Back to Journals » Clinical Ophthalmology » Volume 12
Icare® rebound tonometers: review of their characteristics and ease of use
Authors Nakakura S
Received 18 April 2018
Accepted for publication 17 May 2018
Published 12 July 2018 Volume 2018:12 Pages 1245—1253
DOI https://doi.org/10.2147/OPTH.S163092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Shunsuke Nakakura
Department of Ophthalmology, Saneikai Tsukazaki Hospital, Himeji City, Japan
Abstract: The rebound tonometer has a unique mechanism for measuring intraocular pressure (IOP) and has become popular worldwide due to its ease of use. The most notable advantages are the lack of an air-puff and need for topical anesthesia, ease of operation and transport, and the ability to use it with children. Four rebound tonometers (Icare® TA01i, Icare PRO, Icare HOME, and Icare ic100) are currently available for clinical examination. It is important to understand the characteristics of each tonometer and select the most appropriate one because the IOP values and the purpose of measurement are different. In this review, with the goal of improving the understanding of a range of tonometers, the issues with each device are discussed.
Keywords: rebound tonometer, intraocular pressure, glaucoma, Icare
Introduction
History of the rebound tonometer
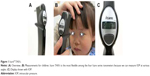
The detailed mechanism of the rebound tonometer was first described in 1997 by Kontiola.1 This type of tonometer is still available as Icare® TA01i (Helsinki, Finland) (Figure 1) released in 2003. The tonometer contains a tiny 1.8-mm diameter plastic ball on a stainless steel wire held in place by an electromagnetic field in a handheld battery-powered unit.2 When the button on the back is pushed, a spring drives the wire and ball forward rapidly. When the ball hits the cornea, the ball and wire decelerate; the deceleration is more rapid if the intraocular pressure (IOP) is high and slower if the IOP is low.2 The speed of deceleration is measured and converted by the device into IOP. The mechanism of this tonometer is superior to that of the Goldmann applanation tonometer (GAT), still regarded as the gold standard tonometer in glaucoma management, because there is no need for topical anesthesia and staining fluorescein, slit lamp mounting, and unnecessary infection care due to the use of a disposable probe. Additionally, this new tonometer does not require an air-puff compared to the conventional noncontact tonometer; therefore, it can easily be used for children or animals. Ophthalmologists and co-medicals treating children with infant or child glaucoma can appreciate the benefits of the rebound tonometer because of the advantages of taking IOP measurements without the need for general anesthesia or sedation.3 However, the disadvantage of Icare TA01i is that the probe can fall out if the tonometer is facing downward. To overcome this problem, an updated version of the rebound tonometer, Icare PRO, was launched in 2010, with updated features including a built-in inclination sensor that enables IOP measurement in the supine position. The new probe (Figure 2) does not fall out when the tonometer is not upright and has improved accuracy, displaying IOP data outputs to the first decimal place.4 Icare PRO is easy to use, especially for measuring IOP when children are sleeping in a supine position in the clinic. Previous studies have implicated peak IOP as a major contributor to glaucoma progression.5,6 Additionally, monitoring 24-hour IOP using a contact sensor tonometer7,8 became a popular research topic around 2010. Consequently, home tonometry also received attention because normal tension glaucoma cannot be diagnosed using only one or two IOP measurements obtained throughout the day. Measuring the diurnal IOP values using home tonometry or during hospitalization is useful for determining the peak IOP and the degree of IOP fluctuation.8,9 A rebound tonometer designed for home/self-use, the IcareONE, was launched in the market, making it possible to determine IOP values outside of office hours.10,11 However, IcareONE has some demerits in that patients can know their approximate IOP values because the measured IOP values are displayed with an 11-point grading system and patients cannot recognize whether the device is held accurately to measure the IOP upright. Therefore, an updated version of the home/self-use tonometer was developed in the form of Icare HOME released in 2014.9,12 Icare HOME has an automatic eye recognition system that identifies the right or left eye, determines whether the instrument is held horizontally, and does not display the measurement data. Icare ic100, an updated version of Icare TA01i, has been available since 2016. Icare ic100 also has an upright position sensor like Icare HOME and Icare PRO, and the IOP value is displayed in a large font. The improvements in this generation of rebound tonometers were focused on preventing the fixation probe from falling, enabling self IOP measurements, and improving the upright position sensor.
