* Tianjiao Lyu, Hua Liu, Lifei Shen, Tianyu Zhou, Weiwei Feng
Department of Gynecology and Obstetrics, Ruijin Hospital, Shanghai Jiaotong University, School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weiwei Feng
Department of Gynecology and Obstetrics, Ruijin Hospital, Shanghai Jiaotong University, School of Medicine, Shanghai, 200025, People’s Republic of China
Tel/Fax +86-21-64370045
Email [email protected]
Purpose: Ovarian cancer, manifested by malignant ascites, is the most lethal gynaecological cancer. Suspended ascites-derived spheroids may contribute to ovarian cancer metastasis. MicroRNAs (miRNAs) are also associated with ovarian cancer metastasis. Here, we aimed to investigate the differentially expressed miRNAs (DE-miRNAs) in ascites-derived spheroids compared with primary tumour tissues, which may regulate ovarian cancer metastasis.
Methods: The DE-miRNAs between ovarian cancer primary tumour tissues and ascites-derived spheroids were identified by GEO2R screening in samples from 3 high-grade serous ovarian cancer (HGSOC) patients of dataset GSE65819. We used MiRTarBase, TargetScanHuman7.2 and STRING to predict the target hub genes of DE-miRNAs and DAVID to perform functional analysis of hub genes. ALGGEN PROMO and TransmiR v2.0 were used to predict transcription factors (TFs) that potentially regulate DE-miRNAs expression. The observed differences in DE-miRNAs expression were validated with samples from 12 HGSOC patients and 2 ovarian cancer cell lines using PCR. The functions of DE-miRNAs on ovarian cancer progression were verified by invasion, adherent, and angiogenesis assays.
Results: Through bioinformatics screening and experimental validation, miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-126-3p and miR-145-5p were identified as being significantly downregulated in ascites-derived spheroids compared with primary tumour tissues. In addition, TFAP2A was identified as a potentially common upstream TF regulating the expression of the above mentioned DE-miRNAs. The overexpression of miR-199a-3p, miR-199b-3p, miR-199a-5p lead to invasion inhibition, and the overexpression of miR-126-3p, miR-145-5p, miR-199a-5p and miR-199b-3p lead to adhesion inhibition of suspended ovarian cancer cells. High-expressed miR-126-3p, miR-199a-3p, miR-199a-5p and miR-199b-3p contributed to apoptosis of suspended ovarian cancer cells.
Conclusion: The downregulated expression of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-126-3p and miR-145-5p in ascites-derived spheroids plays a key role in promoting ovarian cancer progression, which may represent novel molecules for targeted therapy for ovarian cancer.
Keywords: ovarian cancer, metastasis, ascites-derived spheroids, primary tumour tissue, microRNAs
Introduction
Ovarian cancer has become the most lethal gynaecological cancer worldwide. Since there is no obvious clinical presentation in the early phase, ovarian cancer patients are mostly diagnosed in the late stages, presenting with ascites accumulation in the abdominal cavity. The presence of malignant ascites correlates with a deterioration in the quality of life and poor prognosis.1 The ovarian cancer cells are shed from the primary tumour site, subsequently becoming suspended in the ascites and forming spheroids. Finally, the spheroids, which are highly invasive, adhere to the peritoneum and form a new metastasis tumour site. Thus, spheroids appear to play a key role in ovarian cancer implantation metastasis, and the unique expression profile of suspended ascites-derived tumour cells deserves intensive study.
During the process which shed ovarian cancer cells become suspended spheroids, the expression of genes could be regulated by epigenetic plastic happened in this period.2 microRNAs (miRNAs), as the essential member of epigenetic regulation, also involved in this epigenetic plastic process. However, the expression differences of miRNAs between ovarian cancer spheroids and primary ovarian cancer cells had not been studied in-depth.
