Back to Journals » Nature and Science of Sleep » Volume 12
Ideal Time of Day for Risky Decision Making: Evidence from the Balloon Analogue Risk Task
Authors Li M, Mai Z, Yang J, Zhang B , Ma N
Received 5 May 2020
Accepted for publication 6 July 2020
Published 16 July 2020 Volume 2020:12 Pages 477—486
DOI https://doi.org/10.2147/NSS.S260321
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sutapa Mukherjee
Mingzhu Li,1– 5 Zifeng Mai,1– 5 Jiayu Yang,1– 5 Bin Zhang,6 Ning Ma1– 5
1Key Laboratory of Brain, Cognition and Education Sciences (South China Normal University), Ministry of Education, Guangzhou 510631, People’s Republic of China; 2School of Psychology, South China Normal University, Guangzhou 510631, People’s Republic of China; 3Center for Sleep Research, South China Normal University, Guangzhou 510631, People’s Republic of China; 4Center for Studies of Psychological Application, South China Normal University, Guangzhou 510631, People’s Republic of China; 5Guangdong Key Laboratory of Mental Health and Cognitive Science, South China Normal University, Guangzhou 510631, People’s Republic of China; 6Department of Psychiatry, Nanfang Hospital, Southern Medical University, Guangdong-Hong Kong-Macao Greater Bay Area Center for Brain Science and Brain-Inspired Intelligence, Guangzhou 510515, People’s Republic of China
Correspondence: Ning Ma
Center for Sleep Research,Center for Studies of Psychological Application,Guangdong Key Laboratory of Mental Health & Cognitive Science, School of Psychology, South China Normal University, Guangzhou 510631, People’s Republic of China
Email [email protected]
Background: Previous studies have demonstrated that individuals showed higher risk preference in the afternoon than in the morning. However, few studies aimed to explore the alteration of feedback learning effect during risky decision making, which is one of the important psychological processes of real risk behaviors. Moreover, cognitive function altered at the off-peak time due to impaired inhibitory control. The present study is to investigate the time-of-day effect on risky decision making and inhibitory control and whether the alteration of inhibitory control causes the differences in risky decision making across one day.
Participants and Methods: We adopted a within-participants design without extremely chronotype individuals to measure the time-of-day (9 am vs 3 pm) effect on risky decision making by using the Balloon Analogue Risk Task. At the same time, we measured the inhibitory control by using the Go/no-go task.
Results: Our results confirmed that individuals showed higher risk preference in the afternoon than in the morning. In addition, we found that individuals were insensitive to loss and the previous negative feedback in the afternoon. Critically, the results did not reveal any significant correlation between risky decision making and inhibitory control under the regulation of the time-of-day effects, although individuals performed worse on inhibitory control in the afternoon.
Conclusion: The current findings revealed that the time-of-day effect regulated risky decision making and inhibitory control. Individuals act with higher risk preference, less sensitivity to loss as negative feedback, and lower inhibitory control in the afternoon than in the morning. This may reflect the effects of time-of-day on risk propensity and inhibitory control is relatively independent.
Keywords: time-of-day effect, risky decision making, inhibitory control
Introduction
Under the influence of the sleep-wake regulation, the human psychological and physiological functions fluctuate with the time in one day,1–4 which refers to time-of-day effect. This effect has been observed in many cognitive domains, including attention, working memory, inhibition control, as well as risky decision making. Byrne and Murray found that people typically exhibit a higher risk propensity in the afternoon; for example, in a study applied the Balloon Analogue Risk Task (BART), participants pumped more in the afternoon than in the morning and evening.5 Similarly, larks chronotype individuals distinguished by clock-related genes (PER3, NR1D2) have been found to be more adventurous in the afternoon than in the morning.6
Previous research about the time-of-day effect has primarily focused on risk preference. However, risky decision making is a complex behavior. The reinforcement sensitivity theory (RST) posits that the likelihood of risky behavior is affected by the sensitivity to reward (positive feedback) and punishment (negative feedback), and such feedback may differentially affect the subsequent decision.7,8 Individuals tend to become more conservative when a previous decision caused loss during risk-taking.9 People may adjust a current decision according to the outcome of the previous choice, which is known as the feedback learning effect of risk-taking.7,9 However, no research has explored the effect of time-of-day on feedback learning effect during risk-taking.
