Back to Journals » OncoTargets and Therapy » Volume 12
Hypoxia-Induced Cleavage Of Soluble ephrinA1 From Cancer Cells Is Mediated By MMP-2 And Associates With Angiogenesis In Oral Squamous Cell Carcinoma
Authors Ma TT, Wang L, Wang JL, Liu YJ, Chen YC, He HJ, Song Y
Received 24 April 2019
Accepted for publication 18 September 2019
Published 14 October 2019 Volume 2019:12 Pages 8491—8499
DOI https://doi.org/10.2147/OTT.S213252
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Ting-Ting Ma,1,* Lin Wang,2,* Jun-Lin Wang,1 Yan-Jie Liu,1 Yu-Cong Chen,1 Hu-Jie He,1 Yong Song1
1Department of Stomatology, Liuzhou People’s Hospital, Guangxi, People’s Republic of China; 2Department of Oral and Maxillofacial-Head and Neck Oncology, School and Hospital of Stomatology, Wuhan University, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong Song
Department of Stomatology, Liuzhou People’s Hospital, 8 Wenchang Road, Liuzhou 545006, People’s Republic of China
Tel +86 0772 2662705
Email [email protected]
Introduction: ephrinA1 plays important roles in tumor angiogenesis. Matrix metalloproteases (MMPs) can cleave ephrinA1 from the cell membrane into extracellular environment. However, how soluble ephrinA1 is modulated by hypoxia and whether MMPs participate in this hypoxic process remains to be investigated in detail.
Methods: Thirty-seven patients with oral squamous cell carcinoma (OSCC) were included in the present study for HIF-1α, MMP-2, MMP-9 and ephrinA1 detection by immunohistochemistry. Serum samples from 35 patients were collected both preoperatively and postoperatively to confirm the existence of soluble ephrinA1 by ELISA. Block assay and Western blot analysis were further carried out to elucidate the proteolysis mechanism of ephrinA1 under hypoxic condition in vitro.
Results: Our data demonstrated that HIF-1α, MMP-2, MMP-9 and ephrinA1 expressed positively, and correlated with microvessel density in OSCCs, except for MMP-9. The serum expression level of ephrinA1 in OSCC patients decreased significantly after surgical removal of the solid tumors. In vitro experiments indicated that GM6001, a MMP-specific inhibitor, could reduce hypoxia-induced soluble ephrinA1 secretion from SCC cells. Further Western blot analysis confirmed that both HIF-1α and MMP-2 were up-regulated by hypoxia in a similar time-dependent manner, with the MMP-9 expression unchanged during this course.
Conclusion: These results suggested a possible novel mechanism that ephrinA1 secretion is mediated by HIF-1α/MMP-2 signaling cascade which may play pivotal roles in OSCC neovascularization in a paracrine manner.
Keywords: soluble ephrinA1, matrix metalloproteases, hypoxia, angiogenesis, oral squamous cell carcinoma
Introduction
Angiogenesis is a critical process in tumor formation and progression.1,2 Several signaling networks have been proved to contribute to tumor neovascularization, such as vascular endothelial growth factor (VEGF), Tie, Notch, Eph and Robo.3 EphrinA1 and its primary receptor, EphA2, are key members of Eph family which play pivotal roles in tumor angiogenic processes.4–6 Our previous study showed that overexpression of ephrinA1/EphA2 may contribute to tumor angiogenesis and serve as a novel therapeutic target in adenoid cystic carcinoma.7 However, to our knowledge, previous studies mainly focused on the modulation of downstream pathological processes by EphA2/ephrinA1 system,8 how ephrinA1 is regulated in tumor microenvironment is still not well clarified.
