Back to Journals » OncoTargets and Therapy » Volume 13
Icariin Mitigates the Growth and Invasion Ability of Human Oral Squamous Cell Carcinoma via Inhibiting Toll-Like Receptor 4 and Phosphorylation of NF-κB P65
Authors Lei K, Ma B, Shi P, Jin C, Ling T, Li L, He X, Wang L
Received 5 May 2019
Accepted for publication 19 December 2019
Published 10 January 2020 Volume 2020:13 Pages 299—307
DOI https://doi.org/10.2147/OTT.S214514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjay Singh
Ke Lei, 1 Bing Ma, 2 Ping Shi, 2 Che Jin, 3 Tan Ling, 1 Longjiang Li, 4 Xiangyi He, 5 Lunchang Wang 1
1Department of Stomatology, Central Hospital of Guangyuan, Guangyuan 628000, People’s Republic of China; 2Department of Respiratory, Central Hospital of Guangyuan, Guangyuan 628000, People’s Republic of China; 3Endodontics Department, Dental Hospital of Lanzhou, Lanzhou 730000, People’s Republic of China; 4State Key Laboratory of Oral Diseases, Sichuan University, Chengdu 610041, People’s Republic of China; 5Department of Prosthodontics, School of Stomatology, Lanzhou University, Lanzhou 730000, People’s Republic of China
Correspondence: Ping Shi
Department of Respiratory, Central Hospital of Guangyuan, Guangyuan 628000, People’s Republic of China
Tel/Fax +86 839-3222256
Email [email protected]
Background: Oral squamous cell carcinoma (OSCC) is an aggressive malignancy worldwide. Icariin (ICA), an active ingredient of flavonoids, has been demonstrated to possess antitumor activity in diverse cancers. Whereas, the role of ICAin OSCC is still unclear.
Methods: Herein, we investigated the anti-tumor effects of ICA in vitro and in vivo. CCK-8, colony formation and trans-well assay were used to examined viability, proliferation and invasion in SCC-9 and SCC-15 cell lines, respectively. Next, we tested the expression of toll-like receptor 4 (TLR4) and NF-κB P65 by western blot or immunofluorescence staining. Finally, we constructed a xenograft mice model to investigate the effect of ICA in vivo.
Results: In vitro, ICA decreased the human oral squamous cells viability, proliferation and invasion in a concentration-dependent manner. Besides, ICA decreased the phosphorylation level of P65 and down-regulated TLR4 protein. In vivo, compared with control, ICA significantly suppressed the tumor size and weight. In addition, ICA downregulated the levels of Ki67 and VEGF markedly. Dramatically, ICA decreased the phosphorylation level of P65 in tumor tissues.
Conclusion: Taken together, ICA could act as a anticancer drug against OSCC to mitigate the growth and invasion ability, the underlying mechanism may due to the down-regulation of TLR4/NF-κB signaling.
Keywords: icariin, toll-like receptor 4, oral squamous cell carcinomas, NF-κB
Introduction
Oral squamous cell carcinoma (OSCC) is the major form of oral cancer, counting about 90%.1 It has been reviewed that OSCC has kept increasing and the estimated new cases and deaths resulting from OSCC world-wide were 1,688,780 and 600,920, at the end of 2017, respectively.2,3 Despite great advances were made in surgery, chemotherapy and radiotherapy, the 5-year survival rate of OSCC barely changed over the decades.4 The high incidence of OSCC is mainly due to the prevalence of smoking and drinking, which have been proved to be the two major risk factors of OSCC.5 In addition, OSCC is also associated with long-term malnutrition, viral infection and poor oral hygiene.6 Thus, it is critical to reveal the internal mechanism and find new therapeutic strategies for OSCC.
Icariin (ICA) is an active polyphenol extracted from the plant Epimedium genus. As previous researches reported, ICA has a variety of pharmacological benefits including anti-osteoporosis,7 anti-cardiovascular,8 antitumor9,10 and organ protection.11,12 Interestingly, ICA has been proved to suppress ovarian cancer,9 thyroid cancer,10 colon cancer13 and osteosarcoma14 via different signals. However, the anti-OSCC role of ICA has not been discussed yet.