Inhibitory control is one of the core executive functions, which assists people to resist the distraction or lure of irrelevant information.10 Yoon and colleagues proposed that cognitive function altered between peak time and off-peak time and optimal functioning occurring at peak relative to off-peak times, which may be due to impaired inhibitory control.11 Individuals with impaired inhibitory control at off-peak time were difficult or unable to resist the distraction of irrelevant information, which cause undesirable or inappropriate responses. In line with this perspective, Kouchaki and Smith observed that compared to the morning, individuals acted more unethically in the afternoon when self-control (one core aspect of inhibitory control) was too low to resist the temptation to perform unethical but self-profiting behavior.12 Similar results were observed in the study of the time-of-day effect on implicit memory and problem-solving.13,14 Furthermore, other studies revealed that a higher risk tendency was associated with impaired inhibitory control.15–18 Therefore, it is reasonable to speculate that the alteration of risky decision making was modulated by the impaired inhibitory control in the afternoon, because people may find it relatively difficult to resist the impulsivity associated with gain or reward.
The present study is aimed to explore the effect of time-of-day on risky decision making, as well as the feedback learning effect of risk decision-making, and to examine the potential relationship between risk-taking and inhibition control under the regulation of time-of-day effect. We used the Balloon Analogue Risk Task (BART) to measure the risk-taking.7,19,20 The BART provided multiple indexes to assess the risky decision making, not only the risk preference but also the feedback learning effect of learning during risk-taking. In detail, the number of balloon pumps in non-exploded balloons and the number of explosions reflect the risk preference, total gains reflect the performance of task, and the average number of pumps after exploded balloons or unexploded balloons (win or loss) reflect the feedback learning effects.7,21 Meanwhile, we used the Go/no-go task (GNG) to measure the performance of inhibitory control.22,23 The Go/no-go task required individuals to inhibit the prepotent response when the target stimulus appears, which widely applied to measure the inhibitory control function.11 Importantly, because of the interaction effect of chronotypes and time-of-day on risky behavior,6 we recruited individuals neither an extreme morning type nor an extreme evening type and adopted a within-participants design to avoid the potential confounding influence of individual differences.
Based on the previous studies, the hypotheses of the present study were as follows. First, we posited that risky decision making and inhibitory control fluctuated across a day. Individuals showed higher risk propensity and lower inhibitory control in the afternoon compared to in the morning. Second, the change of risky decision making during a day might be related to the alteration of inhibitory control.
Participants and Methods
Participants
Twenty-eight participants (11 males, aged from 18 to 28 years, BMI from 16.53 to 23.37) were included in the study. All participates were right-handed and normal or corrected-to-normal vision. They were assessed by a structural screening questionnaire, consisting with a series of questions about their demographic information, sleep, and health history. The questions included (1) age and gender, (2) left or right-handed, (3) height and weight, (4) normal sleep hours and duration during weekdays and weekends, (5) habits of smoking, alcohol, and caffeine intake, (6) history of shift work and trans-meridian travel, and (7) history of physical, psychiatric, and sleep-related disorders. All participants in the current study reported having no history of physical, psychiatric, and sleep-related disorders. They were non-smokers and were not addicted to caffeine or alcohol. They could not have shift work, transmeridian travel within the three months preceding the experiment. They were required to keep habitually good sleep, such as falling asleep no later than 12:00 am at the midnight, waking up no later than 8:00 am, and regularly having 7–9 hours of sleep every night for 2 weeks preceding the study, as verified by sleep diaries (mean sleep duration of all the participants was 7.93±0.38 hours/day, for detail, see Table 1). They were also instructed not to consume any beverages or foods with caffeine such as coffee and tea, within 48 hours prior to the experiment. The study was conducted in accordance with the international ethical standards for biological rhythm research24 and the Declaration of Helsinki (BMJ 1991; 302: 1194). This study was approved by the Medical Ethical Committee of the South China Normal University (of the principal investigator N.M.). Each participant provided written informed consent, they were paid certain money after finishing the study.
 |
Table 1 The Averaging Results of Sleep Dairy from Two Weeks Preceding the Study |
To measure individuals’ chronotype, we used the Morningness-Eveningness Questionnaire (MEQ).25 Extreme morning type and evening type individuals were excluded. The scores of the participants in the present study were controlled between 30 and 70.25,26
Materials
Balloon Analogue Risk Task (BART)
To measure risk-taking behavior, the BART was used.20 In this task, participants were instructed to inflate a balloon on the screen by pressing the button of “pump” to inflate a balloon or pressing the button of “collect” to stop and collect money. The balloon gets bigger, more points will be collected. But the possibility of the explosion would be higher as pumping more times. Participants can collect the coins of the balloon before the explosion, and lose the coins if balloon bursts. After the experiment, total gains would be converted into cash and paid to the participants (see Figure 1).
