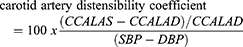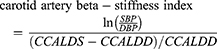Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Ideal Cardiovascular Health and Vascular Phenotype Associations in Mothers with Obesity and Their Six-Year-Old Children
Authors Litwin L, Sundholm JKM, Meinilä J , Kulmala J, Tammelin TH, Rönö K , Koivusalo SB , Eriksson JG, Sarkola T
Received 13 April 2021
Accepted for publication 29 May 2021
Published 13 July 2021 Volume 2021:14 Pages 3187—3197
DOI https://doi.org/10.2147/DMSO.S315402
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ming-Hui Zou
Linda Litwin,1,2 Johnny KM Sundholm,1,3 Jelena Meinilä,4 Janne Kulmala,5 Tuija H Tammelin,5 Kristiina Rönö,6 Saila B Koivusalo,6 Johan G Eriksson,7– 10 Taisto Sarkola1,3
1Children’s Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; 2Department of Congenital Heart Defects and Pediatric Cardiology, FMS in Zabrze, Medical University of Silesia, Katowice, Poland; 3Minerva Foundation Institute for Medical Research, Helsinki, Finland; 4Department of Food and Nutrition, University of Helsinki, Helsinki, Finland; 5LIKES Research Centre for Physical Activity and Health, Jyväskylä, Finland; 6Women’s Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; 7Folkhälsan Research Center, Helsinki, Finland; 8Department of General Practice and Primary Health Care, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; 9Human Potential Translational Research Programme and Department of Obstetrics and Gynaecology, Yong Loo Lin School of Medicine, National University Singapore, Singapore; 10Singapore Institute for Clinical Sciences (SICS), Agency for Science, Technology and Research (A*STAR), Singapore
Correspondence: Linda Litwin
Department of Congenital Heart Defects and Pediatric Cardiology, FMS in Zabrze, Medical University of Silesia, M.Sklodowskiej-Curie 9, Zabrze, 41-800, Poland
Tel +48 322713401
Fax +48 322713401
Email [email protected]
Background: Heredity and family-shared lifestyle contribute to cardiovascular risk, but the magnitude of their influence on arterial structure and function in early childhood is unknown. We aimed to assess associations between child and maternal ideal cardiovascular health, maternal subclinical atherosclerosis, and child arterial phenotype.
Methods: Cross-sectional analysis of 201 mother-child pairs originating from the Finnish Gestational Diabetes Prevention Study (RADIEL) longitudinal cohort was done at child age 6.1 ± 0.5 years with assessments of ideal cardiovascular health (BMI, blood pressure, fasting glucose, total cholesterol, diet quality, physical activity, smoking), body composition, very-high frequency ultrasound of carotid arteries (25 and 35 MHz), and pulse wave velocity.
Results: We found no association between child and maternal ideal cardiovascular health but report evidence of particular metrics correlations: total cholesterol (r=0.24, P=0.003), BMI (r=0.17, P=0.02), diastolic blood pressure (r=0.15, P=0.03), and diet quality (r=0.22, P=0.002). Child arterial phenotype was not associated with child or maternal ideal cardiovascular health. In the multivariable regression explanatory model adjusted for child sex, age, systolic blood pressure, lean body mass, and body fat percentage, child carotid intima-media thickness was independently associated only with maternal carotid intima-media thickness (0.1 mm increase [95% CI 0.05, 0.21, P=0.001] for each 1 mm increase in maternal carotid intima-media thickness). Children of mothers with subclinical atherosclerosis had decreased carotid artery distensibility (1.1 ± 0.2 vs 1.2 ± 0.2%/10 mmHg, P=0.01) and trend toward increased carotid intima-media thickness (0.37 ± 0.04 vs 0.35 ± 0.04 mm, P=0.06).
Conclusion: Ideal Cardiovascular Health metrics are heterogeneously associated in mother-child pairs in early childhood. We found no evidence of child or maternal Ideal Cardiovascular Health effect on child arterial phenotype. Maternal carotid intima-media thickness predicts child carotid intima-media thickness, but the underlying mechanisms remain unclear. Maternal subclinical atherosclerosis is associated with local carotid arterial stiffness in early childhood.
Keywords: cardiovascular disease, atherosclerosis, carotid intima-media thickness, risk factor, child
Plain Language Summary
- Cardiovascular risk factors are heterogeneously associated in mother-child pairs in early childhood.