Toll-like receptor 4 (TLR4) is a member of TLRs, which are necessary mediators of the innate immunity and recognize considerable inflammatory inducers.15 Once activation, TLRs recruit MyD88 (an adaptor molecule) to activate NF-kB pathway. TLR4/NF-κB signaling pathway has been demonstrated to play a critical role in many inflammatory diseases including cancers. Usually, it is activated in diverse diseases and cancer tissues. Also, NF-κB signaling pathway is activated in OSCC cells.16,17 Thus, TLR4/NF-κB is a nice target for OSCC treatment. In present study, relevant researches were performed to figure out the effect of ICA on OSCC and the internal mechanism.
Methods and Materials
Cell Culture and Reagents
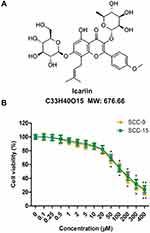
The human SCC-9 and SCC-15 cell lines were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in 1:1 mixture of Dulbecco’s modified Eagle’s medium (DMEM; Gibco Inc., Billings, MT, USA) and Ham’s F12 medium (Gibco) containing 1.2 g/L sodium bicarbonate, 2.5 mM L-glutamine, 15 mM HEPES and 0.5 mM sodium pyruvate supplemented with 400 ng/mL hydrocortisone, and 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin + streptomycin (Gibco). Cell cultures were kept in a 5% CO2 incubator at 37°C. ICA was purchased from ApexBio (Shanghai, China) and the chemical structure was shown in Figure 1A.
Cell Toxicity Assay
Briefly, cell counting kit-8 (CCK-8; Beyotime, Shanghai, China) assay was employed to test cell toxicity of ICA. The SCC-9 and SCC-15 cell lines were treated with gradient concentration of ICA from 0 to 400 μM for 48 h, respectively. CCK-8 assay was performed according to the manufacturer’s instructions .
Colony Formation Assay
SCC-9 and SCC-15 cells were divided into 4 groups and treated with DMSO (Control), 5 μM ICA, 10 μM ICA and 20 μM ICA for 48 h, separately. Colony formation assay was performed as described before.18 Briefly, the base agar containing 0.6% agarose in a 6-well plate was prepared. Next, 1.5 × 104 single cells were mixed with 1 mL complete medium supplemented with 0.3% soft agar (Sigma-Aldrich, USA). Then, the mixture was added to the above 6-well plate. After 5-week culture, the colonies were fixed and stained by PBS containing 4% formaldehyde and 0.005% crystal violet.
Cell Invasion Assay
Cell invasion assay was performed by Trans-well chamber as previous reported.19 Briefly, the upper face of the membrane was covered with Matrigel (BD Biosciences, San Jose, CA, USA) and the upper chamber was filled with 100 μL medium without serum containing 50,000 cells. The lower chamber was filled with 600 μL complete medium with FBS. After 24 h, the undersurface of the polycarbonate membranes was stained by crystal violet and the number of cells was counted visually in six-random fields at 100× magnification under a light microscope.
Western Blot Analysis
SCC-9 and SCC-15 cells were treated as previously described. Cells were treated with RIPA lysis buffer containing cocktail tablets (Sigma Aldrich) and phenylmethanesulfonyl fluoride (PMSF, Sigma Aldrich). Then, the cell extraction was quantified by BCA assay. Next, proteins were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were sealed with 5% skim milk in TBST and probed with specific primary antibodies for 2 h. The primary antibodies were list as followed: Ki67 (#ab16667, Abcam, UK), PCNA (#2586, CST, USA), VEGF (#ab32152, Abcam), Vimentin (#ab8978, Abcam), TLR4 (#14358, CST), Phospho-NF-κB p65 (Ser536, #3033, CST) and NF-κB p65 (#8242, CST). Horseradish peroxidase (HRP)-conjugated secondary antibody anti-mouse IgG, HRP-linked Antibody (#7076, CST) or anti-rabbit IgG, HRP-linked Antibody (#7074, CST) were used to combine with the primary antibodies. Finally, the target proteins were visualized by ECL and ChemiDoc XRS imaging system. GAPDH was chosen as an internal control.