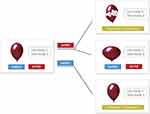 |
Figure 1 Balloon Analogue Risk Task (BART). |
Participants were instructed to pump 45 balloons and the task was self-paced. Considering the potential influence of fatigue (especially during the afternoon session) and keeping the similar duration with GNG task, the maximum number of inflation for each balloon was adapted to 16. Every 1 point could be earned by each successful pump and the explode threshold for each trial was determined randomly from a uniform distribution. Therefore, the probability of burst for a balloon on a given number of pumps is equal to “1/(16-number of pumps)”. The reward value corresponding to each balloon and the total earning during the task were explicitly displayed, but the probability of explosion was blinded to participants. The indexes of this task include the adjusted average number of pumps (no bursted balloons), the number of explosive balloons, total gains, the average number of pumps of no bursted balloons immediately after a positive feedback (gain trial) or a negative feedback (loss trial).
Go/No-Go Task (GNG)
We applied the Go/no-go task to measure inhibitory control. In this task, participants were asked to respond as quickly as possible to the target stimuli (“go” stimuli), and to make no response to the non-target stimuli (“no-go” stimuli). There was a total of 120 trials in two test blocks during each test session (morning or afternoon), and each block included 45 go trials and 15 No-go trials. We used “A” and “O” as the stimuli, and these two letters as target stimuli and non-target stimuli alternately in the two blocks, respectively. The sequence of the two blocks was counterbalanced between participants and conditions (see Figure 2). There was a clear instruction before each test block about the Go and No-go letter. The indexes of the GNG task include mean reaction time of Go trials, hit rate (the number of correct hits on go trials), and false alarm rate (the number of incorrect hits on no-go trials).
 |
Figure 2 Go/no-go Task (GNG) task. Note: The order of task 1 and task 2 was counter-balanced between different participants and sessions. |
Subjective Sleepiness Rating and Objective Vigilant Performance
We measured the subjective sleepiness and objective alerting performance to control the potential confounding influence of the sleepiness on risky decision making. To measure subjective sleepiness, we used the Karolinska Sleepiness Scale (KSS)27 to evaluate participants’ sleepiness from morning to afternoon. The KSS is a 1-item visual analogue scale, scored using a 10-point response range, from 1 (extreme alertness), to 5 (neither alertness nor sleepiness), to 10 (extreme sleepiness).
A 10-min version of the Psychomotor Vigilance Task (PVT)28 was used to test the vigilance of participants in the present study. The PVT is sensitive to sleep loss and circadian rhythmicity29,30 and free of practice effects.31,32 In this task, participants were asked to focus their attention on a red, rectangular box in the middle of a black screen, and to monitor that space for the appearance of a millisecond counter, which appeared at random intervals ranging from 2 s to 10 s. They were instructed to stop the counter as soon as possible by pressing a specified button, after which they would be able to view their reaction time (see Figure 3). Participants were also instructed to avoid anticipating the stimuli so as not to register “false starts” or responses when no stimulus was present on the screen. The indexes of PVT included reaction time and number of lapses (reaction time longer than 500 ms).
 |
Figure 3 Psychomotor Vigilance Task (PVT) task. |
Experimental Procedure
Participants arrived at the laboratory around 8:00 am to adapt to the environment of the lab for 1 h. The laboratory was kept at a constant temperature (26°C) and constant light (50 lux) to alleviate the confounding influences to on participants’ circadian rhythm.4 Participants completed the Karolinska Sleepiness Scale, PVT, GNG task and BART twice, at 9 am and 3 pm, respectively (see Figure 4). As the 10-min PVT may induce mild fatigue, after the PVT, participants allowed to have 3–5 min rest before doing the GNG and BART tasks. Participants could not leave the laboratory, use smartphone/tablet, or nap at any time until they completed the whole experiment. Between the two testing sessions, participants were allowed to talk with the experimenters, read books, and to have lunch at 12:00 at noon. To control for other confounding factors, such as physical activity, the participants allowed to have limited activity in the lab. To avoid the learning effect of Go/No-go task,33 under the supervision of experimenters, participants practiced the Go/No-go task for at least eight times on the two days prior to the experimental day in the same lab where the experiment conducted. The letters in the Go/No-go task during practice sessions were different with the letters during the experimental day. As suggested by White and colleagues,34 the BART has a good test–retest reliability across multiple test sessions. In our study, we provided the instructions for the BART to participants prior to the experiment day, and let them try a few trials to experience both win and loss with respect to pumping the balloons.