  | Figure 2 Probes used in Icare® series. |
The characteristics of each device and IOP values
Icare TA01i
Measurements methods
Icare TA01i is shown in Figure 1A and weighs ~250 g. After instructing the patient to relax and look straight ahead at a specific point to keep the eye steady, IOP measurements were recorded at a distance of 4–8 mm from the center of the cornea along the central corneal axis, and the long probe (Figure 2) was kept in a horizontal position to the corneal center. However, while measuring IOP in children in clinical practice, it is sometimes difficult to measure IOP while keeping the probe in a horizontal position. Clinicians should try and aim for the cornea even if it is the peripheral cornea at various angles because children often look down, up, and away (Figure 1B). Therefore, Icare TA01i is, in a sense, more flexible than the later three instruments (Icare PRO, Icare HOME, and Icare ic100) because it does not include a position sensor.
After six consecutive measurements, the result is given with a letter P on the display followed by the IOP (Figure 1C). The symbols P- and P_ indicate that the SD within the six measurements is greater than normal. If these letters appear, additional measurements should be obtained to ensure accuracy.
Measuring IOP with a patient in the supine position using Icare TA01i is difficult because the probe needs to remain upright. To solve this problem, patients should lie on their left side (ie, left lateral position) when the right eye is measured (and vice versa). The measurement values for the lower eye will be artificially yet significantly higher than those for the upper eye because of the postural change required for the supine position.13
The repeatability for Icare TA01i in the sitting position, recorded as intraclass correlation coefficients (ICC; three sets of IOP readings), was 0.9509 and 0.934.14
IOP tendency compared by GAT using Bland–Altman plots
IOP tendency to GAT was given as the mean IOP difference with SD, if applicable, and 95% limits of agreement (LOA), if applicable.
Previous studies have shown that Icare TA01i gives a slightly higher mean IOP than GAT in the sitting position: 3.35±2.28 mmHg (95% LOA was between −1.12 and 7.81 mmHg),15 1.40±2.19 mmHg (95% LOA was ±4.29 mmHg),16 and 0.6±3.27 mmHg (95% LOA was between −6 and 7 mmHg).17
Additionally, in the supine position, Icare TA01i gives slightly higher mean IOP than GAT: 1.49±2.90 mmHg (95% LOA was between −4.20 and 7.18 mmHg) in our results.18 However, these previous studies enrolled relatively young patients or subjects. In our prospective study of healthy older subjects with a mean age of 72 years, Icare TA01i gave lower mean IOP compared to GAT (−2.46±2.10 mmHg; 95% LOA was between −8.25 and 1.66 mmHg).14 These age-related IOP changes noted using the rebound tonometer were first reported by González-Méijome et al.20 The mean patient age was 45 years (range 18–85 years). They found a negative correlation between age and IOP in the nasal and temporal cornea, but not in the corneal center.20 These results are contradictory to our data18 and those of Sakamoto et al19 (IOP was measured at the corneal center). Sakamoto et al19 reported that older age resulted in the underestimation of IOP measured using GAT with IcareONE, which uses a long probe. Increasing the collagen diameter and larger interfibrillar spacing induced thickening of the peripheral cornea.21 These topographical differences in the stromal collagen and age-related changes may explain the IOP age-related tendency. Grabner et al reported the elastic properties of cornea using dynamic corneal imaging system and that the corneal resistance to indentation was positively correlated to IOP and central corneal thickness, but negatively correlated with age (ie, elderly patients have lower resistance).22 This supports the finding that older patients have lower IOP with Icare TA01i.
Additionally, corneal biomechanical factors such as corneal hysteresis and corneal resistance factor measured by the ocular response analyzer also influenced the IOP measured using Icare TA01i.23,24
Other aspects of the IOP measuring situation that affect the IOP values have also been reported. Beasley et al reported that Icare TA01i is insensitive to small deviations (within 10°) that can occur during the measurements in general ophthalmic practice.25 Takenaka et al reported that IOP measurements at 2 mm from the limbus in the nasal and temporal regions with the probe perpendicular to the cornea were approximately equal to the IOP measured with the GAT.26 These findings are in accordance with the observation that when measuring IOP using Icare TA01i, as long as we kept the probe perpendicular to the cornea, the IOP values were robust.