miRNAs are endogenously expressed, small (19–25 nucleotides), noncoding RNAs that are cleaved from 70 to 100 nucleotide hairpin precursors called pre-miRNAs. Once the pre-miRNAs reach the cytoplasm, under the actions of various enzymatic reactions, they are cleaved into mature miRNAs that downregulate the expression of their targets by inhibiting mRNA translation or promoting mRNAs degradation. miRNAs are estimated to control over 50% of the activities of all protein coding genes and are unsurprisingly involved in ovarian cancer oncogenesis.3 miRNAs can act as potential biomarkers, diagnostic tools and therapeutic targets of ovarian cancer. For instance, the upregulation of miR-92, miR-21 and miR-19a has been used as a sign of ovarian cancer, and cancer cell-secreted microvesicles contain elevated levels of miR-21, which induces myoblast apoptosis and lead to cancer cachexia.4
Bioinformatics approaches have become an effective and systematic means of screening potential target molecules, and the Gene Expression Omnibus (GEO) database harbours a vast amount of gene expression profiles that are useful in distinguishing differentially expressed genes.5 In our study, to identify the differentially expressed miRNAs (DE-miRNAs) between ovarian cancer primary tumour tissue and ascites-derived cancer cells, we screened the miRNA expression profile of the microarray dataset GSE65819. The potential downstream target genes of DE-miRNAs were predicted using miRTarBase and TargetScanHuman7.2, and their biological significances were enriched by gene ontology (GO) annotation and Kyoto Encyclopedia of Gene and Genomes (KEGG) pathway analysis. By drawing a protein–protein interaction (PPI) network, we identified the hub target genes. Subsequently, we used human ovarian cancer samples and ovarian cancer cell lines to verify the results of bioinformatic analysis. The goal of this study was to identify miRNAs that may promote the metastasis of ovarian cancer ascites-derived cancer cells and to further investigate their potential molecular mechanisms by bioinformatic analysis and experimental validation.
Materials and Methods
Selection of Microarray Data from GEO Database
To analyse the differential expression of miRNAs between ovarian cancer primary tumour and ascites-derived tumour cells, we screened GEO datasets and selected the microarray dataset GSE65819 for further study. This dataset is based on the GPL19765 platform (NanoString nCounter miRNA Human v2.1), which contains samples of high-grade serous ovarian cancer (HGSOC) patients. After checking the basic information (including case ID, sample point and sample type) for each sample, we chose data from 3 patients` matched primary tumours and ascites-derived tumour cells, which were collected as soon as the patients were diagnosed as HGSOC without any therapy.
Screen for DE-miRNAs
GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/) can be used to perform modern R language analyses of GEO information under the same trial conditions.5 Therefore, GEO2R was used to screen the DE-miRNAs between primary tumours and ascites-derived tumour cells of ovarian cancer patients. P-value <0.05 and |fold change (FC)|> 2 were set as the criteria for identifying DE-miRNAs.
Prediction of Target Genes
MiRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php) is an experimentally validated miRNA-target interactions database.6 TargetScanHuman7.2 can predict biological targets of miRNAs by searching for the presence of conserved sites that match the seed region of each miRNA.7 We used both miRTarBase and TargetScanHuman7.2 to predict the target downstream genes of the top five most downregulated miRNAs.
Analysis of GO and KEGG Pathway
DAVID bioinformatics resource (https://david.ncifcrf.gov) consists of an integrated biological knowledgebase and analytic tools aimed at systematically extracting biological meaning from large gene lists.8 Therefore, DAVID was used to perform functional and pathway enrichment of the predicted target genes of the five most downregulated miRNAs, including GO and KEGG pathway analysis.
Construction of the PPI Network and DE-miRNA-Hub Gene Network
The STRING database (http://string-db.org) provides uniquely comprehensive coverage and ease of access to both experimental and predicted protein interaction information.9 We used the STRING database to obtain functional associations among the target genes of DE-miRNAs. To identify the hub genes of DE-miRNAs, the Cytoscape (3.7.0) plugin cytohubba was used to calculate the degree of each target gene. The top 20 target genes were identified as hub genes, and the DE-miRNA-hub gene network was established using Cytoscape.
Prediction of Transcription Factors(TFs) of DE-miRNAs
The potential TFs of DE-miRNAs were predicted using ALGGEN PROMO (http://alggen.lsi.upc.es) and TransmiR v2.0 (http://www.cuilab.cn/transmir).10,11 We took the intersection of the results obtained from ALGGEN PROMO and TransmiR v2.0 of each DE-miRNAs and drew a network diagram to display the regulation effects of TFs on DE-miRNAs.
cDNA Synthesis and Quantitative Polymerase Chain Reaction (qPCR)
TRIzol reagent (Invitrogen) was used to extract total RNA from cells from the different treatments. Reverse transcription was performed using a BioTNT microRNA reverse transcription kit (BioTNT, Shanghai, China), and the primers used for reverse transcription of miRNAs were also synthesized by BioTNT Co. Ltd. qPCR was performed using a BioTNT microRNA Real-Time PCR PreMIX kit and a qPCR system (QuantStudio TM 6 Flex, Thermo-ABI). The miRNAs expression levels were normalized to expression of U6 as an internal control and calculated by the ΔΔCt method.