 |
Figure 4 Experimental design and procedure. |
Statistical Analysis
Data were analyzed using SPSS 18.0 software for Windows. The Paired t-tests were performed to compare the time-of-day differences regarding the performances of the BART, PVT, GNG tasks, and the subjective sleepiness rating. The Pearson correlation was performed to explore the relationship between risk-taking behavior- and inhibitory control.
Results
The Time-of-Day Effects of Subjective Sleepiness and Objective Vigilant Performance
There was no significant time-of-day effect with regard to both subjective sleepiness (t(27) = −1.904, p = 0.068, Cohen’s d = −0.36, see Table 2) and objective vigilance, as mean PVT reaction time (t(27) = −0.577, p = 0.569, Cohen’s d = −0.109), and the number of lapses (t(27) = −1.07, p = 0.294, Cohen’s d = −0.202, see Table 2).
 |
Table 2 Descriptive Statistics |
The Time-of-Day Effect of the Balloon Analogue Risk Task (BART)
The paired t-test results showed significant time-of-day effects on the adjusted average number of pumps on unexploded balloons (t(27) = −2.135, p = 0.042, Cohen’s d =−0.404, see Table 2 and Figure 5A), and the adjusted average number of pumps on unexploded balloons in the afternoon (M=6.42, SD=1.41) were significantly more than in the morning (M=6.06, SD=1.16). There was also time-of-day effect on total gains (t(27) = −2.871, p = 0.008, Cohen’s d = −0.543, see Table 2 and Figure 5B), participants got more in the afternoon (M=174.29, SD=13.85) relative to in the morning (M=164, SD=18.1). However, there was no difference in the number of exploded balloons between the morning and afternoon sessions (t(27) = 0.762, p = 0.453, Cohen’s d = 0.144).
Furthermore, we calculated the average number of pumps after exploded balloons and unexploded balloons, representing the positive feedback condition (reactivity to wins) and the negative feedback condition (reactivity to losses) respectively. The paired t-test results showed significant time-of day-effects on the average number of unexploded pumps after negative feedback (t(27) = −2.741, p=0.011, Cohen’s d = −0.518, see Table 2 and Figure 5C). The number of pumps was significantly higher in the afternoon (M=6.24, SD=1.68) than in the morning (M=5.79, SD=1.56). However, no significant time-of-day effects were observed in relation to the positive feedback condition (t (27) = −1.208, p = 0.237, Cohen’s d = −0.228).
The Time-of-Day Effect of Go/No-Go Task
The paired t-test results showed no significant time-of-day effects on the reaction time and hit rate (t (27) = −0.067, p = 0.947, Cohen’s d = 0.013; t (27) = −0.554, p = 0.584, Cohen’s d = −0.105, see Table 2). The results for the false alarm rates did show significant differences between the morning and the afternoon sessions (t (27) = −2.989, p = 0.006, Cohen’s d = −0.565, see Table 2 and Figure 6), with the rate being lower in the afternoon (M=0.82, SD=0.10) than in the morning (M=0.87, SD=0.09). Additionally, we calculated d prime, an index of sensitivity discrimination that indicates the ability to distinguish signals (go trials) from noise (No go trials). However, the analysis did not reveal any significant time-of-day effects (t (27) = 1.977, p = 0.058, Cohen’s d = 0.374).
 |
Figure 6 Time-of-day effect on No-go trials false rate which reflects the level of inhibitory control in Go/no-go task. **p<0.01. |
The relationship between risky decision making and inhibition control under the regulation of the time-of-day effects
We used the delta score (“the index score in the afternoon” minus “the index score in the morning”) to measure the time-of-day effect of risky decision making and inhibitory control. To explore the relationship between risky decision making and inhibitory control under the regulation of the time-of-day effects, we applied Pearson correlations to the delta score of the BART and Go/no-go task. The results showed that risky decision making was not correlated with inhibitory control (see Table 3).