Hypoxia is a common feature in solid tumors and contributes to cancer progression and bad outcome.9,10 A large number of pro-angiogenic factors can be regulated under hypoxic stimulation, including VEGF, platelet-derived growth factor B, plasminogen activator inhibitor-1, etc.11 It has been reported that ephrinA1 mRNA expression can be highly induced by hypoxia in 366 colorectal cancer patients.12 On the other hand, soluble monomeric ephrinA1 can be released from tumor cells and still keeps the ability to activate its receptor, EphA2.13,14 We also detected a marked increase of ephrinA1 expression in hypoxic SCC-9 cells in vitro, and more importantly, ephrin-A1 protein was detected positively in the supernatants from hypoxia groups, suggesting the secretion of soluble ephrin-A1 in hypoxic tumor microenvironment.15 Matrix metalloproteases (MMPs) were involved in proteolytic cleavage of membrane-bound ephrinA1 from cell membranes.16 As hypoxia can potentiate MMP expression in multiple cells,17,18 it is reasonable to suppose that MMPs may also mediate hypoxia-induced releasing of soluble ephrinA1 in oral squamous cell carcinoma (OSCC). Unfortunately, there have not been any available information on the direct relationship among hypoxia, MMPs and soluble ephrinA1 during the modulation of angiogenesis so far.
The present study further investigated soluble ephrinA1 secretion in OSCC patients and the possible mechanism underlying hypoxia modulation of ephrinA1 through MMPs pathway in tumor angiogenesis. Our data provided direct evidence that ephrinA1 could be released from OSCC cells into tumor microenvironment and the level of which was associated with HIF-1α and MMP-2/MMP-9 expression in angiogenic processes. Block assay by GM6001 confirmed that MMP-2 participated in hypoxia regulation of soluble ephrinA1 in OSCC cells in vitro. Our results suggested that hypoxia may promote tumor angiogenesis via a HIF-1α/MMP2/ephrinA1(S) signaling pathway.
Materials And Methods
Materials
Antibodies against MMP-2 (ab37150), MMP-9 (ab76003), HIF-1α (ab51608) were purchased from Abcam (Abcam, MA, US) and ephrin-A1 (sc-911) from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, US).
Patients And Samples
OSCC patients who had never received either chemotherapy or radiotherapy were included in the present study. The diagnosis of all tissue specimens was confirmed by two pathologists. Our studies were approved by the Committees on Ethics in Liuzhou People’s Hospital. All patients provided written informed consent which was conducted in accordance with the Declaration of Helsinki.
Immunohistochemistry
Immunohistochemistry and immunostaining evaluation were performed according to Zhe Shao.7 The dilution rate of primary antibodies are MMP-2 (1:300), MMP-9 (1:300), HIF-1α (1:200), ephrinA1 (1:300). The scoring was conducted independently by two persons.
Cell Culture
The human OSCC cell line, SCC-15 (CRL-1629, ATCC), was kindly provided by Professor Zhengjun Shang (Department of Oral and Maxillofacial-Head and Neck oncology, School and Hospital of Stomatology, Wuhan University), which was approved by the Committees on Ethics in Liuzhou People’s Hospital. The cells were cultured in DMEM/F12 (Hyclone, UT, USA) supplemented with 10% FBS (Gibco, Carlsbad, Calif, USA). All cells were cultured at 37°C in an atmosphere containing 5% CO2.
Hypoxia Experiment
OSCC cells were cultured under hypoxic conditions (1% O2) or normoxic conditions (21% O2) in 5% CO2 at 37°C. Cells were harvested and lysed directly in SDS sample buffer for Western blot analysis. Supernatants were collected and centrifuged to remove the debris at 3000× g for 10 mins. Equal amount of supernatants were loaded to detect the soluble form of eprhinA1 by Western blot analysis.15
Block Assay
Block assay was carried out to evaluate the function of MMPs in hypoxia-induced OSCC cells secretion of ephrin-A1 in vitro. A specific inhibitor, GM6001 (Selleck Chemicals, US, 100μM), was added in the culture medium to block MMPs. The control groups were cultured in the absence of the inhibitor.