Human Samples Collection
The primary tumour tissues and ascites were harvested from patients who were diagnosed with HGSOC in 2017–2019 in the Department of Gynaecology and Obstetrics, Ruijin Hospital. According to the inclusion criteria, (histologically diagnosed to be HGSOC combined with obvious ascites and no treatment before receiving tumour reductive surgery), tissues were collected from 12 patients for further experiments. The average age of these 12 patients were 55. The tumour stage of 5 patients were IIIc, and the other 7 patients were IV. All experiments were approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine, and informed consent was obtained from all patients.
Cell Lines and Culture Conditions
The human ovarian cancer cell line HEY was obtained from the laboratory of Dr Robert Bast at the University of Texas MD Anderson Cancer Center, Houston, TX. The human ovarian cancer cell lines A2780 were obtained from the Shanghai Key Laboratory of Female Reproduction Endocrine Related Disease, Obstetrics and Gynaecology Hospital, Fudan University. The use of A2780 was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine.
For adherent cultures (2D cultures), cells were cultured in 100-mm TC-treated Culture Dishes (430167, Corning) with RPMI 1640 medium containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C under an atmosphere with 5% CO2.
For suspended cultures (3D cultures), cells were cultured in 100-mm Ultra-Low Attachment Culture Dishes (3262, Corning) with RPMI 1640 medium containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C under an atmosphere with 5% CO2.
MiRNA Overexpression
The miRNA mimics and negative control were purchase from RiboBio (Guangzhou, China). We added 5μL of 5nM miRNA mimics and negative control into 6-well plate to make HEY cell lines transfected in the presence of LipofectamineTM 3000 according to the manufacturer’s protocol and verified the overexpression efficiency by qPCR.
TFAP2A Silencing
A TFAP2A-specific siRNA duplex (siTFAP2A, sequence: 5ʹ-GUAGGUCAAUCUCCCUACAtt-3ʹ) was synthesized by Genomeditech (Shanghai, China). NC (5ʹ-UUCUCCGAACGUGUCACGUdTdT-3ʹ) was used as a negative control. For siRNA transfection, 1.5*105 HEY cells/well or 3*105 A2780 cells/well were seeded in a 6-well plate and transfected according to the manufacturer’s protocol and verified the efficiency by qPCR.
Transwell Invasion Assay
The transwell system (24-well insert; pore size 8 mm; Corning Costar) was used to measure the invasive ability of the HEY cell line. The inserts were coated with 50 µL Matrigel at 1:8 dilution (BD Biosciences Pharmingen) overnight, and 3×104 cells suspended in 0.2 mL of fresh medium without FBS were added to the upper well of the chamber. Then, 600 µL of complete medium with 10% FBS was added to the lower well. After incubating for 16 h, the cells on the upper surface of the membrane were swiped off with cotton swabs, and the cells adhering to the lower surface of the inserts were fixed and stained with haematoxylin. The number of stained cells in five representative fields were randomly counted for each insert using an Olympus light microscope at 100× magnification.
Apoptosis Assay
Apoptosis assay was conducted according to the protocol of FITC Annexin V Apoptosis Detection Kit (BD Pharmingen, 556547). Briefly, cells were washed with cold PBS and resuspended in 1x binding buffer at a concentration of 1*106 cells/mL. Add 5 μL of FITC Annexin V and 5μL PI into 100μL above cell suspension. Gently vortex and incubate for 15 min at room temperature in the dark. Then, the samples were analyzed by flow cytometry within 1 hour.
Adherent Assay
The 24-well plate was coated with 200μL Matrigel at 1:50 dilution and air-dried in biosafety cabinet for 6h. Then, the plate was blocked with 200μL serum-free medium for 1h. 3D-cultured cells were trypsinized, counted and resuspended in serum-free medium. 3*104 cells/200μL serum-free medium were added to each Matrigel-coated well and incubated in the incubator for 1.5–2h. Non-adherent cells were removed by washing with PBS. Adherent cells were fixed with 4% paraformaldehyde and stained with 2% crystal violet. Five representative fields of each insert were photographed and counted using an Olympus light microscope at 40×magnification.