Immunofluorescence Staining
Immunofluorescence staining was performed to confirm whether the P65 is activated in SCC-9 and SCC-15 cells. Cells were treated and grouped as previously described. Then, cells were collected and washed with PBS twice and fixed with 4% paraformaldehyde for 20 min at room temperature. Next, the samples were permeabilized with 0.4% TritonX-100 (Sigma-Aldrich) for 10 min, washed with PBS and then sealed with 2% bovine serum albumin (BSA; Solarbio, Beijing, China) in PBS for 1 h at 37°C. After this, the samples were incubated with P65 primary antibody in 1% BSA (Solarbio) in PBS for overnight at 4°C. Then, the samples were washed with PBS and incubated with fluorescein-labeled secondary antibody in 1% BSA (Solarbio) in PBS for 1 h in dark. Finally, the samples were stained with DAPI (1 μg/mL) for 10 min to stain cell nuclei and the cells were visualized by a fluorescence microscope (OLYMPUS, Japan).
Tumorigenesis in vivo
To verify the anti-tumor effect of ICA in vivo, the xenograft models were established in BALB/c mice. BALB/c mice were obtained from Experimental animal center of Sichuan university (Chengdu, China). Briefly, about 3 × 107 SCC-9 cells were suspended in 1 mL Hank’s balanced salt solution (HBSS, Gibco), then 100 μL cell suspension was injected into the left striata of BALB/c mice. Mice were administrated with 40 mg/kg/day ICA (body weight)20 or saline injection intraperitoneally 1 h after SCC-9 cells injection. Mice treated with ICA or saline were sacrificed after injection for 30 days. Tumor weight was measured. All the animal experiments procedures in this study were approved by the Institutional Animal Care and Use Committee of the Central Hospital of Guangyuan and were conducted strictly in accordance with the guidelines of International Guiding Principles for Animal Research.
Immunohistochemistry
Mice tumors were cut into 2-μm sections and embedded with paraffin. Before immunohistochemistry, sections were deparaffinized and rehydrated. Firstly, sections were blocked with 3% H2O2 for 10 min at room temperature followed by incubation with primary antibody against Ki67 and VEGF at 4°C overnight. Secondly, sections were washed with PBS followed by incubation with biotinylated goat anti-mouse immunoglobulin G secondary antibody (Dako, Denmark) at 25°C for 30 min. Thirdly, sections were washed followed by incubation with streptavidin-peroxidase reagent (Dako) and 3, 3ʹ-diaminobenzidine (DAB; Sigma) mixture for 5 min. Finally, sections were counterstained and dehydrated for observation.
Statistics Analysis
Statistically significant differences were evaluated by means of analysis of variance (ANOVA) followed Dunnett’s post hoc test. Data are illustrated in bar graphs, including mean ± SEM. Asterisks/pound denote statistical significance. Statistical significance was considered if p<0.05 in any analysis. All the data were analyzed using Graph Pad Prism 5 (Graph Pad Software, Inc., San Diego, CA).
Results
ICA Inhibited the Viability of Human Oral Squamous Cells
The cytotoxicity assay was performed by CCK-8 assay using SCC-9 and SCC-15 cells treated with ICA at concentrations from 0 to 400 μM for 24 h, respectively. The result from Figure 1B showed that ICA exerted an inhibitory effect on SCC-9 and SCC-15 cells in a concentration-dependent manner. However, ICA at concentrations of 50 μM or higher than 50 μM exhibited obvious cytotoxicity to two-cell lines. Therefore, we chose 3 concentrations of 5 μM, 10 μM and 20 μM for ICA treatment in subsequent experiment.