- Child’s carotid intima-media thickness at 6 years of age is predicted by maternal carotid intima-media thickness, but the underlying mechanisms remain unclear.
- Maternal subclinical atherosclerosis is associated with child’s local carotid arterial stiffness in early childhood.
Introduction
Traditional cardiovascular risk factors contribute to the development and progression of atherosclerosis.1,2 Risk factors tend to cluster and their combination seems to be more predictive of individual cardiovascular risk.3
Ideal Cardiovascular Health (ICVH) was defined by American Heart Association as a set of seven health metrics (body mass index (BMI), blood pressure (BP), fasting glucose, total cholesterol, diet quality, physical activity, smoking) to promote primordial prevention of cardiovascular diseases in children and adults.4 ICVH is inversely associated with subclinical atherosclerosis in adulthood.5 Both ICVH and adverse vascular phenotype are reliable predictors of cardiovascular events and mortality in adults.6–8
Parental cardiovascular disease increases the risk of cardiovascular events in the offspring.9 Both genetic and shared lifestyle-related environmental factors are suggested to be underlying mechanisms, but the magnitude of their contribution is yet to be determined.10,11
The correlation between parental and child ICVH is apparent already in 11–12-year-old children. At this stage, child ICVH correlated with carotid elasticity and inversely correlated with carotid-femoral Pulse Wave Velocity (PWV) but was not reflected in carotid intima-media thickness (IMT).12 However, cardiovascular risk at age 12–18 was associated with increased carotid IMT in the middle age independently of contemporaneous risk factors.13 The evidence on the strength of these associations in early childhood is missing.
In our previous work, we have found no effects of Gestational Diabetes or a maternal lifestyle intervention to prevent Gestational Diabetes on child anthropometrics, body composition, or arterial dimensions and function in early childhood,14 and the focus of this analysis was on the transgenerational trend in cardiovascular risk clustering and its influence on child arterial phenotype. We hypothesized that maternal ICVH and vascular surrogates of cardiovascular disease would be reflected in child ICVH and arterial phenotype during early childhood.
In this study of mothers and their 6-year-old children, we aimed to assess:
- Relationships between maternal and child ICVH.
- Associations between child ICVH and arterial structure and function.
- If maternal ICVH and subclinical atherosclerosis explain child arterial phenotype variability beyond child ICVH.
Materials and Methods
Study Setting
Cross-sectional data were drawn from a six-year follow-up of the Finnish Gestational Diabetes Prevention Study (RADIEL). The original study design has been presented elsewhere.15 Briefly, women planning pregnancy or in the first half of gestation with an increased risk of Gestational Diabetes (obesity and/or history of Gestational Diabetes) were recruited (N=728). The cardiovascular six-year follow-up was designed as an observational study of mother-child pairs with an equal number of mothers with and without Gestational Diabetes, with prespecified cohort size (~200). Invitations were sent to consecutive participants until the limit was reached and 201 dyads were enrolled between June 2015 and May 2017. The follow-up was designed at children age of 5–6 years to ensure cooperation without sedation and included maternal-child dyads assessment of body size and composition, BP, fasting glucose and lipids, physical activity with accelerometers, diet quality and smoking questionnaires (mothers), vascular ultrasound and tonometry, and child echocardiography. The availability of the data is presented in the Supplemental Table S1. The Helsinki University Hospital Ethics Committee for gynecology and obstetrics, pediatrics and psychiatry approved the research protocol (20/13/03/03/2015) for a six-year follow-up assessment. Informed written consent was obtained from all mothers at enrolment. The study was conducted in accordance with the Declaration of Helsinki.