 |
Table 3 The Correlation Between the Time-of-Day Effects on Risky Decision Making and Inhibitory Control |
Discussion
This study aimed to examine the time-of-day effect (9 am and 3 pm) on risky decision making (measured using the BART) and inhibitory control (measured using the Go/no-go task) simultaneously with intermediate chronotype individuals. In line with our hypothesis, participants performed higher risk propensity and insensitivity to negative feedback (loss) and showed lower inhibitory control in the afternoon. However, the results failed to reveal a significant correlation between the diurnal change of inhibitory control and risk-taking. The present study indicated that both risky decision making and inhibitory control are regulated by the time-of-day effect, but these two psychological processes may be relatively independent under the regulation of time-of-day.
According to the reinforcement sensitivity theory, the likelihood of risky behavior was modulated by the sensitivity to reward (gain) and punishment (loss).8 Generally, people were more sensitive to loss than reward.9 To examine this postulation in the context of the present study, we further explored the time-of-day effect on the feedback learning effect of risk-taking. The current results demonstrated that compared with in the morning, individuals pumped more times under the negative feedback condition in the afternoon, but no difference under the positive feedback condition. The higher number of pumps following negative feedback might reflect that individuals did not have the same level of sensitivity to loss in the afternoon as they did in the morning, and they might not make the risky decision based on the preceding feedback.
In line with our expectation, inhibitory control in the Go/no-go task was reduced when the risk propensity was higher in the afternoon. The findings were consistent with previous studies about the time-of-day effect on inhibitory control.35–37 Our results partially support the viewpoints of Yoon,10 that changes in cognitive function at off-peak time is due to impaired inhibitory control. Unfortunately, we did not find any significant relationship between the time-of-day effect on risky decision making and inhibitory control.
As Frings suggested, after prolonged waking times, people may desire greater gains but be increasingly insensitive to loss and be powerless to learn from previous experiences.38 This idea may suggest that the diurnal changes affecting risky decision making are related to increased sleepiness along prolonged waking times. However, our study did not find any significant differences in either the subjective sleepiness or objective PVT performance between the morning and the afternoon. Based on the current results, it seems that subjective sleepiness and objective vigilance do not influence risky decision making in the afternoon. However, future study is warranted to further explore this issue, because subjective sleepiness and objective performance may exist discrepancy under certain situations.29
Another explanation for the diurnal change of risk-taking may be related to the influence of the reward system. According to a dual systems model of risk-taking, the risk behavior is determined by two independent systems: the reward system and the cognitive control system.39,40 Similar to cognitive control, reward function is also modulated by the time-of-day effects. A number of studies have demonstrated that the reward function is more active in the afternoon than at other times in one day.41,42 In the present study, individuals pumped more times in the afternoon, and even after the negative feedbacks. The current finding is consistent with the study of Byrne and Murray, suggesting that people seek more rewards due to the lower potential risk and higher potential reward in the afternoon.6 Therefore, the higher risk preference in the afternoon may be modulated by different levels of reward-seeking across a day.
Limitation and Future Direction
There are some limitations in the current study. First, the sample of the current study was relatively small. This may weaken the extent of the generalization with the present findings, even though the effect size of significant variables of BART and Go/no-go was acceptable. Future study may consider to use larger sample comprising morning and evening types, as well as intermediate types to examine the time-of-day effect on different cognitive functions. Second, participants completed all of the tests in one day. The morning session was consistently prior to the afternoon session, which may bring about practice effects. Although the previous study demonstrated the BART has adequate test–retest reliability,34 a counterbalanced order design with a larger sample of participants might help to verify the current findings. Third, we only selected two time-points with which to measure the performance of participants. It is difficult to demonstrate the variation of risk-taking in one day. In addition, the risky decision making showed a similar diurnal pattern compared with the reward function, which is at the peak in the afternoon than in the morning and evening.41 Future studies should adopt more time-points and combine risk-taking with reward seeking, for instance, measuring performance at 12-h phase differences to account for the circadian effects of risk taking. Finally, we only used behavioral tasks to measure the relationship between risky decision making and inhibitory control under the effect of time-of-day, which may hide the potential relevancy of risky decision making and inhibitory control. In the future, researchers may consider combining with neurophysiologic techniques, such as fMRI and EEG, to better explore the time-of-day effects on risky decision making and inhibitory control.