ICA Inhibited the Colony-Forming Capability of Human Oral Squamous Cells
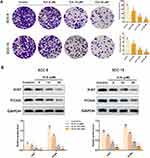
To explore the role of ICA on proliferation of SCC-9 and SCC-15 cells, colony forming assay was performed. As shown in Figure 2A, compared with the matching control group, the capacities of forming colonies were significantly receded by ICA treatment in a concentration-dependent manner. Parallelly, to verify the proliferation inhibitory effect of ICA on two-cell lines, the expression of Ki67 and PCNA proteins were detected by western blot. As a result, ICA down-regulated the Ki67 and PCNA proteins expression in a manner of concentration dependent (Figure 2B).
ICA Inhibited the Invasion of Human Oral Squamous Cells
To elucidate the role of ICA on mobility, we performed cell invasion by trans-well assay. As a result from Figure 3A, the number of invasive cells were significantly reduced by ICA of different concentrations in SCC-9 and SCC-15 cells. Next, we also detected the expression of MMP-9 and Vimentin in two cells, separately. As a result, the data showed that ICA down-regulated the protein levels of MMP-9 and Vimentin in SCC-9 cell. Similarly, this result was supported in SCC-15 (Figure 3B).
ICA Inactivated the TLR4/NF-κB Pathway
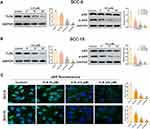
To elucidate the underlying anticancer mechanism of ICA, we tested the expression levels of TLR4/NF-κB axis. As shown in Figure 4A, western blot analysis showed that ICA down-regulated the TLR4 protein expression, and we observed that the downstream NF-κB p65 was phosphorylated in the ICA treatment group, the NF-κB p65 phosphorylation (p-NF-κB p65) level was reduced in a manner of concentration dependent (Figure 4B). Parallelly, the results of SCC-15 were consistent (Figure 4A and B). In particular, we also found that ICA inhibited p65 nuclear translocation. As shown in Figure 4C, the nuclear level of p65 was higher in control group, while ICA treatment significantly reduced the nuclear level of p65 in SCC-9 and SCC-15 via a manner of concentration dependent.
ICA Inhibited the Growth in vivo
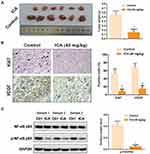
In order to confirm the antitumor effect of ICA in vivo, we established a SCC-9 xenograft tumor model. As shown in Figure 5A, the decreased tumor size and weight were observed in ICA treatment group. As shown in Figure 5B, the expression of Ki67 and VEGF were significantly decreased, compared with control. Importantly, the reduced p-NF-κB p65 level was also exhibited in vivo tumor samples (Figure 5C).
Discussion
Treatment of OSCC still remains a large challenge because of the lack of effective early treatment strategies and high incidence of recurrence.1 The pathological mechanism of OSCC development is associated with unrestricted proliferation and migration of oral squamous cells.21 In present study, we selected SCC-9 and SCC-15 cell lines to be an object for in vitro study. SCC-9 and SCC-15 cells, as squamous carcinoma cells, are widely used in OSCC study. And in vivo study was conducted by establishing SCC-9 ectopic xenograft mice model.
ICA has already identified to confine the growth and mobility in several cancer cells. For instance, ICA was proved to suppress cell cycle transition and cell migration in ovarian cancer cells.9 Besides, ICA was demonstrated to inhibit cell proliferation, migration and invasion in thyroid cancer cells.10 In addition, Tian et al reported that ICA reduced human colon carcinoma cell growth and metastasis activity.13 In this study, we primarily identified that ICA inhibited the proliferation and invasion of SCC-9 and SCC-15 cells and suppressed the tumor growth in vivo. Our present study firstly revealed that ICA could impede the growth and metastasis of OSCC. The in vitro results indicated that the viability, colony formation and invasion of SCC-9 and SCC-15 cells were inhibited by ICA in a manner of concentration dependent. The obtained data was consistent with the early researches. Our in vivo results further verify the tumor growth inhibitory role of ICA, and the data revealed the xenograft tumor size and weight were directly reduced by ICA (40 mg/kg/day) treatment.22 Deeply, we also found that the expression of VEGF and Ki67 were decreased. All in all, ICA inhibited the development of OSCC.