Statistical Analysis
All data are presented as the Mean±SD. A two tailed, unpaired t-test was used to compare the differences between two group by SPSS and GraphPad Prism 8. We considered p value <0.05 as statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
Screening DE-miRNAs Between Ovarian Cancer Primary Tumours and Ascites-Derived Tumour Cells
The microRNA array GSE65819 contains samples collected from HGSOC patients at different time points. The sample types included primary tumour tissue, ascites-derived tumour cells and metastasis tumour tissue. We selected the microRNA array results of the primary tumour tissue and ascites-derived tumour cells harvested from 3 HGSOC patients at the first time they were diagnosed with HGSOC (Figure 1A). Using GEO2R to analyse the DE-miRNAs from the 3 pairs of primary tumour tissue and ascites-derived tumour cells, we identified 69 DE-miRNAs, among which 68 were downregulated and only miR-1245a was upregulated in ascites-derived tumour cells. MiR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p were the top 5 most downregulated miRNA in ascites-derived tumour cells compared with the primary tumour tissues (Figure 1B and C).
Prediction of Target Genes of DE-miRNAs and Pathway Enrichment Analysis
We used miRTarBase and TargetScanHuman7.2 to predict the target downstream genes of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p, resulting in the identification of 796 and 648 probable target genes, respectively. Two hundred and forty-four genes were identified as target genes by taking the intersection. After KEGG pathway analysis, we observed that the target genes of DE-miRNAs were primarily enriched in “proteoglycans in cancer”, “pathways in cancer” and “focal adhesion”. Regarding the GO analysis, “negative regulation of apoptotic”, “phosphatidylinositol mediated signaling”, “positive regulation of transcription from RNA polymerase II promoter” and “positive regulation of cell migration” were the most significant terms in biological process (BP); “cytoplasm”, “SMAD protein complex” and “nuclear chromatin” were the most significant terms in cellular component (CC); and “protein binding”, “protein heterodimerization activity” and “Identical protein binding” were the most significant terms in molecular function (MF). (Figure 2B–E)
Identification of Hub Target Genes of DE-miRNAs
To identify the hub target genes of DE-miRNAs, a PPI network was evaluated by using the STRING database and visualized with Cytoscape. Considering the degree of the target genes, VEGFA, MAPK8, IGF1, SMAD4, SMAD2, GSK3B, MTOR, SMAD3, SIRT1, IGF1R, FGF2, CD44, CAV1, SNAI1, ACTB, PIK3R1, PXN, CDH2, HIF1A and RPS6KB1 were top 20 genes with the highest degree values and were identified as hub genes of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p. The PPI network is shown in Figure 2A.
Identification of Potential Upstream TFs of DE-miRNAs
miRNAs regulate the expression of their target genes, but the expression of miRNAs themselves can also be regulated by TFs. miR-199a-1, miR-199a-2, miR-199b, miR-145 and miR-126 are the pre-miRNAs of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p. We used ALGGEN PROMO and TransmiR v2.0 to predict the probable TFs that regulate the 5 pre-miRNAs mentioned above. After taking the intersection of the TFs predicted by ALGGEN PROMO and TransmiR v2.0 of each pre-miRNA, we found that miR-126 could be regulated by MAZ, AR, USF1, RelA and TFAP2A; miR-145 could be regulated by VDR and TFAP2A; miR-199a-1 could be regulated by TFAP2A; miR-199a-2 could be regulated by AP-1 and YY1; miR-199b could be regulated by TFAP2A (Figure 3A). As a result, we observed that TFAP2A was the common TF that might regulate all 5 pre-miRNAs mentioned above and further affect the expression of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p. Therefore, we down-regulated the expression of TFAP2A in 3D-cultured HEY and A2780. It could be seen that the expression of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p were decreased when TFAP2A was silenced in 3D-cultured HEY and A2780, which meant that TFAP2A could regulate the expression of DE-miRNAs (Figure 3B).
Verification of DE-miRNA Expression Differences Between Primary Tumours and Ascites-Derived Tumour Cells Using Ovarian Cancer Patient Samples and Ovarian Cell Lines
We collected primary tumours and ascites-derived tumour cells from 12 ovarian cancer patients from 2017 to 2019. After evaluating the expression levels of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p, we observed that the expression of miR-199a-3p, miR-199b-3p, miR-199a-5p and miR-145-5p was lower in ascites-derived tumour cells than in the primary tumour (p<0.01). The ascites-derived tumour cells from 11 of 12 patients had a lower miR-199b-3p expression level than that of the primary tumour in (p < 0.01) (Figure 4A).