Icare PRO
Measurements methods
Icare PRO is an updated version of the Icare TA01i rebound tonometer and has been available since 2011. The overall picture is shown in Figure 3A and the weight is ~275 g. The tonometer functions by bouncing a novel magnetized probe off the cornea and measuring the subsequent deceleration of the short probe (Figure 2).
  | Figure 3 Icare® PRO. |
IOP measurements were taken in accordance with the manufacturer’s manual, with the tonometer positioned 3–7 mm from the center of the cornea along the central corneal axis. The position sensor allows IOP measurements in both horizontal and vertical positions. A slight tilt of the instrument in either of the positions prevents the probe from being launched.
After six consecutive measurements, a background color is displayed followed by the IOP. Background colors of yellow or red indicate a slightly high or high SD among the different measurements, respectively. If yellow or red appeared, we repeated this procedure to ensure accuracy, until the IOP obtained was displayed in green, which indicates an accurate final measurement (Figure 3B).
The repeatability of Icare PRO, recorded as ICC (three sets of IOP readings), was 0.88114 and 0.8634 in the sitting position, and 0.6564 in the supine position.
IOP tendency compared by GAT using Bland–Altman plots
Icare PRO gives a mean IOP closer to that obtained with GAT. Previous reports showed that the mean IOP difference between Icare PRO and GAT was −0.38 mmHg (95% LOA was unclear),27 −0.22 mmHg (95% LOA was between −2.8 and 2.4 mmHg),28 0.01±2.16 mmHg (95% LOA was between −4.22 and 4.25 mmHg),10 0.1 mmHg (95% LOA was between −3.6 and 3.8 mmHg),29 and 0.43±2.28 mmHg (95% LOA was between −4.04 and 4.90 mmHg).4
In the supine position, the repeatability of Icare PRO was slightly lower than in the sitting position (ICC of three sets of values; 0.656 vs 0.863); however, the IOP difference between Icare PRO and GAT was only 0.14±2.14 mmHg (95% LOA was between −4.06 and 4.33 mmHg)4 and −0.4 mmHg (95% LOA was between −5.1 and 4.3 mmHg).29
Another factor that affects the measurement of IOP values is manual eyelid manipulation, which increases IOP (about 1.2 mmHg), especially in small palpebral fissure patients.28 It is rare to use a device like Icare PRO with such eyelid manipulation. However, when the examiner opens the eyelids manually and compresses the eyeball as in Baek et al,28 the tendency of the IOP to increase should be kept in mind. The effect of aging on IOP values has not been investigated with this probe and instrument. However, the mean IOP difference between GAT and Icare PRO was small; therefore, the aging effect is expected to be small.
Icare HOME
Measurement methods
Icare HOME is a self-monitoring tonometer designed for home use by glaucoma or glaucoma-suspect patients who require regular IOP monitoring according to the recommendation of an ophthalmologist. Icare HOME is the updated version of IcareONE (no longer available for sale). The IcareONE device displays the results of the measured IOP values with an 11-point grading system and thus allows patients to determine their own IOP values.9 Additionally, to correctly measure the IOP, the subject must hold the tonometer horizontally. However, the patient may be unable to recognize whether the device is held accurately. Therefore, Icare HOME is equipped with eye recognition sensors (that detect which eye is being observed) and positioning sensors (to ascertain whether it is held upright).
An overview of Icare HOME is shown in Figure 4A. The instrument size is ~11×8×3 cm, and the weight is ~150 g. The device includes a long probe (Figure 2) similar to Icare TA01i and Icare ic100, but not Icare PRO. The patient presses the power button and loads the probe. The distance between the tip of the probe and the center of the cornea, along the central corneal axis, is set for the patient at 4–8 mm by tuning the knobs individually. The patient holds the tonometer in front of their face without any vertical or horizontal tilt and presses the measurement button.12 The patient must confirm whether they are holding the device in the correct position by checking for a “green” ring signal (Figure 4B). If the instrument is not held horizontally, the ring signal shows up in “red.” In addition, if the probe is too close or far from the eye, or if the patient’s hair or hands are in the way, the ring signal flashes red with an error beep. The position sensor allows IOP measurements in the horizontal position with green signal indication. A slight malposition of the instrument prevents the probe from being launched.
  | Figure 4 Icare HOME. |
The patient can obtain six consecutive measurements by continually pressing the button or obtain six measurements using single mode until they hear a long beep.31 Finally, the patient must check for the “DONE” light illuminated on the back panel (Figure 4C). The measurement data are not displayed on the panel. Therefore, it is necessary to connect the Icare HOME device to a PC installed with the Icare LINK software program (Icare, Finland Oy, Vantaa, Finland). The tonometer stores information for every complete measurement sequence containing six measurements. The stored information includes the IOP in mmHg, time, date of the measurements, identification of the eye (right or left), and quality level of each measurement. The mean of four IOP values, excluding the maximum and minimum values of six consecutive measurements, is given as the IOP, similar to Icare TA01i. The ICC repeatability (three sets of IOP readings) was 0.8129 in the sitting position.
IOP tendency compared by GAT using Bland–Altman plots
Evaluation of IOP using Icare HOME was performed in two ways: self-measurement or measurement by a second party. Dabasia et al reported that the mean IOP difference between Icare HOME and GAT was −0.3 mmHg (95% LOA was between −5.2 and 4.6 mmHg) by self-measurement, −1.1 mmHg (95% LOA was between −5.3 and 3.2 mmHg) when measured by the partner, and −1.2 mmHg (95% LOA was between −6.3 and 3.9 mmHg) when measured by the trainer.30 Termühlen et al reported that the mean IOP difference between Icare HOME and GAT was −0.8 mmHg (95% LOA was between −7.2 and 5.6 mmHg) with measurement by a second party.12 Chen et al reported the mean IOP difference to be ±1 mmHg with GAT for both eyes (95% LOA was unclear) with self-measurement performed for both visits.31 Pronin et al reported that the IOP difference was −2.5 mmHg (95% LOA was between −4.71 and 9.88 mmHg) with self-measurement and −2.6 mmHg (95% LOA was between −3.48 and 8.8 mmHg) with measurement by a second party compared to GAT.32 Mudie et al reported that the IOP difference between GAT was −0.33 mmHg (95% LOA was unclear) with self-measurement.33 Only Takagi et al reported that the IOP difference between GAT was 0.70 mmHg (95% LOA was between −3.07 and 4.46 mmHg) with self-measurement and 0.91 mmHg (95% LOA was between −2.75 and 4.56 mmHg) as measured by a second party.34
Our previous data also show that the mean Icare HOME IOP was lower than that obtained with GAT 1.03 mmHg (95% LOA was between −5.98 and 3.91 mmHg) by self-measurement.9 Despite using the same long probe for Icare TA01i and examination of reports for various age ranges,9,10,12,30,32,33 the IOP obtained with Icare HOME was slightly lower than that obtained with GAT. This tendency is probably explained by 1) the instrument itself, and not the probe, because the IOP values with both self- and second party measurements are lower than those for GAT. 2) During self-measurements, the subject must confirm that the ring signal at the bottom of the probe is “green”, which is known as accommodation reducing the IOP.35,36 The dominant hand issue may be a concern for IOP measurements by Icare HOME. This is because precisely measuring IOP values in the left eye may be difficult for right-handed patients. However, this concern may not be pertinent in actual measurements.31,32
Diurnal IOP monitoring and its potential
Icare HOME is available to monitor IOP changes outside clinic hours; however, unlike Icare PRO, the IOP cannot be measured in the supine position using this device. Therefore, IOP monitoring can only be performed while the patient is awake, not sleeping.
Before lending the machine to patients, it is important to confirm that the patient can measure IOP by himself or herself after adequate training of taking IOP measurements with Icare HOME.
In our study, healthy young subjects could handle the device and measure diurnal IOP efficiently (100%).9 However, Dabasia et al reported that 71% of glaucoma patients and 83% of healthy subjects correctly measured IOP with Icare HOME (single measurement).30 Additionally, 67.5% of patients with glaucoma or glaucoma suspects (diurnal IOP),37 73% of glaucoma patients (single measurement),32 and 75% of patients with glaucoma or suspected with glaucoma (single measurement)33 accurately measured IOP using Icare HOME. Generally, the complete success of a single measurement by patients with glaucoma or glaucoma suspects is estimated to be about 70%–75%.30,32,33 Therefore, if glaucoma patients attempt diurnal IOP measurements, obtaining complete data will be more difficult. Probable reasons are 1) older patients have less mobility; 2) visual field defects, especially, central visual field disturbance, may affect the patients’ ability to see the “green” ring signal.
Patient satisfaction and compliance
Ease of use is an important factor in using this device for self/home monitoring. Our study included 43 healthy young subjects. Among these, 39.5% reported that the device was “easy to use,” 46.5% selected “normal,” and 13.9% selected “difficult to use.”9 The reasons why the six subjects selected “difficult to use” were “fear of the measurements” (4/6, 66.7%), “difficulty in operating the device” (1/6, 16.7%), and “difficulty because of blinking” (1/6, 16.7%). Dabasia et al reported that among 76 healthy subjects and glaucoma patients, 84% selected “easy to use,” 88% selected “the reading was quick to obtain,” 95% selected “the measurement was comfortable,” and 91% selected “I would use again” and “I would use this device at home.”30
Pronin et al reported that among 79 successful or partially successful patients (performing self-tonometry), 71% stated that “self-tonometry was easy,” and 92% selected “comfortable” and “happy to perform self-tonometry in the future.”32
Overall, the ease of use of Icare HOME for subjects and patients is very high; these results will encourage ophthalmologists to lend Icare HOME to patients for self/home tonometry.
Icare ic100
Measurement methods
Icare ic100 is the newest version of the rebound tonometer and an updated version of Icare TA01i. A picture of Icare ic100 is shown in Figure 5A and it weighs ~230 g. After pressing the “Select” or “Measure” button to turn the tonometer on, a long probe should be loaded, in a similar manner to Icare TA01i and Icare HOME. Similar to Icare TA01i and Icare PRO, after instructing the patient to relax and look straight ahead at a specific point to keep the eye steady, IOP measurements were taken at a 4–8 mm distance from the center of the cornea along the central corneal axis. Icare ic100 has a positioning sensor unlike Icare TA01i. Therefore, the probe should be kept in a horizontal position closer to the corneal center. If malpositioned, the probe cannot be launched by the position sensor. Icare ic100 has an automatic measurement mode (six consecutive blasts by pushing the button deep once) or manual mode (single blast by pushing the button lightly, and six measurements). After six consecutive measurements with a long beeping sound, the result appears on the plain display with a green circle (OK), a yellow circle (acceptable variation), and “repeat” (too much variation) (Figure 5B). It is slightly difficult for left-handed clinicians to see the display. Additionally, in our limited impressions with recent use, Icare ic100 is more difficult to use for measuring IOP in children, patients in wheelchairs, and handicapped persons, compared to Icare TA01i because of its strict position sensor.
IOP tendency compared by GAT using Bland–Altman plots
A literature search on PubMed on April 16, 2018 revealed no results on studies using Icare ic100. We conducted a cross-sectional study to compare the IOP tendency between Icare ic100 and GAT. Forty-five healthy subjects were enrolled from April 1 to April 16, 2018. The mean age was 29.7 years (range 22–48 years) with 15 men (33%).
IOP was measured twice in a randomized manner using GAT and Icare ic100, and the mean of two measurements was used for subsequent analysis. Our results showed that the mean IOP for Icare ic100 was 13.0±2.8 mmHg (range 3–18) and the IOP for GAT was 15.6±2.8 mmHg (range 9–24). The mean IOP difference between Icare ic100 and GAT was −2.53±2.77 mmHg (95% LOA was between −7.95 and 2.89 mmHg) (Figure 6A). The mean IOP obtained with Icare ic100 was significantly lower than that with GAT (P<0.001, by paired t-test). Scatterplot of IOPs from Icare ic100 and GAT (Figure 6B) showed that GAT gives higher IOPs than Icare ic100 at almost IOP’s range. The repeatability (ICC, two sets of IOP readings) of Icare ic100 was 0.94 (95% CI 0.89–0.97) in the sitting position.
  | Figure 6 (A) Box plots with mean IOP of Icare ic100 and GAT. IOP was significantly lower with the Icare ic100 than with GAT by about 2.5 mmHg. (B) Scatterplot of IOPs from Icare ic100 and GAT. |
Conclusion and future directions
In this review, we presented only the mean IOP differences compared to GAT. In general, the IOP tendency between two instruments is also evaluated by Bland–Altman analysis with 95% LOA. However, Bland–Altman analysis could not be found in some references. The Icare series is developing and spreading globally in clinical practice, with improved usage. To measure IOP correctly, a position sensor is useful to keep the instrument upright within the appropriate distance from the corneal center. However, this leads to contradictions because the patients who critically require IOP to be measured using the Icare series cannot remain motionless and quiet. Therefore, in our practice, the first Icare TA01i is still useful, especially in children who are awake. The characteristics of Icare tonometers are summarized in Table 1. Clinicians should pay attention to the patients’ characteristics and choose the appropriate Icare tonometer. However, measuring IOP with Icare tonometers has greater merits than ever before.
Acknowledgments
The author thanks his co-workers Etsuko Terao, Yuki Fujio, Yasuko Fujisawa, Kanae Matsuya, Yui Kobayashi, Asuka Noguchi, and Ryo Aoki for obtaining data.
Disclosure
The author reports no conflicts of interest in this work.
References
Kontiola A. A new electromechanical method for measuring intraocular pressure. Doc Ophthalmol. 1996–1997;93(3):265–276. | ||
Stamper RL. A history of intraocular pressure and its measurement. Optom Vis Sci. 2011;88(1):E16–E28. | ||
Grigorian F, Grigorian AP, Olitsky SE. The use of the iCare tonometer reduced the need for anesthesia to measure intraocular pressure in children. J AAPOS. 2012;16(6):508–510. | ||
Nakakura S, Mori E, Yamamoto M, Tsushima Y, Tabuchi H, Kiuchi Y. Intradevice and interdevice agreement between a rebound Tonometer, Icare PRO, and the Tonopen XL and Kowa hand-held applanation tonometer when used in the sitting and supine position. J Glaucoma. 2015;24(7):515–521. | ||
Zeimer RC, Wilensky JT, Gieser DK, Viana MA. Association between intraocular pressure peaks and progression of visual field loss. Ophthalmology. 1991;98(1):64–69. | ||
Wilensky JT, Gieser DK, Dietsche ML, Mori MT, Zeimer R. Individual variability in the diurnal intraocular pressure curve. Ophthalmology. 1993;100(6):940–944. | ||
Kakaday T, Hewitt AW, Voelcker NH, Li JS, Craig JE. Advances in telemetric continuous intraocular pressure assessment. Br J Ophthalmol. 2009;93(8):992–996. | ||
Mansouri K, Medeiros FA, Tafreshi A, Weinreb RN. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucoma. Arch Ophthalmol. 2012;130(12):1534–1539. | ||
Noguchi A, Nakakura S, Fujio Y, et al. A pilot evaluation assessing the ease of use and accuracy of the new self/home-tonometer IcareHOME in healthy young subjects. J Glaucoma. 2016;25(10):835–841. | ||
Moreno-Montañés J, Martínez-de-la-Casa JM, Sabater AL, Moralez-Fernandez L, Sáenz C, Garcia-Feijoo J. Clinical evaluation of the new rebound tonometers Icare PRO and Icare ONE compared with the Goldmann tonometer. J Glaucoma. 2015;24(7):527–532. | ||
Halkiadakis I, Stratos A, Stergiopoulos G, et al. Evaluation of the Icare-ONE rebound tonometer as a self-measuring intraocular pressure device in normal subjects. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1207–1211. | ||
Termühlen J, Mihailovic N, Alnawaiseh M, Dietlein TS, Rosentreter A. Accuracy of measurements with the iCare HOME rebound tonometer. J Glaucoma. 2016;25(6):533–538. | ||
Lee JY, Yoo C, Jung JH, Hwang YH, Kim YY. The effect of lateral decubitus position on intraocular pressure in healthy young subjects. Acta Ophthalmol. 2012;90(1):e68–e72. | ||
Kato Y, Nakakura S, Matsuo N, et al. Agreement among Goldmann applanation tonometer, iCare, and Icare PRO rebound tonometers; non-contact tonometer; and Tonopen XL in healthy elderly subjects. Int Ophthalmol. 2018;38(2):687–696. | ||
García-Resúa C, González-Meijome JM, Gilino J, Yebra-Pimentel E. Accuracy of the new ICare rebound tonometer vs. other portable tonometers in healthy eyes. Optom Vis Sci. 2006;83(2):102–107. | ||
Nakamura M, Darhad U, Tatsumi Y, et al. Agreement of rebound tonometer in measuring intraocular pressure with three types of applanation tonometers. Am J Ophthalmol. 2006;142(2):332–334. | ||
van der Jagt LH, Jansonius NM. Three portable tonometers, the TGDc-01, the ICARE and the Tonopen XL, compared with each other and with Goldmann applanation tonometry. Ophthalmic Physiol Opt. 2005;25(5):429–435. | ||
Nakakura S, Mori E, Yamamoto M, Tsushima Y, Tabuchi H, Kiuchi Y. Intraocular pressure of supine patients using four portable tonometers. Optom Vis Sci. 2013;90(7):700–706. | ||
Sakamoto M, Kanamori A, Fujihara M, Yamada Y, Nakamura M, Negi A. Assessment of IcareONE rebound tonometer for self-measuring intraocular pressure. Acta Ophthalmol. 2014;92(3):243–248. | ||
González-Méijome JM, Jorge J, Queirós A, et al. Age differences in central and peripheral intraocular pressure using a rebound tonometer. Br J Ophthalmol. 2006;90(12):1495–1500. | ||
Boote C, Dennis S, Newton RH, Puri H, Meek KM. Collagen fibrils appear more closely packed in the prepupillary cornea: optical and biomechanical implications. Invest Ophthalmol Vis Sci. 2003;44(7):2941–2948. | ||
Grabner G, Eilmsteiner R, Steindl C, Ruckhofer J, Mattioli R, Husinsky W. Dynamic corneal imaging. J Cataract Refract Surg. 2005;31(1):163–174. | ||
Chui WS, Lam A, Chen D, Chiu R. The influence of corneal properties on rebound tonometry. Ophthalmology. 2008;115(1):80–84. | ||
Jorge JM, González-Méijome JM, Queirós A, Fernandes P, Parafita MA. Correlations between corneal biomechanical properties measured with the ocular response analyzer and ICare rebound tonometry. J Glaucoma. 2008;17(6):442–448. | ||
Beasley IG, Laughton DS, Coldrick BJ, Drew TE, Sallah M, Davies LN. Does rebound tonometry probe misalignment modify intraocular pressure measurements in human eyes? J Ophthalmol. 2013;2013:791084. | ||
Takenaka J, Mochizuki H, Kunihara E, Tanaka J, Kiuchi Y. Intraocular pressure measurement using rebound tonometer for deviated angles and positions in human eyes. Curr Eye Res. 2012;37(2):109–114. | ||
Güler M, Bilak Ş, Bilgin B, Şimşek A, Capkin M, Hakim Reyhan A. Comparison of intraocular pressure measurements obtained by Icare PRO rebound tonometer, Tomey FT-1000 noncontact tonometer, and Goldmann applanation tonometer in healthy subjects. J Glaucoma. 2015;24(8):613–618. | ||
Baek SU, Ha A, Kim YK, Jeoung JW, Park KH. Effect of manual eyelid manipulation on intraocular pressure measurement by rebound tonometry. Br J Ophthalmol. Epub 2018 Feb 2. | ||
Jablonski KS, Rosentreter A, Gaki S, Lappas A, Dietlein TS. Clinical use of a new position-independent rebound tonometer. J Glaucoma. 2013;22(9):763–767. | ||
Dabasia PL, Lawrenson JG, Murdoch IE. Evaluation of a new rebound tonometer for self-measurement of intraocular pressure. Br J Ophthalmol. 2016;100(8):1139–1143. | ||
Chen E, Quérat L, Åkerstedt C. Self-tonometry as a complement in the investigation of glaucoma patients. Acta Ophthalmol. 2016;94(8):788–792. | ||
Pronin S, Brown L, Megaw R, Tatham AJ. Measurement of intraocular pressure by patients with glaucoma. JAMA Ophthalmol. 2017;135(10):1–7. | ||
Mudie LI, LaBarre S, Varadaraj V, et al. The Icare HOME (TA022) Study: performance of an intraocular pressure measuring device for self-tonometry by glaucoma patients. Ophthalmology. 2016;123(8):1675–1684. | ||
Takagi D, Sawada A, Yamamoto T. Evaluation of a new rebound self-tonometer, Icare HOME: comparison with Goldmann applanation tonometer. J Glaucoma. 2017;26(7):613–661. | ||
Armaly MF, Rubin ML. Accommodation and applanation tonometry. Arch Ophthalmol. 1961;65:415–423. | ||
Read SA, Collins MJ, Becker H, et al. Changes in intraocular pressure and ocular pulse amplitude with accommodation. Br J Ophthalmol. 2010;94(3):332–335. | ||
Huang J, Katalinic P, Kalloniatis M, Hennessy MP, Zangerl B. Diurnal intraocular pressure fluctuations with self-tonometry in glaucoma patients and suspects: a clinical trial. Optom Vis Sci. 2018;95(2):88–95. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.