TLR4/NF-κB signaling pathway is positively correlated with inflammation. Cancer, in a kind, is an inflammation disease. Therefore, inflammation environment is beneficial for cancer development. In addition, TLR4 is overexpressed in a variety of cancer cells, including hepatocellular carcinoma,23 ovarian and breast cancer cells.24 In our study, TLR4 was overexpressed in SCC-9 and SCC-15 cells as well as in xenograft tumor tissues of OSCC, suggesting that TLR4 may be an oncogene in OSCC. Proverbially, p65 is a subunit of the NF-κB, which can be activated by phosphorylation under oxidative stress and inflammation environment. After being activated, p65 was transferred into the nucleus to regulate inflammation-related genes.25 In this study, p65 was greatly increased in nucleus, which suggested that TLR4/NF-κB signaling pathway is activated in OSCC cells and tumors. Dramatically, in the present study, ICA decreased the expression of TLR4 and the p-P65 NF-κB in a manner of concentration dependent in vitro and in vivo. Previous studies confirmed that the inhibitory effect of ICA on TLR4 expression,26 and discussed its inhibitory role on NF-κB.27 Herein, combined with previous reports, we explored whether ICA was involved in TLR4/NF-κB pathways in OSCC, and we first demonstrated that ICA inhibited the growth and invasion of OSCC via TLR4/NF-κB pathway.
In summary, our present study revealed that ICA could suppress the viability, colony formation and invasion of OSCC, the internal mechanism might be related to the inhibition of TLR4/NF-κB signaling pathway. Our study indicated the potential drug target of OSCC and enriched the pharmacological activities of ICA. However, whether ICA connects with other pathways or modulates multiple pathways to act OSCC requires further study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liang X, Deng M, Zhang C, et al. Combined class I histone deacetylase and mTORC1/C2 inhibition suppresses the initiation and recurrence of oral squamous cell carcinomas by repressing SOX2. Cancer Lett. 2019;454:108–119. doi:10.1016/j.canlet.2019.04.010
2. He B, Lin X, Tian F, Yu W, Qiao B. MiR-133a-3p inhibits oral squamous cell carcinoma (OSCC) proliferation and invasion by suppressing COL1A1. J Cell Biochem. 2018;119(1):338-346. doi:10.1002/jcb.26182
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
4. Marsh D, Suchak K, Moutasim KA, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223(4):470–481. doi:10.1002/path.2830
5. Tanaka F, Yamamoto K, Suzuki S, et al. Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut. 2010;59(11):1457–1464. doi:10.1136/gut.2009.205724
6. Van ZA, Bunn BK. Clinical features of oral cancer. SADJ. 2012;67(10):566–569.
7. Hwang E, Lin P, Ngo HT, et al. Icariin and icaritin recover UVB-induced photoaging by stimulating Nrf2/ARE and reducing AP-1 and NF-κB signaling pathways: a comparative study on UVB-irradiated human keratinocytes. Photochem Photobiol Sci. 2018;17(10):1396–1408. doi:10.1039/C8PP00174J
8. Zheng Y, Lu L, Yan Z, et al. mPEG-icariin nanoparticles for treating myocardial ischaemia. Artif Cells Nanomed Biotechnol. 2019;47(1):801–811. doi:10.1080/21691401.2018.1554579
9. Wang P, Zhang J, Xiong X, et al. Icariin suppresses cell cycle transition and cell migration in ovarian cancer cells. Oncol Rep. 2019;41(4):2321–2328. doi:10.3892/or.2019.6986
10. Fang L, Xu W, Kong D. Icariin inhibits cell proliferation, migration and invasion by down-regulation of microRNA-625-3p in thyroid cancer cells. Biomed Pharmacother. 2019;109:2456–2463. doi:10.1016/j.biopha.2018.04.012