To investigate whether this phenomenon could also be observed using ovarian cancer cell line models, we use 2D and 3D cultures to mimic the growth states of primary tumours ascites-derived tumour cells, respectively. The results showed that compared with the 2D culture, miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p were decreased in the 3D-cultured HEY cell line. The expression of miR-199a-3p, miR-199b-3p, miR-145-5p and miR-126-3p but not miR-199a-5p was decreased in the 3D-cultured A2780 cell line (Figure 4B).
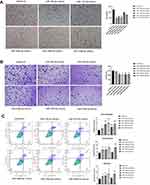
Effects of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miRNA-126-3p on Invasive, Adherent and Apoptosis Abilities of Ovarian Cancer Cells
The KEGG pathway and GO function enrichment showed in Figure 2B and C revealed that positive regulation of cell migration, focal adhesion and negative regulation of apoptotic process were dominant functions of target genes belongs to DE-miRNAs. Therefore, we used transwell invasion assay, adhesion assays and apoptosis assay to investigate the effects of DE-miRNAs on regulating the apoptosis and metastatic capacity of 3D-cultured HEY cell line. After overexpressing miR-199a-3p, miR-199b-3p and miR-199a-5p, the invasion ability of 3D-cultured HEY cells were inhibited (p = 0.0398, p = 0.0485 and p=0.0409, respectively, Figure 5A). The adhesive abilities of 3D-cultured HEY cells were abrogated when miR-126-3p, miR-145-5p, miR-199a-5p and miR-199b-3p were overexpressed respectively (p = 0.0028, p = 0.0009, p = 0.0010 and p = 0.0171, respectively), which meant that miR-126-3p, miR-145-5p, miR-199a-5p and miR-199b-3p could suppress adhesion ability of ovarian cancer cell (Figure 5B). As it is showed in Figure 5C, miR-126-3p, miR-199a-3p and miR-199a-5p could promote the apoptosis of 3D-cultured HEY cells in both early apoptosis phase and late apoptosis phase (p=0.0036, p = 0.0124 and p = 0.0077, respectively). Besides, miR-199b-3p could also enhance the apoptosis of 3D-cultured HEY cells, but mainly in early apoptosis phase (p = 0.0323). In summary, miR-199a-3p, miR-199b-3p and miR-199a-5p could inhibit suspended ovarian cancer cells invasion, while miR-126-3p, miR-145-5p, miR-199a-5p and miR-199b-3p could inhibit suspended ovarian cancer cells adhesion. And miR-126-3p, miR-199a-3p, miR-199a-5p and miR-199b-3p contributed to the apoptosis of suspended ovarian cancer cells.
Discussion
The primary metastatic route of ovarian cancer is peritoneal dissemination, which is responsible for the greatest morbidity and mortality in ovarian cancer patients. Tumour cells shed from the primary tumour and become suspended in ascites to implant in the peritoneum and form new metastasis tumour sites. Thus, shed tumour cells carried in ascites are important for metastasis process. MiRNAs are small, highly conserved noncoding RNA molecules that play an important role in ovarian cancer metastasis. They are also considered to be potential biomarkers for monitoring ovarian cancer.12 However, little research had focused on miRNA expression profile variations in ovarian cancer cells from ascites shed from primary tumour sites. Therefore, the goal of the present study was to elucidate the dominant differential miRNAs in ascites-derived tumour cells compared with the primary tumour sites and determine the contribution of these miRNAs to ovarian cancer metastasis.