Vascular Ultrasound
Very-high resolution ultrasound images of the carotid arteries were obtained by one skilled investigator (TS) using 25 MHz and 35 MHz transducers with the Vevo 770 system, and using UHF22, UHF48 (similar center frequencies) with the Vevo MD system (VisualSonics, Toronto, Canada) for the final 52 mother-child pairs. The common carotid artery was imaged bilaterally 1 cm proximal to the carotid bulb at rest in the supine position. High-quality cine images covering 3–4 cardiac cycles were acquired using the highest frequency able to visualize the far-wall. The images were analyzed offline using Vevo 3.0.0 (Vevo 770) and VevoLab (Vevo MD) software with manual electronic calipers.16 Lumen diameter and IMT were measured using the leading–leading edge technique in end-diastole by one experienced observer (JKMS) blinded to subject characteristics (Supplemental Figure S1). We have previously reported intra-observer coefficients of variations for very-high resolution ultrasound measurements in children and adults ranging as 1.2–3.7% for lumen diameter, and 6.9–9.8% for IMT and inter-observer coefficients of variations 1.5–4.6% for lumen diameter, 6.0–10.4% for IMT. Carotid IMT Z-scores adjusted for age and sex were calculated using healthy Caucasian non-obese children references.17
Carotid lumen diameter was measured in both peak systole and end-diastole to assess carotid artery beta-stiffness index and carotid artery distensibility coefficient. Systolic and diastolic BP for elastic property calculations were recorded during ultrasound imaging in supine position from the right arm with oscillometry (Dinamap ProCare 200, GE) using appropriately sized cuffs. Carotid artery distensibility coefficient and carotid artery beta-stiffness index were calculated from the carotid artery using the formulas:
where CCALAS and CCALAD are common carotid artery lumen area in systole and diastole, respectively; CCALDS and CCALDD are common carotid artery lumen diameter in systole and diastole, respectively; and SBP and DBP are systolic and diastolic BP.18 Intra-observer coefficients of variations were 5.4% for carotid artery distensibility coefficient and 5.9% for carotid artery beta-stiffness index, and inter-observer coefficients of variations were 11.9% for carotid artery distensibility coefficient and 12.8% for carotid artery beta-stiffness index.
Maternal carotid arteries were further screened for plaques using a conventional high-resolution ultrasound Vivid 7 (GE) equipped with a 12 MHz linear transducer. The carotid arteries were screened bilaterally starting from the common carotid arteries proximal to the bulb, throughout the bifurcation and the proximal parts of the internal and external carotid arteries. Plaques were defined according to Mannheim consensus as 1. a focal thickening of the vascular wall of either 0.5 mm or 50% of the surrounding IMT or 2. a total arterial wall thickness exceeding 1.5 mm.19 Plaque presence was assessed dichotomously. Repeat measurements were independently performed on a subset of images (N=40) by both primary observer (JKMS) to assess intra-observer variability and by a second observer (TS) to assess inter-observer variability. Cohen’s κ for intra-observer variability and inter-observer variability were 0.89 and 0.83, respectively.
PWV
PWV was measured by a trained research nurse to assess regional arterial stiffness using mechanosensors (Complior Analyse, Alam Medical, Saint-Quentin-Fallavier, France) at rest in supine position.20 Sensors were set at the right carotid, right radial and right femoral arteries to assess central (right carotid-femoral) and peripheral (right carotid-radial) transit times. The direct distance between recording sites was measured using a tape measure to the nearest 0.1 cm. The distance right carotid-femoral was multiplied by 0.8 and subsequently used in central PWV calculations. Repeat recordings were performed in supine position. Two recordings were obtained with a third recording performed in the setting of a higher than 0.5 m/s (10%) difference between measurements. In the setting of more than two measurements the results with the lowest tolerance values were used in analyses, tolerance being a quality parameter to quantify variability in pulse waves during recording. The mean of at least two measurements was used in the final analyses. PWV was measurable in 168 children. The coefficient of variation for repeat measurements was 3.5% for carotid-femoral PWV and 4.8% for carotid-radial PWV (N=55).
Maternal Vascular Score
A set of three binary metrics was used to reflect subclinical atherosclerosis in mothers: carotid plaque presence, carotid IMT adjusted for age and exceeding 90th percentile within our sample, and carotid-femoral PWV above 90th percentile matched for age decade and optimal BP.21
ICVH Metrics
ICVH is a set of seven binary metrics, with cumulative range from 0 to 7 (the higher the score, the better the compliance with the guidelines).4 The ICVH metrics applied in the present study were consistent with the original definition (with three modifications - Supplemental Table S2) and included:
- Maternal smoking: never or quit > 12 months;
- BMI – children iso-BMI < 25 kg/m2,22 mothers BMI < 25 kg/m2;
- Child physical activity > 60 min of moderate-to-vigorous activity/day;
- Diet quality – children Finnish Children Healthy Eating Index > 60%,23 mothers Healthy Food Intake Index > 60%;24
- Total cholesterol – children <170 mg/dL, mothers <200 mg/dL without treatment;
- Blood pressure – children systolic and diastolic BP < 90th percentile,25 mothers < 120/80 mmHg without treatment;
- Fasting glucose < 100 mg/dL without treatment.