Conclusion
In the present study, we found that the time-of-day effect regulated risky decision making and inhibitory control. Individuals act with higher risk preference, less sensitivity to loss as negative feedback, and lower inhibitory control in the afternoon compared to in the morning. Furthermore, the alteration of risky decision making was not caused by the impaired inhibitory control in the afternoon, which suggests that the effects of time-of-day on risk propensity and inhibitory control may be relatively independent. Future studies may consider combining the reward function with neuroimaging methods to explore the diurnal variations of risky decision making. The current findings extend the understanding of the time-of-day effect on risky decision making and suggest that individuals may consider their optimal time when making an important decision since the time-of-day effect regulates decision-making behavior.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Blake MJ. Time of day effects on performance in a range of tasks. Psychon Sci. 1967;9(6):349–350. doi:10.3758/BF03327842
2. West R, Murphy KJ, Armilio ML, Craik FI, Stuss DT. Effects of time of day on age differences in working memory. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):P3–10. doi:10.1093/geronb/57.1.P3
3. Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: circadian rhythms in human cognition. Cogn Neuropsychol. 2007;24(7):755–789. doi:10.1080/02643290701754158
4. Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155–190. doi:10.1016/B978-0-12-396971-2.00007-5
5. Byrne JE, Murray G. Diurnal rhythms in psychological reward functioning in healthy young men: ‘wanting’, liking, and learning. Chronobiol Int. 2017;34(2):287–295. doi:10.1080/07420528.2016.1272607
6. Ingram KK, Ay A, Kwon SB, et al. Molecular insights into chronotype and time-of-day effects on decision-making. Sci Rep. 2016;6:29392. doi:10.1038/srep29392
7. Schmitz F, Manske K, Preckel F, Wilhelm O. The multiple faces of risk-taking. Eur J Psychol Assess. 2016;32(1):17–38. doi:10.1027/1015-5759/a000335
8. Corr PJ. Reinforcement Sensitivity Theory (RST): introduction. In: Corr PJ, editor. The Reinforcement Sensitivity Theory of Personality. New York: Cambridge University Press; 2008:1–43. doi:10.1017/CBO9780511819384.002
9. Xu S, Fang Z, Rao H. Real or hypothetical monetary rewards modulates risk taking behavior. Acta Psychol Sin. 2013;45(8):874–886. doi:10.3724/sp.J.1041.2013.00874
10. Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–168. doi:10.1146/annurev-psych-113011-143750
11. Yoon C, May CP, Hasher L. Aging, circadian arousal patterns, and cognition. In: Park D, Schwartz N, editors. Cognitive Aging: A Primer. East Sussex: Psychology Press; 1998:151–170. doi:10.4324/9780203345115
12. Kouchaki M, Smith IH. The morning morality effect: the influence of time of day on unethical behavior. Psychol Sci. 2014;25(1):95–102. doi:10.1177/0956797613498099
13. Wieth MB, Zacks RT. Time of day effects on problem solving: when the non-optimal is optimal. Think Reason. 2011;17(4):387–401. doi:10.1080/13546783.2011.625663
14. May CP, Hasher L, Foong N. Implicit memory, age, and time of day: paradoxical priming effects. Psychol Sci. 2005;16(2):96–100. doi:10.1111/j.0956-7976.2005.00788.x
15. Li Q, Nan W, Taxer J, Dai W, Zheng Y, Liu X. Problematic internet users show impaired inhibitory control and risk taking with losses: evidence from stop signal and mixed gambles tasks. Front Psychol. 2016;7:370. doi:10.3389/fpsyg.2016.00370
16. Yao YW, Wang LJ, Yip SW, et al. Impaired decision-making under risk is associated with gaming-specific inhibition deficits among college students with Internet gaming disorder. Psychiatry Res. 2015;229(1–2):302–309. doi:10.1016/j.psychres.2015.07.004
17. Noel X, Bechara A, Dan B, Hanak C, Verbanck P. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology. 2007;21(6):778–786. doi:10.1037/0894-4105.21.6.778
18. Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage. 2013;71:275–283. doi:10.1016/j.neuroimage.2013.01.025
19. Lauriola M, Panno A, Levin IP, Lejuez CW. Individual differences in risky decision making: a meta-analysis of sensation seeking and impulsivity with the balloon analogue risk task. J Behav Decis Mak. 2014;27(1):20–36. doi:10.1002/bdm.1784
20. Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). J Exp Psychol Appl. 2002;8(2):75–84. doi:10.1037//1076-898x.8.2.75
21. Ashenhurst JR, Bujarski S, Jentsch JD, Ray LA. Modeling behavioral reactivity to losses and rewards on the Balloon Analogue Risk Task (BART): moderation by alcohol problem severity. Exp Clin Psychopharmacol. 2014;22(4):298–306. doi:10.1037/a0036837
22. Donders FC. On the speed of mental processes. Acta Psychol (Amst). 1969;30:412–431. doi:10.1016/0001-6918(69)90065-1
23. Wessel JR. Prepotent motor activity and inhibitory control demands in different variants of the go/no-go paradigm. Psychophysiology. 2017;55(3):e12871. doi:10.1111/psyp.12871
24. Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–1929. doi:10.3109/07420528.2010.516381