11. Chen HA, Chen CM, Guan SS, Chiang CK, Wu CT, Liu SH. The antifibrotic and anti-inflammatory effects of icariin on the kidney in a unilateral ureteral obstruction mouse model. Phytomedicine. 2019;59:152917. doi:10.1016/j.phymed.2019.152917
12. Angeloni C, Barbalace MC, Hrelia S. Icariin and its metabolites as potential protective phytochemicals against alzheimer’s disease. Front Pharmacol. 2019;10:271. doi:10.3389/fphar.2019.00271
13. Tian M, Yang S, Yan X. Icariin reduces human colon carcinoma cell growth and metastasis by enhancing p53 activities. Braz J Med Biol Res. 2018;51(10):e7151. doi:10.1590/1414-431X20187151
14. Ren Y, Zhu F, Liu Z. Inhibitory effect of icariin on osteosarcoma cell proliferation via the Wnt/β-catenin signaling pathway. Oncol Lett. 2018;16(2):1405–1410. doi:10.3892/ol.2018.8821
15. Jiang Y, He L, Ju C, et al. Isolation and expression of grass carp toll-like receptor 5a (CiTLR5a) and 5b (CiTLR5b) gene involved in the response to flagellin stimulation and grass carp reovirus infection. Fish Shellfish Immunol. 2015;44(1):88–99. doi:10.1016/j.fsi.2015.01.024
16. Wei LY, Lee JJ, Yeh CY, et al. Reciprocal activation of cancer-associated fibroblasts and oral squamous carcinoma cells through CXCL1. Oral Oncol. 2019;88(undefined):115–123. doi:10.1016/j.oraloncology.2018.11.002
17. Bano N, Yadav M, Mohania D, Das BC, Ahmad A. The role of NF-κB and miRNA in oral cancer and cancer stem cells with or without HPV16 infection. PLoS One. 2018;13(10):e0205518. doi:10.1371/journal.pone.0205518
18. Yang D, Cheng D, Tu Q. HUWE1 controls the development of non-small cell lung cancer through down-regulation of p53. Theranostics. 2018;8(13):3517–3529. doi:10.7150/thno.24401
19. Zhang P, Wang C, Ma T, You S. O-GlcNAcylation enhances the invasion of thyroid anaplastic cancer cells partially by PI3K/Akt1 pathway. Onco Targets Ther. 2015;8(2):3305–3313. doi:10.2147/OTT.S82845
20. Liu B, Xu C, Wu X, et al. Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience. 2015;294:193–205. doi:10.1016/j.neuroscience.2015.02.053
21. Liu S, Liu LH, Hu WW, Wang M. Long noncoding RNA TUG1 regulates the development of oral squamous cell carcinoma through sponging miR-524-5p to mediate DLX1 expression as a competitive endogenous RNA. J Cell Physiol. 2019;234(11):20206–20216. doi:10.1002/jcp.28620
22. Zhang DC, Liu JL, Ding YB, Xia JG, Chen GY. Icariin potentiates the antitumor activity of gemcitabine in gallbladder cancer by suppressing NF-κB. Acta Pharmacol Sin. 2013;34(2):301–308. doi:10.1038/aps.2012.162
23. Zhou S, Du R, Wang Z, et al. TLR4 increases the stemness and is highly expressed in relapsed human hepatocellular carcinoma. Cancer Med. 2019;8(5):2325–2337. doi:10.1002/cam4.2070
24. Zandi Z, Kashani B, Poursani EM, et al. TLR4 blockade using TAK-242 suppresses ovarian and breast cancer cells invasion through the inhibition of extracellular matrix degradation and epithelial-mesenchymal transition. Eur J Pharmacol. 2019;853:256–263. doi:10.1016/j.ejphar.2019.03.046
25. DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246(1):379–400. doi:10.1111/j.1600-065X.2012.01099.x
26. Wu J, Zhou J, Chen X, et al. Attenuation of LPS-induced inflammation by ICT, a derivate of icariin, via inhibition of the CD14/TLR4 signaling pathway in human monocytes. Int Immunopharmacol. 2012;12(1):74–79. doi:10.1016/j.intimp.2011.10.015
27. Ma P, Zhang S, Su X, Qiu G, Wu Z. Protective effects of icariin on cisplatin-induced acute renal injury in mice. Am J Transl Res. 2015;7(10):2105–2114.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.