Diet quality was assessed with the Finnish Children Healthy Eating Index (range 1–42) in children and Healthy Food Intake Index (range 0–17) in mothers. Both indexes covered 4 out of 5 categories included in the original diet metric (with the exception of sodium intake).23,24 The cut-off value for the ideal vs non-ideal diet quality was defined as above 60% to reflect the original metric definition (ideal if more than 3 out of 5 criteria were met). Child BMI was defined as non-ideal if exceeding the sex-specific cut-off value for overweight children in reference to the recent healthy pediatric Finnish population (girls 87.7th percentile, boys 78.2th percentile) which is slightly different from the 85th percentile in the original metric.22 Maternal physical activity was excluded due to the considerable drop out and very low discriminating value (Supplemental Table S1, ICVH criterium fulfilled in 96% of mothers). ICVH was subjectively divided into the following categories: low (children 0–3, mothers 0–2), intermediate (children 4, mothers 3–4), and high (children and mothers 5–6) providing the opportunity to compare different categories.
BMI and Body Composition
Height and weight were measured with electronic devices (Seca GmbH & Co. KG, Germany) to the nearest 0.1 cm and 0.1 kg. Child BMI Z-scores were generated with reference to the recent Finnish population dataset.22 Body composition was assessed by bioelectrical impedance (InBody 720, InBody Bldg, Korea).
BP
Resting BPs were measured in the sitting position from the right arm with adequate cuffs with oscillometry (Omron M6W, Omron Healthcare Europe B.V., The Netherlands). Mean systolic and diastolic BP were calculated from the two lowest measurements (out of a minimum of three). Child BP Z-scores were calculated in reference to the guidelines.25
Blood Sample
Blood samples of plasma glucose and lipids were taken in the fasting state. Results from 3 children with uncertain fasting compliance (concurrently excessively high triglycerides, fasting glucose, and low glycated hemoglobin A1c (HbA1c)) were excluded from the analysis. Total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were assessed with enzymatic assays, plasma glucose with enzymatic hexokinase assay, and HbA1c with immunoturbidimetric analyzer (Roche Diagnostics, Basel, Switzerland).
Diet Quality, Physical Activity, Maternal Smoking, Medical History and Education
Mother’s dietary intake was assessed with food frequency questionnaires and was further evaluated with Healthy Food Intake Index. The Healthy Food Intake Index was previously validated as a useful tool to reflect compliance with the Nordic Nutrition Recommendations26 in the original RADIEL cohort.24 Briefly, it contains eleven components covering consumption of vegetables, fruit and berries, high-fiber grains, fish, milk, cheese, cooking fat, fat spread, snacks, sugar-sweetened beverages and fast food. The higher score reflects better compliance with recommendations. Child’s diet quality was assessed with a 3-day food record and further evaluated with the Finnish Children Healthy Eating Index. The Finnish Children Healthy Eating Index was previously validated in the Finnish pediatric population.23 It includes five categories of food: vegetables, fruits and berries; oils and margarine; foods containing high amounts of sugar; fish and fish dishes; and skimmed milk. The food consumption was scored so that the higher the consumption the higher the score, except for foods containing high amounts of sugar the scoring was reversed. Before scoring, the intake was adjusted for energy intake by dividing the intake (grams) by energy intake (kcal). The higher the score, the better the child diet quality.
Moderate-to-vigorous physical activity (MVPA) was measured with the hip-worn accelerometer in children (ActiGraph GT3X, ActiGraph, Pensacola, USA), and armband in mothers (SenseWear ArmBand Pro 3). Monitors were instructed to be worn during waking and sleeping hours, but sleeping time was eliminated from the analysis. Monitors on children collected data at 30 Hz sample rate. The data were normally filtered, converted into 10 s epoch counts and analyzed using Evenson (2008) cut-point (≥2296 cpm).27 Monitors on mothers collected MET values in 60 s epochs. MVPA was calculated as MET values over 3. Valid measurement was defined as at least 2 weekdays and 1 weekend day (with a minimum of 480 minutes of recording per day) for a child and 3 weekdays and 1 weekend day (with a minimum of 720 minutes of recording per day) for a mother. MVPA time was calculated as a weighted mean value [(average MVPA min/day of weekdays × 5 + average MVPA min/day of weekend day × 2)/7] and, in addition, as % of the total wearing time. Recent data on physical activity in Finnish population were used as a reference.28
Information on maternal smoking, chronic diseases, medications, and educational attainment were obtained with a questionnaire.