The results showed that the expression changes of miRNAs in ascites-derived tumour cells primarily involved their downregulation, with miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p exhibiting the most significant changes. Through the functional analysis of these miRNAs, we observed that they may be involved in the regulation of invasive, adherent and apoptosis abilities of ascites-derived tumour cells. Several studies have investigated the relationship of the above miRNAs with ovarian cancer. Lower miR-199a expression was shown to be significantly correlated with a poor prognosis of ovarian cancer.13 As the mature miRNAs of miR-199a, miR-199a-3p and miR-199a-5p can also manipulate ovarian cancer development. Most studies of miR-199a-3p have shown that it can enhance the cisplatin sensitivity of ovarian cancer cells via a unique mechanism, such as targeting ITGB814 or DDR1.15 MiR-199a-3p and miR-199a-5p were also demonstrated to be ovarian cancer suppressors by inhibiting cancer cell proliferation and invasion.16,17 MiR-199a-3p was also shown to inhibit ovarian cancer proliferation and invasion by targeting PLXNB2.18 The interaction network of noncoding RNAs is essential for tumour progression. Several noncoding RNAs, such as CircMUC16,19 lncRNA LUCAT120 and lncRNA NORAD21 promote ovarian cancer progression through miR-199a-3p and miR-199a-5p. Pan et al reported that miR-126 was underrepresented in exosomes from the plasma of epithelial ovarian cancer patients compared with that of healthy women.22 MiR-145 was shown to serve as an outstanding biomarker of ovarian cancer since it is significantly downregulated in the sera of ovarian cancer patients compared to healthy women.23 It was also reported that miR-145 can suppress ovarian cancer cell growth and invasion by targeting CCD2, E2F324 and TRIM2.25 Many noncoding RNAs such as lncRNA-ROR26 and circ-ITCH27 play a role in regulating ovarian cancer though miR-145. However, little research has been performed on miR-145-5p, which is its mature miRNA. Our results showed that miR-199a-3p, miR-199a-5p and miR-199b-3p could suppress the invasion of spheroid ovarian cancer cells and miR-126-3p, miR-145-5p, miR-199a-5p and miR-199b-3p could suppress the adhesion of spheroid ovarian cancer cells. In addition, miR-126-3p, miR-199a-3p, miR-199a-5p and miR-199b-3p contributed to the apoptosis of spheroid ovarian cancer cell. These findings might explain why spheroid cells had an enhanced capability in metastasis and a required anoikis resistance.28–30
Then, the differential expressions of these five DE-miRNAs obtained from bioinformatics screening were further verified in human sample and ovarian cancer cell lines. Using 12 pairs of primary tumour tissues and ascites-derived tumour cells collected from ovarian cancer patients to validate the observed expression differences in the identified DE-miRNAs, we showed that miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-126-3p and miR-145-5p were significantly decreased in suspended ascites-derived tumour cells compared with primary tumour tissues, supporting the bioinformatics screening results. In addition, we performed adherent and suspended cultivation of ovarian cancer cells lines to mimic the growth environment characteristics of primary tumour tissue and ascites-derived tumour cells to further validate the decreased expression of the identified DE-miRNAs when ovarian tumour cells are suspended in ascites. 3D-cultured HEY cell lines showed decreased expression of 5 miRNAs as compared to 2D-culture. However, only 4 microRNAs except for miR-145-5p were reduced the expression level in the 3D-cultured A2780 cell lines, which did not totally consistent with HEY cell lines and patient sample validation results. Therefore, we presume that the tumour microenvironment may play a role in DE-miRNA expression change when ovarian cancer cells shed from the primary tumour and become suspended in ascites, since the survival of ovarian cancer cells depends on numbers of other components, such as stromal cells and immune cells in the tumour microenvironment. The mechanism associated with this process requires further in-depth study.
Since miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p were significantly downregulated in ascites-derived ovarian cancer tumour cells, the mechanism associated with this phenomenon was intriguing. The level of mature miRNA expression is associated with that of its precursor. For example, the mature miRNAs miR-199a-3p and miR-199a-5p are formed after cleavage from their precursor pre-miR-199a (one in chromosome 1 called miR-199a-2, another in chromosome 19 called miR-199a-1).31 Therefore, we predicted the probable upstream TFs that could potentially regulate the pre-miRNAs of DE-miRNAs. As the network diagram showed in Figure 3A, TFAP2A may be the common TF that can control the expression of all the pre-miRNAs of the identified DE-miRNAs. However, the ability of TFAP2A to regulate the abovementioned pre-miRNAs has yet to be reported. In our study, we found that the expression of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-145-5p and miR-126-3p in 3D-cultured ovarian cell lines was decreased when TFAP2A was down-regulated. According to the results of previous studies, TFAP2A favours the survival of ovarian cancer patients, with a potential mechanism being that TFAP2A may suppress ovarian cancer invasion and peritoneal carcinomatosis.32
In summary, in the present study, we confirmed that the expression of miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-126-3p and miR-145-5p was reduced in ascites-derived tumour cells compared with primary tumour tissues, which may contribute to ovarian cancer peritoneal metastasis by promoting invasion, adherent and resisting apoptosis. TFAP2A was predicted to be a common transcription factor and could regulate the expression of the above 5 miRNAs. In addition, the tumour microenvironment might give rise to the aberrant expression of miRNAs in ascites-derived tumour cells, and the underlying mechanisms need to be researched by further studies.