25. Horne JA, Ostberg OJ. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110.
26. Kerkhof GA. Inter-individual differences in the human circadian system: a review. Biol Psychol. 1985;20:83–112. doi:10.1016/0301-0511(85)90019-5
27. Shahid A, Wilkinson K, Marcu S, Shapiro CM. Karolinska Sleepiness Scale (KSS). In: Shahid A, Wilkinson K, Marcu S, Shapiro CM, editors. STOP, THAT and One Hundred Other Sleep Scales. New York: Springer; 2011:209–210. doi:10.1007/978-1-4419-9893-4_47
28. Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20(4):267. doi:10.1093/sleep/20.4.267
29. Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Archives Italiennes de Biologie. 2001;139(3):253–267. doi:10.4449/aib.v139i3.503
30. Dorrian J, Rogers N, Dinges DF. Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep Deprivation. Boca Raton: CRC Press; 2004:39–70. doi:10.3109/9780203998007-4
31. Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34(5):581–591. doi:10.1093/sleep/34.5.581
32. Basner M, Hermosillo E, Nasrini J, et al. Repeated administration effects on psychomotor vigilance test performance. Sleep. 2018;41(1):zsx187. doi:10.1093/sleep/zsx187
33. Schapkin SA, Falkenstein M, Marks A, Griefahn B. Practice-related effects in a go-nogo task. Percept Mot Skills. 2007;105(3):1275–1288. doi:10.2466/PMS.105.7.1275-1288
34. White TL, Lejuez CW, de Wit H. Test-retest characteristics of the Balloon Analogue Risk Task (BART). Exp Clin Psychopharmacol. 2008;16(6):565–570. doi:10.1037/a0014083
35. García A, Ramírez C, Martínez B, Valdez P. Circadian rhythms in two components of executive functions: cognitive inhibition and flexibility. Biol Rhythm Res. 2012;43(1):49–63. doi:10.1080/09291016.2011.638137
36. Hartley LR, Shirley E. Color-name interference at different times of day. J Appl Psychol. 1976;61(7):119–122. doi:10.1037//0021-9010.61.1.119
37. Manly T, Lewis GH, Robertson IH, Watson PC, Datta A. Coffee in the cornflakes: time-of-day as a modulator of executive response control. Neuropsychologia. 2002;40(1):1–6. doi:10.1016/s0028-3932(01)00086-0
38. Frings D. The effects of sleep debt on risk perception, risk attraction and betting behavior during a blackjack style gambling task. J Gambl Stud. 2012;28(3):393–403. doi:10.1007/s10899-011-9266-9
39. Humphrey G, Dumontheil I. Development of risk-taking, perspective-taking, and inhibitory control during adolescence. Dev Neuropsychol. 2016;41(1–2):59–76. doi:10.1080/87565641.2016.1161764
40. Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52(3):216–224. doi:10.1002/dev.20445
41. Byrne JEM, Hughes ME, Rossell SL, Johnson SL, Murray G. Time of day differences in neural reward functioning in healthy young men. J Neurosci. 2017;37(37):8895–8900. doi:10.1523/JNEUROSCI.0918-17.2017
42. Hasler BP, Forbes EE, Franzen PL. Time-of-day differences and short-term stability of the neural response to monetary reward: a pilot study. Psychiatry Res. 2014;224(1):22–27. doi:10.1016/j.pscychresns.2014.07.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