Data Analysis
Data are presented as mean ± SD, median (interquartile range) or as a count (percentage). All continuous variables were assessed for normal distribution based on histograms and normal Q-Q plots.
Independent samples t-test, Mann–Whitney U-test, one-way ANOVA, Kruskal–Wallis, and chi-square test were used as appropriate to compare groups (mothers vs child, boys vs girls, or low vs intermediate vs high ICVH).
The univariate associations between child and maternal characteristics were explored using Pearson’s or Spearman rank-order correlation coefficients.
Multivariable linear regression modelling was used to build explanatory models of child HDL cholesterol and carotid IMT. Variables selection was based on correlation and expert clinical judgement, avoiding significant multicollinearity in the model, and including potential confounders. Multicollinearity was assessed with the Variance Inflation Factor, with a maximum value of 1.9. Interactions were analyzed with multivariable linear regression.
Two-tailed P ≤ 0.05 was set as significant, with except for P ≤ 0.01 in the correlation analyses for determinants of child carotid IMT.
Statistical analysis was performed with SPSS, IBM, version 25 and GraphPad Prism version 8.4.3.
Results
Study Population
Participant characteristics are presented in Table 1 and Supplemental Table S3. Child BMI Z-score and BP Z-scores were increased compared to the reference population. Detailed data on child arterial morphology were reported in our previous work.14 Only 15 (12%) children and 5 (2.7%) mothers met all the criteria for ICVH (Supplemental Figures 2 and 3, Supplemental Tables S4–S6).
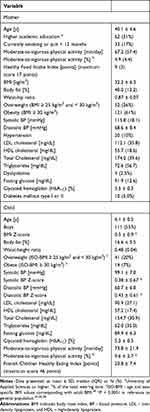 |
Table 1 Study Participants |
Associations Between Maternal and Child ICVH
Maternal and child cumulative ICVH scores were associated only among boys (boys: rs=0.32, P=0.01; girls: rs=−0.18, P 0.2). When analyzed as continuous variables, maternal-child univariate correlation analyses were significant for measures of lipids, HbA1C, adiposity, diastolic BP, and diet quality (Supplemental Figure S4–S10).
Child and maternal LDL, HDL, and total cholesterol were associated (r=0.23, P=0.003; r=0.35, P<0.0001; r=0.24, P=0.003, respectively, Figure 1). When stratified for child sex, correlation between child and maternal LDL and total cholesterol remained significant only in boys (Supplemental Table S7). Triglycerides and HDL cholesterol were associated with body fat percentage in girls (rs=0.34, P=0.004; r=−0.37, P=0.002, respectively, Figure 1, Supplemental Table S8).
We found a significant correlation between child and maternal HbA1C (r=0.27, P=0.004), though no association was noted for fasting glucose (P=0.4). Child BMI Z-score, but not body fat percentage, weakly correlated with maternal BMI and waist–hip ratio (r=0.17, P=0.02; r=0.18, P=0.02, respectively). Child diastolic BP Z-score weakly correlated with maternal diastolic BP (r=0.15, P=0.03). Finnish Children Healthy Eating Index correlated with maternal Healthy Food Intake Index (r=0.22, P 0.002). This relationship was observed only in boys (r=0.31, P=0.001).
Results were consistent after exclusion of mothers on hypertension, hypercholesterolemia, or hyperglycemia treatment.
Association Between Child ICVH and Vascular Structure and Function
Detailed arterial phenotypes are presented in Supplemental Table S9. Child vascular structure was independent of child characteristics (Supplemental Table S10). We did not observe any association between child ICVH and vascular structure or function. In the analysis of children stratified for ICVH score rank, we observed increased carotid IMT Z-score only in children with intermediate-score as compared to low-score (mean ± SD; intermediate-score 0.41 ± 0.63 vs low-score −0.07 ± 0.71, P = 0.03, Supplemental Table S11).
Associations Between Maternal Characteristics and Child Vascular Phenotype
Maternal ICVH was not related to child vascular phenotype (Supplemental Tables S10 and S12). Child and maternal carotid IMT were correlated (Figure 2), but maternal-child correlations between different vascular stiffness parameters were not statistically significant (Supplemental Table 9, Supplemental Figure S11). In the multivariable regression explanatory model adjusted for child sex, age, systolic BP, lean body mass, and body fat percentage maternal carotid IMT was the only independent predictor of child carotid IMT (adjusted R2 = 0.08). Child carotid IMT increased by 0.1 mm (95% CI 0.05, 0.21, P = 0.001) for each 1 mm increase in maternal carotid IMT (Supplemental Table S13). Child sex did not moderate the effect.