Conclusions
In summary, miR-199a-3p, miR-199b-3p, miR-199a-5p, miR-126-3p and miR-145-5p were significantly downregulated in ascites-derived ovarian cancer cells compared with primary tumour tissues. TFAP2A may be a common upstream TF that can regulate the expression of the identified DE-miRNAs. The results of the present study suggest that increased expression of miR-199a-3p, miR-199b-3p and miR-199a-5p inhibits the invasion of suspended ascites-derived tumour cells and increased miR-126-3p, miR-145-5p, miR-199a-5p and miR-199b-3p inhibit the adhesion of suspended ascites-derived tumour cells. Overexpression of miR-126-3p, miR-199a-3p, miR-199a-5p and miR-199b-3p contributed to apoptosis of suspended ascites-derived tumour cells. Further study on the identified DE-miRNAs and their functions could be useful in the treatment of ovarian cancer.
Abbreviations
miRNAs, microRNAs; DE-miRNAs, differentially expressed miRNAs; TFs, transcription factors; GEO, Gene Expression Omnibus; KEGG, Kyoto Encyclopedia of Gene and Genomes; PPI, protein–protein interaction; HGSOC, high-grade epithelial ovarian cancer; BP, biological process; CC, cellular component; MF, molecular function.
Data Sharing Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE65819. Other raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Approval and Informed Consent
All experiments were approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University, School of Medicine.
Consent for Publication
The participant has consented to the submission of the article to the journal.
Acknowledgments
We would like to thank all the patients that agreed with dedicating their ovarian cancer samples. We wish them good health. In addition, we appreciated to all the efforts that was made by the surgeons in our department. And we would like to express our gratitude to the support given by Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (Grant No. 20172003), Ruijin Youth NSFC Cultivation Fund (Grant No. 2019QNPY02014), Guangci Distinguished Young Scholars Training Program of Shanghai Jiaotong University School of Medicine affiliated Ruijin Hospital (GCQN-2019-B12) and Nature Science Foundation of Shanghai(20ZR1433700).
Funding
This work was supported by the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (Grant No. 20172003), Ruijin Youth NSFC Cultivation Fund (Grant No. 2019QNPY02014), Guangci Distinguished Young Scholars Training Program of Shanghai Jiaotong University School of Medicine affiliated Ruijin Hospital (GCQN-2019-B12) and Nature Science Foundation of Shanghai(20ZR1433700).
Disclosure
The authors declare that there is no conflict of interest.
References
1. Kim S, Kim B, Song YS. Ascites modulates cancer cell behavior, contributing to tumor heterogeneity in ovarian cancer. Cancer Sci. 2016;107(9):1173–1178. doi:10.1111/cas.12987
2. Bapat SA, Jin V, Berry N, et al. Multivalent epigenetic marks confer microenvironment-responsive epigenetic plasticity to ovarian cancer cells. Epigenetics. 2010;5(8):716–729. doi:10.4161/epi.5.8.13014
3. Deb B, Uddin A, Chakraborty S. miRNAs and ovarian cancer: an overview. J Cell Physiol. 2018;233(5):3846–3854. doi:10.1002/jcp.26095
4. He WA, Calore F, Londhe P, Canella A, Guttridge DC, Croce CM. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc Natl Acad Sci U S A. 2014;111(12):4525–4529. doi:10.1073/pnas.1402714111
5. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41(Database issue):D991–D995. doi:10.1093/nar/gks1193
6. Chou CH, Shrestha S, Yang CD, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46(D1):D296–d302. doi:10.1093/nar/gkx1067
7. Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005. doi:10.7554/eLife.05005
8. Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi:10.1038/nprot.2008.211
9. Szklarczyk D, Franceschini A, Kuhn M, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39(Databaseissue):D561–D568. doi:10.1093/nar/gkq973
10. Tong Z, Cui Q, Wang J, Zhou Y. TransmiR v2.0: an updated transcription factor-microRNA regulation database. Nucleic Acids Res. 2019;47(D1):D253–d258. doi:10.1093/nar/gky1023
11. Farré D, Roset R, Huerta M, et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31(13):3651–3653. doi:10.1093/nar/gkg605
12. Staicu CE, Predescu DV, Rusu CM, et al. Role of microRNAs as clinical cancer biomarkers for ovarian cancer: a short overview. Cells. 2020;9(1):169. doi:10.3390/cells9010169
13. Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14(9):2690–2695. doi:10.1158/1078-0432.CCR-07-1731
14. Cui Y, Wu F, Tian D, et al. miR-199a-3p enhances cisplatin sensitivity of ovarian cancer cells by targeting ITGB8. Oncol Rep. 2018;39(4):1649–1657.