ELISA
To assess the existence and amount of ephrinA1 in extracellular environment, soluble ephrinA1 levels in OSCC patients’ serum were measured with sandwich ELISA as previously reported.19
Western Blot Analysis
Protein samples were harvested in lysis buffer containing protease inhibitor cocktail (Roche, Germany). Equal amount or volume of protein was loaded and separated in 8% SDS-PAGE. Then, proteins were transferred to polyvinylidene difluoride membrane (Millipore Co., Billerica, MA, USA) at 200 mA for 2 hrs (100mA, 1 hr for ephrinA1) at 4°C. After blockage in TBS containing 5% BSA (w/v) and 0.1% Tween-20 for 1 hr, membranes were incubated with appropriate primary antibodies at 4°C overnight. The dilution ratios of primary antibodies were as in the following: ephrinA1 (1:300), HIF-1α (1:1000), MMP-2 (1:1000), MMP-9 (1:1000), β-actin (1:1000). After five 5 mins washes with TBST, the blocks were treated with horseradish peroxide-conjugated secondary antibody (dilution 1:10,000) (Jackson Immunoresearch Laboratories) for 1 hr at room temperature. Finally, bands were recorded by using ECL plus Western blotting detection reagents (Beyotime, People’s Republic of China).15
Statistical Analysis
Data were collected and analyzed by using SPSS 17.0 software (SPSS Inc.) through Paired t-test or ANOVA. P-values less than 0.05 were considered statistically significant.
Results
HIF-1α/MMP/ephrinA1 Were Involved In OSCC Angiogenesis
Serial tissue specimens from 37 OSCC patients were taken into immunohistochemistry examination. EphrinA1, MMP-2, MMP-9, HIF-1α staining were observed positively in cancer cells, with little immunoreactivity of the surrounding connective tissue (Figure 1) and noncancerous tissues (Supplementary Figure 1). The proportion of moderate to strong staining were 86.84% (33/38) for HIF-1α, 81.58% (31/38) for ephrinA1, 73.68% (28/38) for MMP-2, 52.63% (20/38) for MMP-9, with their mean score as showing 5.03±1.26, 4.86±1.38, 4.60±1.50 and 3.38±1.16, respectively (Supplementary IHC data).
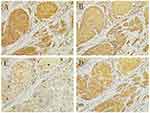 |
Figure 1 HIF-1α (A), MMP-2 (B), MMP-9 (C) and ephrinA1 (D) expressed positively in OSCCs. (100X). |
Anti-CD34 antibody was used to detect the vascular endothelial cells around cancer cells. Pearson correlation analysis was carried out to evaluate the relationship among ephrinA1, MMP-2, MMP-9, HIF-1α and MVD. As shown in Figure 2A–C, ephrinA1, MMP-2, MMP-9 and HIF-1α expression were significantly correlated with each other (P<0.01). The expression levels of ephrinA1, MMP-2 and HIF-1α correlated remarkably with the MVD (P<0.01), except MMP-9 (P=0.07) (Figure 2D–G) (Supplementary IHC data).
These results suggested that HIF-1α/MMP-2/ephrinA1 participates in OSCC angiogenesis and the crosstalk among them may play an important role in this process.
EphrinA1 Expressed Positively In Serum Of OSCC Patients
Although soluble ephrinA1 has been confirmed in serum of multiple malignancies, no previous studies have addressed the secretion of ephrinA1 from OSCC in detail. Our ELISA data showed positive expression of ephrinA1 in serum of 35 OSCC patients, both pre- and post-operatively (Figure 3A). More importantly, ephrinA1 protein level was much lower 2 weeks after surgery (0.56±0.06 ng/mL) than that before the surgery (0.68±0.06 ng/mL), presenting a significant decreased expression of soluble ephrinA1 upon removal of the tumor tissue in most cases (Figure 3B). These outcomes provided direct evidence that ephrinA1 could be secreted from OSCC cells into tumor microenvironment in vivo (Supplementary ELISA data).
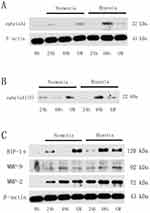
Hypoxia Up-Regulated Expression And Secretion Of ephrinA1 In Cancer Cells
As shown in Figure 4A, both membrane-bound ephrinA1 and soluble ephrinA1 were up-regulated significantly in SCC cells under hypoxic stimulation in comparison with the normoxia group, which was consistent with our previous reports.15 Intriguingly, after exposure to GM6001 (100μM), a specific MMP inhibitor, ephrinA1 protein was decreased remarkably in supernatants from hypoxic cancer cells (Figure 4B). These findings suggested that MMPs play a critical role in hypoxia-induced SCC cells secretion of ephrinA1 in tumor microenvironment.
HIF-1α/MMP-2 Were Required For Cleavage Of ephrinA1 In Hypoxic Cancer Microenvironment
In order to determine whether MMPs were involved in hypoxia modulation of ephrinA1 expression in SCC cells, two key members of MMP family including MMP-2 and MMP-9 were further investigated by Western blot analysis. Compared with normoxia-stimulated cancer cells, SCC cells exposed to hypoxia were observed a marked increase in MMP-2 expression in a time-dependent manner, with the maximum effect at timepoint 48 hrs, while the MMP-9 expression was relatively lower and kept stable during this course. More importantly, MMP-2 expression was also accompanied with a similar HIF-1α expression tendency in SCC cells, the level of which started to increase at 24 hrs, arrived at its top point at 48 hrs (Figure 4C).
Taken together, we could come to a conclusion that hypoxia induces the secretion of ephrinA1 from OSCC cells via a HIF-1α/MMP-2 dependent signaling cascade in its angiogenic process.
Discussion
Sufficient data demonstrated that ephrinA1/EphA2 system are key factors in tumor angiogenesis, such as ovarian cancer20 and endometrial cancer.21 As supporting evidence, our results also confirmed a significant correlation between ephrinA1 expression and MVD in OSCC. EphrinA1 was first recognized as a membrane-bound ligand through glycosylphosphatidylinositol linkage to cell surface,4,5 cell to cell contact is needed to exert its function to activate EphA2, which means a juxtacrine mechanism in tumor microenvironment. However, further study found a functional, soluble, unclustered monomeric form of ephrinA1 could be released from the plasma membrane into cell medium and still kept the ability to induce phosphorylation and internalization of EphA2 in tumor cells.13,22 It was reported that soluble monomeric ephrinA1 from cancer cells could activate EphA2 to increase angiogenesis in bladder cancer.23 Similarly, our previous study presented the existence of soluble ephrinA1 in supernatants of SCC cells under hypoxic condition and its potential to induce morphological changes of U-251 cells in vitro.15 Although ephrinA1 secretion has been proved by many in vitro studies, relatively little is known whether soluble ephrinA1 can be truely released from solid tumor into extra cellular environment in vivo, especially in OSCC, or not. In the present study, ELISA assay showed that most patients experienced a marked down-regulation of ephrinA1 in serum upon removal of the tumor by surgery, which provided direct evidence on cleavage of ephrinA1 from cancer cells into tumor microenvironment. These findings suggest that soluble ephrinA1 may act as a paracrine molecule in promoting OSCC angiogenesis.
Although the function of soluble ephrinA1 in tumor angiogenesis has been well investigated, the mechanisms by which it is modulated in tumor biology still need to be elucidated in detail. Hypoxia is one of the most important characteristics in solid tumors. Hypoxia-inducible factors (HIFs) play essential roles in adaptive cellular response to tumor hypoxic microenvironment.24 HIF-1α is the most important factor responsible for activating transcriptional responses under hypoxia and can induce the expression of a variety of pro-angiogenic factors.11 We found that HIF-1α expressed positively in most cases of OSCC and correlated significantly with tumor angiogenesis. It has been reported that MMPs mediate cleavage of ephrinA1 from glioblastoma cells into the culture medium.16 In order to further investigate the functional involvement of MMPs in hypoxia-stimulated ephrinA1 releasing, the effect of GM6001 on ephrinA1 secretion in SCC cells was determined. After culture in hypoxia for 48 hrs, soluble ephrinA1 in supernatants was elevated significantly. Exposure to GM6001 inhibited hypoxia-induced expression of soluble ephrinA1 apparently, indicating that MMPs also participated in controlling cleavage of ephrinA1 under hypoxic condition.
MMPs are pivotal factors in cancer invasion and angiogenesis through matrix remodeling.25 They are highly expressed in multiple human malignancies. Among them, MMP-2 and MMP-9 are the most efficient ones in initiating collagen breakdown18 and can achieve robust cleavage of ephrinA1 in GBM cells.16 Therefore, MMP-2 and MMP-9 expression in OSCC specimens and their changes under hypoxic stimulation were examined in depth in the present study. Our results suggested that up-regulation of MMP-2 mainly contributes to hypoxia-induced ephrinA1 secretion in OSCC angiogenesis. In addition, we documented in this study that MMP-9 may not act as an important signaling molecule in the same process. In keeping with our findings, high expression and activation of MMP-2 have been detected in human pancreatic cancer tissues and the level of which correlated to tumor invasion and metastasis significantly.26 These results revealed that MMP2-dependent signaling pathway involves in hypoxia modulation of angiogenesis through increasing soluble ephrinA1 in OSCCs.
In conclusion, the most significant results of the present study showed that 1) HIF-1α, MMP-2 and ephrinA1 expressed positively and correlated significantly with angiogenesis in OSCCs; 2) solid tumor can secret ephrinA1 into extracellular microenvironment in vivo; 3) MMPs mediate hypoxia-induced ephrinA1 releasing from tumor cells; 4) HIF-1α/MMP-2 participates in cleavage of ephrinA1 from membranes in tumor angiogenesis. These results reported here clarify HIF-1α/MMP-2/ephrinA1(S) as a novel mechanism underlying hypoxia modulation of angiogenesis in OSCCs.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 81560436). We thank Professor Zheng-jun Shang for providing SCC cell lines and Dr Xiao-cheng Zhou, Hui Wang for technical support (Department of Oral and Maxillofacial-Head and Neck oncology, School and Hospital of Stomatology, Wuhan University).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ronca R, Benkheil M, Mitola S, et al. Tumor angiogenesis revisited: regulators and clinical implications. Angiogenesis. 2017;37:1231–1274.
2. Kuol N, Stojanovska L, Apostolopoulos V, et al. Role of the nervous system in tumor angiogenesis. Cancer Microenviron. 2018;11:1–11. doi:10.1007/s12307-018-0207-3
3. Ziyad S, Iruela-Arispe ML. Molecular mechanisms of tumor angiogenesis. Genes Cancer. 2011;2:1085–1096. doi:10.1177/1947601911432334
4. Beauchamp A, Debinski W. Ephs and ephrins in cancer: ephrin-A1 signalling. Semin Cell Dev Biol. 2012;23:109–115. doi:10.1016/j.semcdb.2011.10.019
5. Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res. 2008;6:1795–1806. doi:10.1158/1541-7786.MCR-08-0244
6. Chen Z, Oh D, Biswas KH, et al. Spatially modulated ephrinA1: ephA2signaling increases local contractility and global focal adhesion dynamics to promote cell motility. Proc Natl Acad Sci U S A. 2018;115:E5696–e705. doi:10.1073/pnas.1719961115
7. Shao Z, Zhu F, Song K, et al. EphA2/ephrinA1 mRNA expression and protein production in adenoid cystic carcinoma of salivary gland. J Oral Maxillofac Surg. 2013;71:869–878. doi:10.1016/j.joms.2012.10.026
8. Kang M, Jeong W, Bae H, et al. Bifunctional role of ephrin A1-Eph system in stimulating cell proliferation and protecting cells from cell death through the attenuation of ER stress and inflammatory responses in bovine mammary epithelial cells. J Cell Physiol. 2018;233:2560–2571. doi:10.1002/jcp.26131
9. DE Lima PO, Jorge CC, Oliveira DT, Pereira MC. Hypoxic condition and prognosis in oral squamous cell carcinoma. Anticancer Res. 2014;34:605–612.
10. Isa AY, Ward TH, West CM, et al. Hypoxia in head and neck cancer. Head Neck. 2014;27:622–639.
11. Lv X, Li J, Zhang C, et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis. 2017;4:19–24. doi:10.1016/j.gendis.2016.11.003
12. Yamamoto H, Tei M, Uemura M, et al. Ephrin-A1 mRNA is associated with poor prognosis of colorectal cancer. Int J Oncol. 2013;42:549–555. doi:10.3892/ijo.2012.1750
13. Wykosky J, Palma E, Gibo DM, et al. Soluble monomeric EphrinA1 is released from tumor cells and is a functional ligand for the EphA2 receptor. Oncogene. 2008;27:7260–7273. doi:10.1038/onc.2008.328
14. Xu Q, Lin WC, Petit RS, et al. EphA2 receptor activation by monomeric Ephrin-A1 on supported membranes. Biophys J. 2011;101:2731–2739. doi:10.1016/j.bpj.2011.10.039
15. Song Y, Zhao XP, Song K, et al. Ephrin-A1 is up-regulated by hypoxia in cancer cells and promotes angiogenesis of HUVECs through a coordinated cross-talk with eNOS. PLoS One. 2013;8:e74464. doi:10.1371/journal.pone.0074464
16. Beauchamp A, Lively MO, Mintz A, et al. EphrinA1 is released in three forms from cancer cells by matrix metalloproteases. Mol Cell Biol. 2012;32:3253–3264. doi:10.1128/MCB.06791-11
17. Kumar P, Teece S, Choi KY, et al. Hypoxia potentiates MMP-2 expression in human carotid atherosclerotic plaques and human macrophages. Atherosclerosis Supp. 2018;32:95. doi:10.1016/j.atherosclerosissup.2018.04.289
18. Liu Y, Zhang H, Yan L, et al. MMP-2 and MMP-9 contribute to the angiogenic effect produced by hypoxia/15-HETE in pulmonary endothelial cells. J Mol Cell Cardiol. 2018;121:36–50. doi:10.1016/j.yjmcc.2018.06.006
19. Mohamed IH, Giorgio C, Bruni R, et al. Polyphenol rich botanicals used as food supplements interfere with EphA2–ephrinA1 system. Pharmacol Res. 2011;64:464–470. doi:10.1016/j.phrs.2011.06.008
20. Lin YG, Han LY, Kamat AA, et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332–340. doi:10.1002/cncr.22415
21. Merritt WM, Kamat AA, Hwang JY, et al. Clinical and biological impact of EphA2 overexpression and angiogenesis in endometrial cancer. Cancer Biol Ther. 2010;10:1306–1314. doi:10.4161/cbt.10.12.13582
22. Alford S, Watson-Hurthig A, Scott N, et al. Soluble ephrin a1 is necessary for the growth of HeLa and SK-BR3 cells. Cancer Cell Int. 2010;10:41. doi:10.1186/1475-2867-10-41
23. Chu M, Zhang C. Inhibition of angiogenesis by leflunomide via targeting the soluble ephrin-A1/EphA2 system in bladder cancer. Sci Rep. 2018;8:1539. doi:10.1038/s41598-018-19788-y
24. Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143:512–519. doi:10.1111/imm.12380
25. Shay G, Lynch CC, Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44-46:200–206. doi:10.1016/j.matbio.2015.01.019
26. Binker MG, Binker-Cosen AA, Richards D, et al. Hypoxia–reoxygenation increase invasiveness of PANC-1 cells through Rac1/MMP-2. Biochem Biophys Res Commun. 2010;393:371–376. doi:10.1016/j.bbrc.2010.01.125
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.