Maternal vascular score correlated with child carotid artery distensibility coefficient and β-stiffness index (rs=−0.21, P=0.007, rs=0.16, P=0.04, respectively, Supplemental Table S10). Children born to mothers with vascular score 1–3 as compared with children of mothers with score 0 had lower carotid artery distensibility coefficient (mean ± SD, 1.1 ± 0.2 vs 1.2 ± 0.2%/10 mmHg, P=0.01) and trend toward increased carotid artery beta-stiffness index (median (IQR), 3.0 (0.7) vs 2.8 (0.7), P=0.052) and carotid IMT (mean ± SD, 0.37 ± 0.04 vs 0.35 ± 0.04 mm, P=0.06) (Figure 3, Supplemental Table S14).
 |
Figure 3 Child vascular phenotype stratified for maternal vascular score. Data presented as mean + SD, P with independent samples t-test (A and C) and Mann–Whitney U-test (B). Significant results are bolded (P ≤ 0.05). Maternal vascular score: range 0–3, a set of three binary metrics: carotid plaque presence, carotid intima-media thickness adjusted for age and exceeding 90th percentile within our sample, and carotid-femoral pulse wave velocity above 90th percentile matched for age decade and optimal blood pressure.21 Abbreviation: IMT, intima-media thickness. |
Combination of maternal scores (ICVH, vascular score), as well as child and maternal scores were not associated with child arterial phenotype (Supplemental Table S10).
Discussion
In this cross-sectional analysis of mothers and their 6-year-old children, we studied associations of child ICVH, maternal ICVH, and maternal subclinical atherosclerosis with child arterial structure and function. The main finding is that only maternal subclinical atherosclerosis and neither child nor maternal conventional cardiovascular risk factors were associated with adverse alterations of vascular phenotype in early childhood. This novel insight into early child vascular development adds to our understanding of the transgenerational implications of subclinical atherosclerosis.
We report evidence of decreased carotid artery distensibility and trends toward increased carotid artery beta-stiffness index and carotid IMT in children of mothers with vascular surrogates of cardiovascular disease. There was, however, no direct association between maternal and child vascular function metrics, and we hypothesize that inclusion of maternal plaques in the vascular score has significantly increased its predictive value.
We observed a positive association between child and maternal carotid IMT; however, mechanisms remain unclear, as child carotid IMT was independent of child and maternal characteristics. The association between child ICVH score rank and carotid IMT showed inconsistency, as we did not observe any difference between low and high ICVH.
We are aware that additional factors could play a role, including child head circumference, which could be an important predictor of carotid artery size during the early growth period. Further, our results could possibly be attributed to unmeasured factors influencing the fetal vascular development. However, we previously reported no effect of maternal pre-pregnancy overweight/obesity and Gestational Diabetes on child carotid IMT in early childhood.14 Further studies are warranted to explore arterial structure and function accounting for child growth and genetic background.
The reported associations are consistent with previous studies in adolescents, which provided evidence of parent–child vascular phenotype associations, including carotid IMT, although body size was not adjusted for in the analyses.29 This was further confirmed by the considerable heritability of carotid IMT and arterial stiffness in adults.30,31
Observed associations between maternal subclinical atherosclerosis and child vascular phenotype were not extended by maternal ICVH. This is in line with previous studies where a considerable proportion of the variation in child vascular phenotype was explained by genetic factors independent of parent and child conventional cardiovascular risk factors.29
Further, the observed vascular changes were independent of child ICVH, suggesting predominant influence of a heritability background during early childhood. The contribution of environment-related factors seems to evolve with child age, as significant associations between child vascular function and ICVH have been previously reported in a large cross-sectional cohort study of children aged 11–12 years.12
The study sample was derived from the RADIEL study and due to the high prevalence of maternal obesity and Gestational Diabetes these results are not representative of the general population but reflect findings in populations with maternal obesity. In our previous work, we have found no evidence of fetal cardiovascular programming related to Gestational Diabetes.14 Due to the lack of consensus on cardiovascular risk or metabolic health assessments during early childhood,32 we consider applying ICVH during early childhood in combination with novel vascular ultrasound technology validated for this particular age group a novel research approach. We introduced minor modifications to the original American Heart Association ICVH metrics in order to include a comprehensive score based on the available young pediatric Finnish data set. Considerable child cumulative ICVH attrition due to parental resistance toward child blood testing could have biased our results, but challenges with child metabolic assessment are common in young childhood research settings. Our results could be biased by unmeasured factors, including seasonal variation in physical activity, sedentary behaviors, screen time, sleep patterns, and child passive smoking. The analysis in this study was limited by the lack of paternal and sibling’s data, which could improve our understanding of transgenerational cardiovascular risk trends in families. However, the comprehensively assessed homogenous and relatively large sample strengthen our conclusions on the reported transgenerational vascular phenotype patterns.
