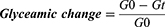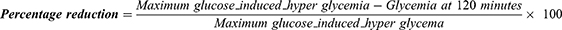Back to Journals » Journal of Experimental Pharmacology » Volume 14
Hypoglycemic, Antihyperglycemic, and Toxic Effects of Physalis peruviana L. Aqueous and Methanolic Leaf Extracts in Wistar Rats
Authors Kasali FM , Kadima JN, Tusiimire J, Agaba AG
Received 30 December 2021
Accepted for publication 31 May 2022
Published 7 June 2022 Volume 2022:14 Pages 185—193
DOI https://doi.org/10.2147/JEP.S356533
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Paola Rogliani
Félicien Mushagalusa Kasali,1– 3 Justin Ntokamunda Kadima,3 Jonans Tusiimire,2 Amon Ganafa Agaba4
1Pharm-Bio Technology and Traditional Medicine Center, Mbarara University of Science and Technology, Mbarara, Uganda; 2Department of Pharmacy, Mbarara University of Science and Technology, Mbarara, Uganda; 3Department of Pharmacy, Official University of Bukavu, Bukavu, Democratic Republic of the Congo; 4Department of Pharmacology and Therapeutics, Mbarara University of Science and Technology, Mbarara, Uganda
Correspondence: Félicien Mushagalusa Kasali, Pharm-Bio Technology and Traditional Medicine Center, Mbarara University of Science and Technology, Mbarara, Uganda, Tel +256 750919712, Email [email protected]
Background: Physalis peruviana L. (Solanaceae) is a plant widely used in traditional medicine systems to manage various diseases, including diabetes mellitus, which remains a global health problem in developing and developed countries. This study aimed to scientifically evaluate its antidiabetic bioactivity and short-term toxicity in rats.
Methods: We prepared various doses (100, 200, 400 mg/kg) of aqueous and methanolic leaf extracts for the antidiabetic study, and a dose of 2000 mg/Kg was prepared for the acute toxicity test. The first group that evaluated the hypoglycemic effect consisted of forty normoglycemic Wistar rats aged 7– 8 months old with a weighted average of 265.8 ± 24.6 g. The second group consisted of intraperitoneal glucose-loaded male animals to evaluate the antihyperglycemic effect. The third group contained two groups of normoglycemic female rats (n = 3), aged 3 and 4 months old (weight average: 187.45 ± 14.82 g), treated for 14 days with aqueous and methanolic extracts (2 g/kg b.w) to assess mortality and toxic effects. Blood samples were taken at 30, 60, 90, and 120 min post-treatment in hypoglycemic and antihyperglycemic evaluations. Glibenclamide (5 mg/kg) was used as a reference drug. The control animals in each group did not receive the extracts.
Results: In hypoglycemic rats, 100 mg/kg of aqueous and methanolic extracts significantly lowered the fasting blood glucose level by 13.92% (p < 0.0001) and 21.95% (p < 0.01), respectively, compared to the control group. In glucose tolerance test group, methanolic extracts significantly reduced hyperglycemia by 54.55% (p < 0.0001), 46.50% (p < 0.0001), 39.78% (p < 0.0001) at 400, 200 and 100 mg/kg b.w, respectively, compared to control; aqueous extract 400 mg/kg reduced hyperglycemia by 39.44% (p < 0.05). At the 2000 mg/kg dose, leaf aqueous and methanolic extracts did not show any signs of intoxication and mortality.
Conclusion: Crude aqueous and methanolic leaf extracts of P. peruviana ambrosioides appeared safe at 2000 mg/kg and have bioactivity in controlling the blood glucose levels, supporting their use in treating diabetes.
Keywords: Physalis peruviana, leaf extracts, antidiabetic bioactivity, toxicity, rats
Introduction
Diabetes mellitus (DM) constitutes a global health problem worldwide, the prevalence of which is worryingly increasing. The number of deaths in 2021 is estimated at 6.7 million. In 2030 and 2045, the diabetic population is estimated to increase to 643 and 783 million, respectively. During this period, the number of people with diabetes is estimated to increase by 46%; meanwhile, the world’s population will have grown by 20%. About 5.5% and 10.8% of people live in low- and middle-income countries.1,2 One of the features of diabetes is hyperglycemia, which increases the risk of diabetes and associated complications. Chronic diabetes is associated with dysfunction and failure of different organs in long-term damage, especially the eyes, kidneys, nerves, heart, and blood vessels.3 Current oral antidiabetic (OADs) synthesis origin is expensive and not easily accessible in developing countries. Besides, many OADs possess several adverse reactions and do not always provide excellent long-term glycemic control. For this reason, the use of medicinal plants as by-products is more on the rise in developing countries4 compared to wealthy countries. Medicinal plants are wholly integrated into traditional therapeutic systems.
Scientifically, validating the antidiabetic bioactivity of any natural plants used locally in traditional health systems. Ethnobotanical, chemical, and pharmacological studies have been carried out worldwide to analyze their rich phytochemical compounds and their beneficial biological activities.5
Recent data show about 1.7 million people suffer from DM in the Democratic Republic of Congo (DRC), ranking fourth in the top ten countries with diabetes cases in Africa.6 Ethnobotanical studies have described many plants as potential sources of antidiabetic compounds, of which Physalis peruviana L (Solanaceae), locally known as “Mbuma,” “Mbupuru,” “Umuhire,” P. peruviana is a native plant from the Andean region and a semi-upright herbaceous shrub or perennial, producing a group of branched stems. The plant is used worldwide to treat cancer, malaria, sexual problems, conjunctivitis, cataract, splenomegaly, gastritis, inflammation, jaundice, gout, glaucoma, gastro-intestinal disorders, diverse infections, pains, human immunodeficiency virus/Acquired immunodeficiency syndrome (HIV/AIDS), etc.7,8 Aqueous leaf extracts of P. peruviana have been evaluated in guinea pigs using the Oral Glucose Tolerance Test (OGTT)9 and diabetic alloxan-induced model.10 Furthermore, its hydro-alcoholic extract’s antidiabetic properties and its fractions have been evaluated in streptozotocin-induced diabetic Wistar rats.11
This study aimed to evaluate the hypoglycemic and antihyperglycemic bioactivity of aqueous and methanolic extracts and their acute toxicity in rats.
Materials and Methods
Chemical and Reagents
Carboxymethyl cellulose (Shanghai Huaxuan, China), Chloroform LR (GriffchemTM, India), Dextrose monohydrate (Loba Chemie, India), Glibenclamide (Nobel, Turkey), Glucose intravenous infusion 50% (Abacus Parenteral Drugs-Uganda, Uganda), Methanol 99.5% (Loba Chemie, India), n-butanol 99% (Loba Chemie, India), Physiological solution (Abacus Parenteral Drugs, Uganda), Picric acid 10% (BDH Limited Poole, England), and Sodium Chloride Extra Pure (Loba Chemie, India).
Plant Material
Fresh leaves of P. peruviana were collected in Lwiro (2°14ʹ24 “S, 28°47ʹ50 “E), located 50 Km from Bukavu city (in the Eastern part of the DRC), between April and October 2019. The leaf part was air-dried at room temperature and then manually grounded to a fine powder. Plant materials were identified by the botanist Mr. Gentil IRAGI, from the Department of Biology of the Center for Research in Natural Sciences/Lwiro, and voucher specimens were deposed in the herbarium under number LWI113898883.
Preparation of Plant Extracts
Aqueous Extract
The infusion was prepared by mixing 250 g of plant material with 250 mL distilled water for 20 min; then, 9 liters of boiling distilled water was added and kept stand at room temperature for 30 minutes under occasional shaking and stirring. The extract was filtered using cotton wool and concentrated to dryness under reduced pressure using a rotary evaporator (IKA® RV 10, Germany) at 40°C. The crude residue was frozen (−80°C) and lyophilized using Benchtop Freeze Dryer (FD-ICL, Japan).
Methanolic Extraction
Cold maceration in methanol was prepared according to the standard method.12 Thus, 250 g of dry powder was repeatedly macerated into 3 liters of methanol for 48 hours under occasional shaking and stirring. After filtration, the methanolic solution was filtered using cotton wool and concentrated to dryness with a rotary evaporator (IKA® RV 10, Germany) under reduced pressure at 40°C.
Extracts Yields
The extraction yield was calculated as g of dry residue per 100 g of dry plant mass. The dry residues obtained from both aqueous (AEPP) and methanolic (MEPP) extracts were weighed (75.39 g: yield 30.15%) and (15.01 g: yield 6.00%), respectively. They were then kept in a dark container in the freezer at −20°C until further pharmacological investigations.
Experimental Animals
Normoglycemic male Wistar rats (weight average: 265.8± 24.6 g) were used for hypoglycemic and antihyperglycemic evaluations and females (weight average: 187.45 ± 14.82 g) for acute oral toxicity. All rats were maintained in the animal house at the Animal Research Facility of the Department of Pharmacology, Faculty of Medicine of Mbarara University of Science and Technology under standardized conditions (the temperature at 23.8 ± 1.9°C; relative humidity: 63.6 ± 6.6% and 12 h daylight/dark cycle) with free access to pelleted animal food, and drinking water ad libitum. Their feed contained carbohydrates, proteins, calcium, vitamins, and water.13
Experimental Sample Size Determination
Forty were divided into a randomization sequence of 8 groups with 5 rats, each using the Experimental Design Assistant (EDA) tool. A technician not aware of the intervention did the assignment to treatment groups. Investigators were aware of the treatment groups; blinding was impossible because of the difference in physical aspects between the control and treated groups.14
Determination of the Blood Glucose Levels
The fasting blood glucose (FBG) concentration was taken using a glucometer (Accu-Chek®, South Africa) and tips by the enzymatic glucose oxidase method applied to blood. The blood samples were collected from the end of the tail. The basal level of glucose was collected after 6 hours of morning fasting (8:00 am to 2:00 pm).
Evaluation of Hypoglycemic Activity in Normoglycemic Rats
Fifty normoglycemic male rats (aged 7–8 months old) were randomly divided into ten groups (n = 5/group), and pre-treatment blood glucose levels were obtained. They were orally administered with either carboxymethylcellulose 1%, glibenclamide, or different doses of AECA and MECA. The blood samples were collected from the tails before treatment (GBCT0) and then at 30, 60, 90, and 120 min post-treatment, GBC30, GBC40, GBC90, GBC120.15 The randomized rats involved in this present study were divided into the following groups:
Group 1-Normal control; rats received carboxymethylcellulose 1% (1 mL/100g b.w)
Group 2-Reference; rats, received glibenclamide (5 mg/kg b.w)
Group 3-Normal rats treated with 100 mg/kg b.w of aqueous extract of P. peruviana
Group 4-Normal rats treated with 200 mg/kg b.w of aqueous extract of P. peruviana
Group 5-Normal rats treated with 400 mg/kg b.w of aqueous extract of P. peruviana
Group 6-Normal rats treated with 100 mg/kg b.w of methanolic extract of P. peruviana
Group 7-Normal rats treated with 200 mg/kg b.w of methanolic extract of P. peruviana
Group 8-Normal rats treated with 400 mg/kg b.w of methanolic extract of P. peruviana
Glycemic change (in percentage) was calculated as a function of time according to the following formula:
where G0 and Gt represent (zero time or 0 h) glycemic values before and at 30, 60, 90, and 120 minutes of oral administration of the plant extracts, respectively.16
Assessment of Antihyperglycemic Activity
The antihyperglycemic effect was assessed by PGTT in 40 rats randomly divided into 8 groups of 5 rats. All animals were fasted in the morning (6 hrs) with free access to water.17,18 Before glucose loading in the morning, glycemia was determined (GCT0). The animals received orally carboxymethylcellulose 1%, extract doses, and glibenclamide doses for control, test, and reference groups. Then, 4 mL/kg b.w of glucose solution 50% containing 2 g was given intraperitoneally at 30 min after an oral administration of plant extracts at different doses or glibenclamide or carboxymethylcellulose 1%. Blood samples were taken at 30, 60, 90, and 120 minutes after glucose. The animals were grouped and treated as earlier described in the hypoglycemic evaluation. The percentage reduction of glucose-induced hyperglycemia by the treatment and control groups was determined from the formula given below:19
Acute Toxicity Study
According to the Organization for Economic Cooperation and Development (OECD) guidelines no. 425, the acute toxicological study was carried out based on the limited dose test of the up and down procedure. Test Guidelines related to an Acute Oral Toxicity under a computer-guided Statistical Programme-AOT425statPgm, version 1.0,20 at a limit dose of 2000 mg/kg body weight/oral route and default of Sigma at 0.5. The protocol was used as described by Kale et al21 with a few modifications. