Back to Journals » Clinical and Experimental Gastroenterology » Volume 8
IBS-like symptoms in patients with ulcerative colitis
Received 12 October 2014
Accepted for publication 17 November 2014
Published 19 February 2015 Volume 2015:8 Pages 101—109
DOI https://doi.org/10.2147/CEG.S58153
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Andreas M. Kaiser
David J Gracie,1 Alexander C Ford1,2
1Leeds Gastroenterology Institute, St James’s University Hospital, 2Leeds Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, UK
Abstract: Ulcerative colitis (UC) and irritable bowel syndrome (IBS) are chronic gastrointestinal disorders that, until recently, have been considered dichotomous conditions falling on either side of a functional-organic divide. However, persistent gastrointestinal symptoms, akin to those of IBS, are observed in up to one in three patients with quiescent UC. Whether these lower gastrointestinal symptoms are secondary to coexistent IBS or occult UC disease activity is uncertain, but when objective evidence of disease activity is lacking, escalation of conventional pharmacotherapy in such patients is often ineffective. The etiologies of both UC and IBS remain unclear, but dysregulation of the enteric nervous system, an altered microbiome, low-grade mucosal inflammation, and activation of the brain–gut axis is common to both; this suggests that some overlap between the two conditions is plausible. How best to investigate and manage IBS-type symptoms in UC patients remains unclear. Studies that have assessed patients with UC who meet criteria for IBS for subclinical inflammation have been conflicting in their results. Although evidence-based treatments for IBS exist, their efficacy in UC patients reporting these types of symptoms remains unclear. Given the disturbances in gut microbiota in UC, and the possible role of the brain–gut axis in the generation of such symptoms, treatments such as probiotics, fecal transfer, antidepressants, or psychological therapies would seem logical approaches to use in this group of patients. However, there are only limited data for all of these therapies; this suggests that randomized controlled trials to investigate their efficacy in this setting may be warranted.
Keywords: irritable bowel syndrome, ulcerative colitis, antidepressants, psychological therapies, probiotics
Introduction
Ulcerative colitis (UC) is a chronic inflammatory condition affecting the colon and rectum with an incidence of 8–14 per 100,000 people and a prevalence of 120–200 per 100,000 people in Western populations.1 The etiology of the condition is not fully understood, but is thought to be related to a combination of immune dysregulation, host genetic factors, environmental factors, altered mucosal permeability, and disturbances in the gut microbiome.2 Given that UC is a lifelong condition with no cure, patients suffering from the disease are faced, potentially, with many years of chronic gastrointestinal (GI) symptoms.
The natural history of UC is that of quiescent symptoms, interspersed with episodes of active disease or flare-ups. A flare-up of disease activity may be classified as mild, moderate, or severe, with treatment strategies tailored accordingly. In keeping with the chronic nature of the condition, medical management of UC is aimed at inducing and maintaining remission of disease activity by using a combination of certain types of drugs, such as glucocorticosteroids, oral and topical 5-aminosalicylic acids, and thiopurines, with other types of medications, such as ciclosporin and infliximab, reserved for acute severe disease.3–8
In contrast to UC, irritable bowel syndrome (IBS) is a highly prevalent condition, with a worldwide population prevalence of between 10% and 20%.9 The cardinal features of IBS are a change in bowel habit in the presence of abdominal pain or discomfort. Patients are subtyped according to the predominant stool pattern they report: IBS with diarrhea; IBS with constipation; or mixed IBS if the stool pattern fluctuates between the two. As IBS is a functional GI disorder, without any known organic explanation, the condition is diagnosed by using symptom-based diagnostic criteria, with the current gold standard being the Rome III criteria,10 although recent evidence suggests that the accuracy of these criteria for predicting the presence of IBS is only modest.11
Overlap between IBS and UC
Typically, a flare-up of disease activity in UC manifests itself with an alteration in bowel habit, which may be associated with abdominal pain or discomfort. Traditional management dictates that a change in symptoms in individuals with UC should prompt an evaluation that includes assessments by using all of the following: 1) clinical disease activity scoring systems, such as the simple clinical colitis activity index,12 to assess disease activity; 2) serum and/or fecal biomarkers of disease activity, including C-reactive protein and fecal calprotectin (FCP); and 3) endoscopic visualization of the colonic mucosa with histopathological interpretation of biopsy specimens.
These tools may aid the decision-making process, in terms of the need to modify or to escalate pharmacotherapy. However, when UC patients present with lower GI symptoms in the absence of biochemical, endoscopic, and histopathological evidence of disease activity, the clinician is faced with a dilemma regarding further management, as this lack of information then raises the possibility of either subclinical UC activity or coexistent IBS in a patient with known UC. Escalation of therapy in this situation may be advocated by some experts, but the use of immunomodulator therapies or biological agents is not without the risk of adverse events.13–16 Furthermore, the use of biological agents carries with it significant financial implications. In addition, in clinical trials, patients with inflammatory bowel disease (IBD) without objective evidence of disease activity often do not respond to these agents as well as those with definitive evidence of active inflammation.17,18
Given that both IBS and UC are chronic diseases that may present with similar symptoms and given that individuals with IBS are often diagnosed on the grounds of symptoms alone by using an imperfect gold standard,11 there is the potential for a missed diagnosis of UC in patients thought to have IBS. This is further complicated by the fact that some individuals with IBS, particularly those with postinfectious IBS, have been shown to display evidence of low-grade mucosal inflammation,19–22 suggesting that this may be a contributing factor in the development of these symptoms. This has led some experts to propose that the classical view that IBS and IBD are mutually exclusive conditions falling on either side of a functional–organic divide is too simplistic. Instead, a biopsychosocial model of IBD–IBS has been put forward,23 although this approach remains controversial. A recent systematic review and meta-analysis, which pooled data from several cross-sectional surveys and case-control studies, estimated that around one in three UC patients reported symptoms compatible with IBS,24 with the odds for reporting these type of symptoms four times higher in patients with UC felt to be in clinical remission, compared with controls without UC.
The implication of all of this for clinical practice is that there may be significant difficulty for the physician in distinguishing between UC patients with genuine IBS and those who have ongoing subclinical inflammation secondary to occult disease activity. To complicate matters further, even studies that have specifically examined this issue are conflicting. In one cross-sectional survey of IBD patients in clinical remission, significantly higher levels of FCP were observed among those who reported symptoms compatible with IBS than among those without those symptoms.25 However, in another study of similar design,26 there was no difference in median FCP levels between those who reported IBS-type symptoms and those who did not; this finding suggests that subclinical inflammation was not the cause of IBS-type symptoms in this cohort of patients.
Pathological basis
Given that there is a fourfold greater prevalence of IBS-type symptoms in IBD patients than in healthy controls,24 it follows that UC patients who display these symptoms may share common risk factors with the IBS population for their development. To date, the etiology of IBS is unclear, but it is thought to arise from a combination of psychological and organic pathologies. The classical view of IBS as a centrally driven condition has been superseded by an increasing body of evidence suggesting that the cause of IBS is multifactorial. There is now a realization that low-grade mucosal inflammation,27 an altered microbiome,28 increased intestinal permeability,29 and genetic factors contribute to the development of IBS;30 all of these factors are common also to UC (Figure 1).
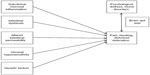  | Figure 1 Proposed etiology of IBS-type symptoms in UC. |
Mucosal inflammation, dysbiosis, intestinal permeability, and visceral hypersensitivity
Mucosal inflammation is the hallmark of UC. However, recent advances in the understanding of the etiology of IBS suggest that subclinical mucosal inflammation and increased mucosal barrier permeability may play a role in the development of symptoms. Studies have demonstrated higher levels of circulating proinflammatory cytokines in peripheral blood25,31 and higher levels of proinflammatory cell infiltrates in the intestinal mucosa of IBS patients20 than in patients in the control group. The exact cause of this inflammation is uncertain, but may be related to an alteration in the gut microbiome, with evidence of a dysbiosis in IBS, and a relative abundance of pro-inflammatory species compared with healthy individuals without IBS.32
The advent of 16s ribosomal RNA gene sequencing has provided a specific and inexpensive method of studying microbial diversity in the gut and with it further investigating the association of altered gut microbial composition with the development of various GI diseases including IBS,33 IBD,34 and colorectal cancer.35 The mechanism by which gut microbes may be able to affect intestinal permeability and thus propagate symptoms compatible with IBS is complex, but dysregulation of the enteric nervous system in response to an altered microbiome has been proposed as a cause.36,37
It is suggested that dysbiosis is associated with an increase in the expression of toll-like receptors in the intestinal epithelium, which are responsible for the recognition of bacterial lipopolysaccharides,38 and that the expression of these receptors, in combination with the presence of proinflammatory bacterial species, induces activation of the enteric nervous system and results in mucosal inflammation, altered expression of tight junction proteins,39 epithelial barrier dysfunction, increased mucosal permeability, and consequent visceral hypersensitivity and stimulation of the brain–gut axis. Moreover, the complex interactions between gut microbes, the enteric nervous system, and the brain–gut axis has also been implicated in the development of stress, anxiety, and depression in IBS patients;40 with some evidence to suggest this interplay may also affect the prevalence of psychological illness, even in individuals without any evidence of GI disease.41
In a prospective case-control study of patients with IBS or quiescent IBD (including 18 patients with UC) and healthy controls undergoing ileocolonoscopy, biopsy specimens were taken and questionnaires were completed to assess the severity of IBS-type symptoms in all participants.42 Biopsy specimens were assessed for proinflammatory cell infiltrates, including mast cells, intraepithelial lymphocytes, and eosinophils. Immunohistochemistry was performed for CD-117 and CD-3. Colonic paracellular permeability and tumor necrosis factor (TNF)-α levels were quantitatively assessed. Messenger RNA (mRNA) expression of tight junction proteins ZO-1, a-catenin, and occludin were also measured. The authors demonstrated higher paracellular permeability in UC patients with IBS-type symptoms than in those without those symptoms. The authors also reported that paracellular permeability in IBS patients was comparable to that in those with quiescent UC with IBS symptoms, whereas paracellular permeability in quiescent UC patients without IBS-type symptoms was similar to that of controls. The presence of IBS-type symptoms was associated with increased paracellular permeability and reduced tight junction protein mRNA expression universally. Mast cell infiltrates were higher in IBS and UC groups than in the controls, but intraepithelial lymphocytes were demonstrated in higher numbers for all IBD groups than for the IBS groups and for the controls; this finding suggests that subclinical inflammation is implicated in the etiology of these symptoms, especially because TNF-α mRNA expression was higher in the subgroup of IBD patients with IBS-type symptoms.
The chronic, recurrent mucosal inflammation characteristic of UC may also lead to visceral afferent hypersensitivity. This could, in turn, lead to symptoms compatible with IBS because of abnormal neuronal responses that cause hyperalgesia and allodynia, along with abnormal local reflexes and that result in altered GI motility and secretion. Evidence to support this comes from a rat model of colitis43 and, more recently, a barostat study conducted among UC patients in remission.44 The latter study demonstrated a positive correlation between rectal perception thresholds and IBS-like symptoms. The investigation also showed that compared to the healthy control patients, UC patients had more mast cells in their colonic mucosa and had a higher percentage of these mast cells in close proximity to nerve fibers; a similar finding was previously described by Barbara et al for IBS patients.21
Stress, anxiety, and depression and the brain–gut axis – a bidirectional relationship?
Several of the studies in the aforementioned meta-analysis24 also demonstrated a negative impact of the presence of IBS-type symptoms on both mood and quality of life in patients with IBD.45–51 Stress, anxiety, and depression are common in both IBS and IBD.52–55 However, the effect of psychological comorbidity on the natural history of these conditions remains controversial. Prior understanding of the cause of these conditions led health care professionals to assume that IBS was a centrally mediated process but that UC was a condition restricted to the colon and rectum. Evidence now exists to suggest that the relationship between stress and flare-ups of disease activity in the two conditions may be more complex and that the presence of psychological comorbidity is associated with greater symptom severity and more flare-ups of disease activity in both.56–58
A proposed explanation is that psychological comorbidity results in a stress response, which may contribute to worsening outcomes in these conditions. This stress response results from a complex interaction between different, interconnected parts of the brain that include the hypothalamus, amygdala, and hippocampus. Communication between these areas results in the activation of the autonomic nervous system and the hypothalamus–pituitary axis. Activation of the hypothalamus–pituitary axis results in an increased secretion of glucocorticosteroids from the zona fasciculata of the adrenal cortex, while increased sympathetic autonomic activity results in increased secretions of the catecholamines epinephrine and norepinephrine from the adrenal medulla. This interaction between descending autonomic nerves, the enteric nervous system, and endocrine pathways is referred to as the brain–gut axis.59
The perception of visceral pain is thought to involve the spinothalamic, spinoreticular, and spinomesencephalic tracts.60 Interestingly, the central coordinating center for each of these pathways involves the limbic system, which also serves to mediate emotional responses; this supports the theory that psychological as well as physiological pathology contributes to the development of functional GI symptoms in IBS and UC. It follows that psychological health, stress, anxiety, and depression symptoms and visceral hypersensitivity are interrelated and, therefore, that mood may influence the generation and perception of symptoms.
Longitudinal follow-up studies have suggested that there is a higher risk of developing anxiety or depression among people without mood disorders who report GI symptoms compatible with IBS at baseline. These studies have also suggested an increased likelihood of developing GI symptoms de novo among people who, at baseline, demonstrate anxiety or depression but not GI symptoms.61 This bidirectional effect of the brain–gut pathway seen in functional GI disorders raises the possibility that the relationship between the brain and the gut may also be bidirectional in UC and that coexistent anxiety or depression, if unrecognized or untreated, may have a role in the generation of symptoms compatible with IBS in UC patients.
Evidence to support a bidirectional relationship between the brain and gut in UC comes mainly from animal models. Mice with chronic GI inflammation develop behavioral changes akin to mood disorders in humans.62 Studies have demonstrated that in murine models of quiescent colitis, the induction of depression can reactivate inflammation of the colonic mucosa,63 which can be attenuated by the administration of antidepressant drugs, and that this is mediated via interference with the inhibition of proinflammatory macrophage activity by the vagus nerve.64 In humans, there is some evidence to suggest that acute psychological stress induces the production of proinflammatory cytokines in the serum and mucosa of UC patients.65 Small retrospective studies of the effect of psychological counseling or antidepressants have been conducted in patients with UC who acted as their own controls before and after the institution of these interventions, and these studies have demonstrated fewer relapses of disease activity and less utilization of glucocorticosteroids following their introduction.66,67 In addition, in a recent study, patients with UC demonstrated an improvement in overall depression scores following the commencement of anti-TNF-α or immunomodulator therapy for active disease.68
Treatment strategies for IBS-type symptoms in UC
Given that patients with a confirmed diagnosis of IBS take more sickness-related absences from work than those without bowel symptoms,69 that IBS costs almost US$1 billion in direct costs per year and another $50 million in indirect costs per year,70 and that patients with IBS consume >50% more health care resources than matched controls without IBS,71 it seems that proactive treatment of these types of symptoms in patients with UC is also needed.
Whether IBS-type symptoms in UC relate to subclinical disease activity, or true IBS, the response to conventional therapies of patients with IBD without objective evidence of disease activity who enter clinical trials of these drugs is often attenuated.17,18 This suggests that other management strategies are required. For IBS, the evidence base for effective therapies has been summarized previously.72 Soluble fiber, antispasmodics (including peppermint oil), antidepressants, psychological therapies, and probiotics all appear to be of some benefit.73–76 Given the potential role of an abnormal microbiome and the possible influence of the brain–gut axis on the development of IBS-type symptoms in UC, it would perhaps not be unreasonable to adopt some of the strategies physicians use when treating patients with IBS (Table 1).
Probiotics
Probiotics are live or attenuated microorganisms that may have beneficial effects in humans and that have been widely studied in IBS. They are thought to have their actions via the modulation of the GI flora, anti-inflammatory properties,77 and the ability to modulate visceral hypersensitivity,78–80 although it is important to point out that these effects are often species- and strain-specific. They have also been shown to be able to exert beneficial effects on mood via the brain-gut axis.81 Their use in UC patients with IBS-type symptoms may therefore be an attractive option. A meta-analysis identified numerous randomized controlled trials (RCTs) of probiotics in UC,82 but a variety of species and strains were utilized; hence, the conclusions able to be derived were limited. However, Escherichia coli Nissle 1917 did appear to be of benefit in preventing relapse of disease activity in quiescent UC. Unfortunately, none of the included studies addressed the use of probiotics in the treatment of IBS-type symptoms in UC.
Fecal transfer
Fecal transfer has been used with some success in other illnesses that are characterized by the presence of dysbiosis, such as pseudomembranous colitis caused by Clostridium difficile.83–85 A recent systematic review of case series examining the efficacy of fecal transfer for the treatment of IBD included 111 patients86 and suggested an overall improvement in disease activity in almost 90% of UC cases. However, none of the included studies examined the effect of fecal transfer on IBS-type symptoms in UC patients and, to date, no RCTs of the efficacy of fecal transfer for the management of UC have been published.
Antidepressants and the management of IBS symptoms in UC
Tricyclic antidepressants and selective serotonin reuptake inhibitors are more effective than placebos in treating IBS.75 A systematic review of publications reporting the efficacy of antidepressant medications in the maintenance and induction of remission of IBD was published by Mikocka-Walus et al in 2006.87 The review included six case reports, one nonrandomized, open-label study, and one letter that, collectively, reported beneficial effects of bupropion, paroxetine, amitriptyline, and phenelzine. However, the studies were small, and all except one was conducted in patients with Crohn’s disease rather than UC.
We are aware of only one study reporting the efficacy of tricyclic antidepressants in UC patients with ongoing symptoms who had no objective evidence of disease activity. It is important to point out that patients in this retrospective study were not screened formally by using validated questionnaires to confirm whether or not they met symptom-based criteria for IBS.88 Outcomes, which were based on self-reported symptom severity by participants who were using a Likert scale, appeared to be good, with at least a moderate improvement in symptoms in 56% of UC patients. Symptom response among those with UC was similar to that observed in a control group of IBS patients.
Psychological therapies in UC
In addition to antidepressants, psychological therapies have also been shown to be beneficial in IBS.74 These include cognitive behavioral therapy (CBT) and gut-directed hypnotherapy, and both are recommended by national guidelines for the management of IBS.72,89 However, evidence for their use as an effective treatment for UC, particularly in those with IBS-type symptoms, is lacking. A Cochrane review investigated the efficacy of psychotherapy, patient education, and relaxation techniques for IBD.90 Outcomes assessed included health-related quality of life, coping, emotional status, and disease activity. In total, 21 studies were included, but there was no clear benefit identified for any of the psychological interventions in adults with UC for any of the outcomes of interest.
Another systematic review91 of 16 studies of psychological interventions, including stress management, psychodynamically informed therapy, CBT, and hypnosis assessed their effects on anxiety and depression, quality of life, and IBD activity. CBT and psychodynamically informed therapy were beneficial for anxiety and depression, but they appeared to have no effect on disease activity, whereas hypnotherapy, used in two studies, demonstrated a beneficial effect on disease activity, but not anxiety, depression, or quality of life.91
More recently, Keefer et al92 reported the results of an RCT of gut-directed hypnotherapy in quiescent UC. Patients were randomized to seven sessions of either hypnotherapy or an attention control. The authors reported that those receiving hypnotherapy were significantly more likely to remain in clinical remission, although there were no differences in quality of life, perceived stress, or psychological factors between the two treatment arms. Again, it is important to point out that no study to date has examined the effect of psychological therapies on the treatment of IBS-type symptoms in UC.
Conclusion
IBS-type symptoms in quiescent UC are common. There remains uncertainty regarding the cause of these symptoms, although low-grade mucosal inflammation secondary to subclinical UC activity remains a distinct possibility. Notwithstanding this, while the etiologies of IBS and UC remain elusive, there is consensus that both conditions result from a combination of genetic factors, disordered intestinal immunity, low-grade mucosal inflammation, and an altered microbiome, and that stress triggers flare-ups of disease activity via the brain–gut axis. Together, these factors suggest that a significant overlap between the two conditions is biologically plausible.
Targets for therapeutic intervention may exist, however, and arise from the proposed interactions between the microbiome, the enteric nervous system, and brain–gut axis that we have discussed. Manipulating the fecal microbiota, either via the use of probiotics or fecal transfer, may yet prove to be beneficial in UC patients with IBS-type symptoms, but more research is required. Moreover, an increasing acceptance that the relationship between IBS, stress, anxiety, and depression may be bidirectional, as well as an understanding that this may also be the case in UC, suggests that RCTs of antidepressant medications and psychological therapies are warranted, not only to assess improvement in IBS-type symptoms, but also as a means of preventing of relapse of disease activity in those with quiescent UC, particularly if coexistent mood disorders are identified.
Disclosure
The authors report no conflicts of interest in this work.
References
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. | |
Ford AC, Moayyedi P, Hanauer SB. Ulcerative colitis. BMJ. 2013;346:f432. | |
Gracie DJ, Ford AC. Evidence-based management of ulcerative colitis. Minerva Gastroenterol Dietol. 2012;58(2):87–99. | |
Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):601–616. | |
Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):590–599. | |
Ford AC, Khan KJ, Sandborn WJ, Hanauer SB, Moayyedi P. Efficacy of topical 5-aminosalicylates in preventing relapse of quiescent ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(5):513–519. | |
Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):644–659, quiz 660. | |
Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011; 106(4):630–642. | |
Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–721. | |
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. | |
Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Validation of the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gastroenterology. 2013; 145(6):1262–1270. | |
Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43(1):29–32. | |
Williams CJ, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-α therapy in inflammatory bowel disease. Aliment Pharmacology Ther. 2014;39(5):447–458. | |
Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108(8):1268–1276. | |
Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374(9701):1617–1625. | |
Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141(5):1621–1628.e1–e5. | |
Reinisch W, Wang Y, Oddens BJ, Link R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35(5):568–576. | |
Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–1395. | |
Sundin J, Rangel I, Kumawat AK, Hultgren-Hörnquist E, Brummer RJ. Aberrant mucosal lymphocyte number and subsets in the colon of post-infectious irritable bowel syndrome patients. Scand J Gastroenterol. 2014;49(9):1068–1075. | |
Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122(7):1778–1783. | |
Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. | |
Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. | |
Long MD, Drossman DA. Inflammatory bowel disease, irritable bowel syndrome, or what?: A challenge to the functional-organic dichotomy. Am J Gastroenterol. 2010;10(8)5:1796–1798. | |
Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2012;107(10):1474–1482. | |
Keohane J, O’Mahony C, O’Mahony L, O’Mahony S, Quigley EM, Shanahan F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: A real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105(8):1789–1794, quiz 1795. | |
Berrill JW, Green JT, Hood K, Campbell AK. Symptoms of irritable bowel syndrome in patients with inflammatory bowel disease: examining the role of sub-clinical inflammation and the impact on clinical assessment of disease activity. Aliment Pharmacol Ther. 2013;38(1):44–51. | |
Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46(4):421–431. | |
Kassinen A, Krogius-Kurikka L, Mäkivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133(1):24–33. | |
Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20(11–12):1317–1322. | |
Ek WE, Reznichenko A, Ripke S, et al. Exploring the genetics of irritable bowel syndrome: a GWA study in the general population and replication in multinational case-control cohorts. Gut. Epub September 23, 2014. | |
McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther. 2011;33(9):1045–1052. | |
Lee KN, Lee OY. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J Gastroenterol. 2014;20(27):8886–8897. | |
Hong SN, Rhee PL. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J Gastroenterol. 2014;20(10):2470–2481. | |
Walujkar SA, Dhotre DP, Marathe NP, Lawate PS, Bharadwaj RS, Shouche YS. Characterization of bacterial community shift in human Ulcerative Colitis patients revealed by Illumina based 16S rRNA gene amplicon sequencing. Gut Pathog. 2014;6:22. | |
Akin H, Tözün N. Diet, microbiota, and colorectal cancer. J Clin Gastroenterol. 2014;48 Suppl 1:S67–S69. | |
Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146(1–2):41–46. | |
Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G775–G785. | |
Brint EK, MacSharry J, Fanning A, Shanahan F, Quigley EM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;106(2):329–336. | |
Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. | |
Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146(6):1500–1512. | |
Vitetta L, Bambling M, Alford H. The gastrointestinal tract microbiome, probiotics, and mood. Inflammopharmacology. 2014;22(6):333–339. | |
Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63(5):744–752. | |
La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9(12):2791–2795. | |
van Hoboken EA, Thijssen AY, Verhaaren R, et al. Symptoms in patients with ulcerative colitis in remission are associated with visceral hypersensitivity and mast cell activity. Scand J Gastroenterol. 2011;46(7–8):981–987. | |
Simrén M, Axelsson J, Gillberg R, Abrahamsson H, Svedlund J, Björnsson ES. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97(2):389–396. | |
Ansari R, Attari F, Razjouyan H, et al. Ulcerative colitis and irritable bowel syndrome: relationships with quality of life. Eur J Gastroenterol Hepatol. 2008;20(1):46–50. | |
Barratt SM, Leeds JS, Robinson K, et al. Reflux and irritable bowel syndrome are negative predictors of quality of life in coeliac disease and inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:159–165. | |
Bryant RV, van Langenberg DR, Holtmann GJ, Andrews JM. Functional gastrointestinal disorders in inflammatory bowel disease: Impact on quality of life and psychological status. J Gastroenterol Hepatol. 2011;26(5):916–923. | |
Farrokhyar F, Marshall JK, Easterbrook B, Irvine EJ. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis. 2006;12(1):38–46. | |
Minderhoud IM, Oldenburg B, Wismeijer JA, van Berge Henegouwen GP, Smout AJ. IBS-like symptoms in patients with inflammatory bowel disease in remission: relationships with quality of life and coping behavior. Dig Dis Sci. 2004;49(3):469–474. | |
Piche T, Ducrotté P, Sabate JM, et al. Impact of functional bowel symptoms on quality of life and fatigue in quiescent Crohn’s disease and irritable bowel syndrome. Neurogastroenterol Motil. 2010;22(6):e626–e174. | |
Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122(4):1140–1156. | |
Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15(7):1105–1118. | |
Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65(4):528–533. | |
Panara AJ, Yarur AJ, Rieders B, et al. The incidence and risk factors for developing depression after being diagnosed with inflammatory bowel disease: a cohort study. Aliment Pharmacol Ther. 2014;39(8):802–810. | |
Lackner JM, Ma CX, Keefer L, et al. Type, rather than number, of mental and physical comorbidities increases the severity of symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(9):1147–1157. | |
Shah E, Rezaie A, Riddle M, Pimentel M. Psychological disorders in gastrointestinal disease: epiphenomenon, cause or consequence? Ann Gastroenterol. 2014;27(3):224–230. | |
Mittermaier C, Dejaco C, Waldhoer T, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med. 2004;66(1):79–84. | |
Jones MP, Dilley JB, Drossman D, Crowell MD. Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil. 2006;18(2):91–103. | |
Drossman DA. Functional abdominal pain syndrome. Clin Gastroenterol Hepatol. 2004;2(5):353–365. | |
Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain – gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61(9):1284–1290. | |
Bercik P, Verdu EF, Foster JA, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139(6):2102–2112. | |
Ghia JE, Blennerhassett P, Deng Y, Verdu EF, Khan WI, Collins SM. Reactivation of inflammatory bowel disease in a mouse model of depression. Gastroenterology. 2009;136(7):2280–2288. | |
Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118(6):2209–2218. | |
Mawdsley JE, Macey MG, Feakins RM, Langmead L, Rampton DS. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology. 2006;131(2):410–419. | |
Goodhand JR, Greig FI, Koodun Y, et al. Do antidepressants influence the disease course in inflammatory bowel disease? A retrospective case-matched observational study. Inflamm Bowel Dis. 2012;18(7):1232–1239. | |
Wahed M, Corser M, Goodhand JR, Rampton DS. Does psychological counseling alter the natural history of inflammatory bowel disease? Inflamm Bowel Dis. 2010;16:664–669. | |
Horst S, Chao A, Rosen M, et al. Treatment with immunosuppressive therapy may improve depressive symptoms in patients with inflammatory bowel disease. Dig Dis Sci. Epub February 2, 2014. | |
Drossman DA, Li Z, Andruzzi E, et al. US householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38(9):1569–1580. | |
Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136(2):376–386. | |
Inadomi JM, Fennerty MB, Bjorkman D. Systematic review: the economic impact of irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18(7):671–682. | |
Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109 Suppl 1:S2–S26, quiz S27. | |
Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109(9):1367–1374. | |
Ford AC, Quigley EM, Lacy BE, et al. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-analysis. Am J Gastroenterol. 2014;109(10):1547–1561. | |
Ford AC, Quigley EM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. 2014;109(9):1350–1365, quiz 1366. | |
Ford AC, Talley N, Spiegel BMR, et al. Effect of fibre, antispasmodics, and peppermint oil in irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337(7683):1388–1392. | |
O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–551. | |
Kamiya T, Wang L, Forsythe P, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191–196. | |
Verdú EF, Bercík P, Bergonzelli GE, et al. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 2004;127(3):826–837. | |
Verdú EF, Bercik P, Verma-Gandhu M, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182–190. | |
Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144(7):1394–1401. | |
Jonkers D, Penders J, Masclee A, Pierik M. Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs. 2012;72(6):803–823. | |
Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087. | |
van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. | |
Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071. | |
Sha S, Liang J, Chen M, et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39(10):1003–1032. | |
Mikocka-Walus AA, Turnbull DA, Moulding NT, Wilson IG, Andrews JM, Holtmann GJ. Antidepressants and inflammatory bowel disease: a systematic review. Clin Pract Epidemiol Ment Health. 2006;2:24. | |
Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol. 2014;48(5):423–429. | |
Spiller R, Aziz Q, Creed F, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56(12):1770–1798. | |
Timmer A, Preiss JC, Motschall E, Rücker G, Jantschek G, Moser G. Psychological interventions for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2011;2:CD006913. | |
Knowles SR, Monshat K, Castle DJ. The efficacy and methodological challenges of psychotherapy for adults with inflammatory bowel disease: a review. Inflamm Bowel Dis. 2013;19(12):2704–2715. | |
Keefer L, Taft TH, Kiebles JL, Martinovich Z, Barrett TA, Palsson OS. Gut-directed hypnotherapy significantly augments clinical remission in quiescent ulcerative colitis. Aliment Pharmacol Ther. 2013;38(7):761–771. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