Conclusions
ICVH metrics are heterogeneously associated in mother-child pairs in early childhood. We found no evidence of child or maternal Ideal Cardiovascular Health effect on child arterial phenotype. Maternal carotid IMT predicts child carotid IMT, but the underlying mechanisms remain unclear. Maternal subclinical atherosclerosis is associated with local carotid arterial stiffness in early childhood.
Abbreviations
ICVH, ideal cardiovascular health; BMI, body mass index; BP, blood pressure; PWV, Pulse Wave Velocity; IMT, intima-media thickness; RADIEL, Finnish Gestational Diabetes Prevention Study; HbA1C, glycated hemoglobin A1C; LDL, low-density lipoprotein; HDL, high-density lipoprotein; MVPA, moderate-to-vigorous physical activity.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author [LL], upon reasonable request.
Ethics Approval and Informed Consent
The Helsinki University Hospital Ethics Committee for gynecology and obstetrics, pediatrics and psychiatry approved the research protocol (20/13/03/03/2015) for the six-year follow-up assessment. Informed written consent was obtained from all mothers at enrolment. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The study nurses, Maria Finne and Hanna Oksa, are acknowledged for excellent coordination of study visits and data collection management.
Author Contributions
LL assisted with cardiovascular data collection during the follow-up and was responsible for data management and analysis, and drafting of the manuscript. JS assisted in data collection, data management and analysis. JM, JK, TT assisted with data management. KR managed the data of the primary recruitment and assisted with follow-up data management. SK coordinated and recruited the original cohort. JE coordinated the follow-up study. The cardiovascular data were gathered by TS, who supervised data analysis and writing of the paper. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Funding
This study has been supported by grants from the Sigrid Juselius Foundation, the Medical Society of Finland, Medicinska understödsföreningen Liv och Hälsa, Finnish Foundation for Pediatric Research, the Stockmann Foundation, and the National Science Centre, Poland (research project No 2018/29/N/NZ7/00991).
Disclosure
The authors report no conflicts of interest in this work.
References
1. McGill HC, McMahan CA, Herderick EE, et al. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. Arterioscler Thromb Vasc Biol. 2000;20(3):836–845. doi:10.1161/01.ATV.20.3.836
2. Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med. 1998;338(23):1650–1656. doi:10.1056/nejm199806043382302
3. Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. doi:10.1161/CIRCULATIONAHA.105.548206
4. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi:10.1161/CIRCULATIONAHA.109.192703
5. Laitinen TT, Pahkala K, Magnussen CG, et al. Lifetime measures of ideal cardiovascular health and their association with subclinical atherosclerosis: the Cardiovascular Risk in Young Finns Study. Int J Cardiol. 2015;185:186–191. doi:10.1016/j.ijcard.2015.03.051
6. Aneni EC, Crippa A, Osondu CU, et al. Estimates of mortality benefit from ideal cardiovascular health metrics: a dose response meta-analysis. J Am Heart Assoc. 2017;6(12). doi:10.1161/JAHA.117.006904
7. O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340(1):14–22. doi:10.1056/NEJM199901073400103
8. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121(4):505–511. doi:10.1161/CIRCULATIONAHA.109.886655
9. Lloyd-Jones DM, Nam BH, D’Agostino RB, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a Prospective Study of parents and offspring. J Am Med Assoc. 2004;291(18):2204–2211. doi:10.1001/jama.291.18.2204
10. Lechner K, von Schacky C, McKenzie AL, et al. Lifestyle factors and high-risk atherosclerosis: pathways and mechanisms beyond traditional risk factors. Eur J Prev Cardiol. 2020;27(4):394–406. doi:10.1177/2047487319869400
11. McPherson R, Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ Res. 2016;118(4):564–578. doi:10.1161/CIRCRESAHA.115.306566