15. Deng Y, Zhao F, Hui L, et al. Suppressing miR-199a-3p by promoter methylation contributes to tumor aggressiveness and cisplatin resistance of ovarian cancer through promoting DDR1 expression. J Ovarian Res. 2017;10(1):50. doi:10.1186/s13048-017-0333-4
16. Kinose Y, Sawada K, Nakamura K, et al. The hypoxia-related microRNA miR-199a-3p displays tumor suppressor functions in ovarian carcinoma. Oncotarget. 2015;6(13):11342–11356. doi:10.18632/oncotarget.3604
17. Liu X, Yao B, Wu Z. miRNA-199a-5p suppresses proliferation and invasion by directly targeting NF-κB1 in human ovarian cancer cells. Oncol Lett. 2018;16(4):4543–4550.
18. Xiang G, Cheng Y. MiR-126-3p inhibits ovarian cancer proliferation and invasion via targeting PLXNB2. Reprod Biol. 2018;18(3):218–224. doi:10.1016/j.repbio.2018.07.005
19. Gan X, Zhu H, Jiang X, et al. CircMUC16 promotes autophagy of epithelial ovarian cancer via interaction with ATG13 and miR-199a. Mol Cancer. 2020;19(1):45. doi:10.1186/s12943-020-01163-z
20. Liu HZ, Liu GY, Pang WW, Zhang H, Zeng ZJ, Wang HJ. LncRNA LUCAT1 promotes proliferation of ovarian cancer cells by regulating miR-199a-5p expression. Eur Rev Med Pharmacol Sci. 2020;24(4):1682–1687.
21. Xu C, Zhu LX, Sun DM, Yao H, Han DX. Regulatory mechanism of lncRNA NORAD on proliferation and invasion of ovarian cancer cells through miR-199a-3p. Eur Rev Med Pharmacol Sci. 2020;24(4):1672–1681.
22. Pan C, Stevic I, Müller V, et al. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Mol Oncol. 2018;12(11):1935–1948. doi:10.1002/1878-0261.12371
23. Liang H, Jiang Z, Xie G, Lu Y. Serum microRNA-145 as a novel biomarker in human ovarian cancer. Tumour Biol. 2015;36(7):5305–5313. doi:10.1007/s13277-015-3191-y
24. Hua M, Qin Y, Sheng M, et al. miR‑145 suppresses ovarian cancer progression via modulation of cell growth and invasion by targeting CCND2 and E2F3. Mol Med Rep. 2019;19(5):3575–3583.
25. Chen X, Dong C, Law PT, et al. MicroRNA-145 targets TRIM2 and exerts tumor-suppressing functions in epithelial ovarian cancer. Gynecol Oncol. 2015;139(3):513–519. doi:10.1016/j.ygyno.2015.10.008
26. Li J, Zhang S, Wu L, Pei M. Interaction between LncRNA-ROR and miR-145 contributes to epithelial-mesenchymal transition of ovarian cancer cells. Gen Physiol Biophys. 2019;38(6):461–471. doi:10.4149/gpb_2019028
27. Hu J, Wang L, Chen J, et al. The circular RNA circ-ITCH suppresses ovarian carcinoma progression through targeting miR-145/RASA1 signaling. Biochem Biophys Res Commun. 2018;505(1):222–228. doi:10.1016/j.bbrc.2018.09.060
28. Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastases: biology and pathology. Gynecol Oncol. 2009;113(1):143–148. doi:10.1016/j.ygyno.2008.11.032
29. Lyu T, Jiang Y, Jia N, et al. SMYD3 promotes implant metastasis of ovarian cancer via H3K4 trimethylation of integrin promoters. Int J Cancer. 2020;146:1553–1567.
30. Tang MK, Zhou HY, Yam JW, Wong AS. c-Met overexpression contributes to the acquired apoptotic resistance of nonadherent ovarian cancer cells through a cross talk mediated by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2. Neoplasia. 2010;12(2):128–138. doi:10.1593/neo.91438
31. Gu S, Cheung HH, Lee TL, Lu G, Poon WS, Chan WY. Molecular mechanisms of regulation and action of microRNA-199a in testicular germ cell tumor and glioblastomas. PLoS One. 2013;8(12):e83980. doi:10.1371/journal.pone.0083980
32. Sumigama S, Ito T, Kajiyama H, et al. Suppression of invasion and peritoneal carcinomatosis of ovarian cancer cells by overexpression of AP-2alpha. Oncogene. 2004;23(32):5496–5504. doi:10.1038/sj.onc.1207723
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.