12. Liu RS, Wake M, Grobler A, et al. Cross-sectional associations between ideal cardiovascular health scores and vascular phenotypes in 11- to 12-year-olds and their parents: the Longitudinal Study of Australian Children. Int J Cardiol. 2019;277:258–265. doi:10.1016/j.ijcard.2018.11.020
13. Raitakari OT, Juonala M, Kähönen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. J Am Med Assoc. 2003;290(17):2277–2283. doi:10.1001/jama.290.17.2277
14. Sundholm JKM, Litwin L, Rönö K, Koivusalo SB, Eriksson JG, Sarkola T. Maternal obesity and gestational diabetes: impact on arterial wall layer thickness and stiffness in early childhood - RADIEL study six-year follow-up. Atherosclerosis. 2019;284:237–244. doi:10.1016/j.atherosclerosis.2019.01.037
15. Rönö K, Stach-Lempinen B, Klemetti MM, et al. Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL). BMC Pregnancy Childbirth. 2014;14(1):70. doi:10.1186/1471-2393-14-70
16. Sarkola T, Redington A, Keeley F, Bradley T, Jaeggi E. Transcutaneous very-high-resolution ultrasound to quantify arterial wall layers of muscular and elastic arteries: validation of a method. Atherosclerosis. 2010;212(2):516–523. doi:10.1016/j.atherosclerosis.2010.06.043
17. Sarkola T, Manlhiot C, Slorach C, et al. Evolution of the arterial structure and function from infancy to adolescence is related to anthropometric and blood pressure changes. Arterioscler Thromb Vasc Biol. 2012;32(10):2516–2524. doi:10.1161/ATVBAHA.112.252114
18. Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5(3):243–255. doi:10.1007/s12265-012-9359-6
19. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). Cerebrovasc Dis. 2012;34(4):290–296. doi:10.1159/000343145
20. Pereira T, Maldonado J. Comparative study of two generations of the complior device for aortic pulse wave velocity measurements. Blood Press Monit. 2010;15(6):316–321. doi:10.1097/MBP.0b013e32833f5685
21. Mattace-Raso FUS, Hofman A, Verwoert GC, et al. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338–2350. doi:10.1093/eurheartj/ehq165
22. Saari A, Sankilampi U, Hannila M-L, Kiviniemi V, Kesseli K, Dunkel L. New Finnish growth references for children and adolescents aged 0 to 20 years: length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann Med. 2011;43(3):235–248. doi:10.3109/07853890.2010.515603
23. Kyttälä P, Erkkola M, Lehtinen-Jacks S, et al. Finnish Children Healthy Eating Index (FCHEI) and its associations with family and child characteristics in pre-school children. Public Health Nutr. 2014;17(11):2519–2527. doi:10.1017/S1368980013002772
24. Meinilä J, Valkama A, Koivusalo SB, et al. Healthy Food Intake Index (HFII) - validity and reproducibility in a gestational-diabetes-risk population. BMC Public Health. 2016;16(1):680. doi:10.1186/s12889-016-3303-7
25. Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. doi:10.1542/peds.2017-1904
26. Nordic Council of Ministers. Nordic Nutrition Recommendations 2012 – Integrating Nutrition and Physical Activity.
27. Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26(14):1557–1565. doi:10.1080/02640410802334196
28. Matarma T, Tammelin T, Kulmala J, Koski P, Hurme S, Lagström H. Factors associated with objectively measured physical activity and sedentary time of 5–6-year-old children in the STEPS Study. Early Child Dev Care. 2017;187(12):1863–1873. doi:10.1080/03004430.2016.1193016
29. Ryder JR, Pankratz ND, Dengel DR, et al. Heritability of vascular structure and function: a Parent–Child Study. J Am Heart Assoc. 2017;6(2):2. doi:10.1161/JAHA.116.004757
30. North KE, MacCluer JW, Devereux RB, et al. Heritability of carotid artery structure and function: the Strong Heart Family Study. Arterioscler Thromb Vasc Biol. 2002;22(10):1698–1703. doi:10.1161/01.ATV.0000032656.91352.5E
31. Xiang AH, Azen SP, Buchanan TA, et al. Heritability of subclinical atherosclerosis in Latino families ascertained through a hypertensive parent. Arterioscler Thromb Vasc Biol. 2002;22(5):843–848. doi:10.1161/01.ATV.0000015329.15481.E8
32. Pacor JM, Younus A, Malik R, et al. Prevalence of ideal cardiovascular health metrics in children & adolescents: a systematic review. Prog Pediatr Cardiol. 2016;43:141–146. doi:10.1016/j.ppedcard.2016.09.002
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.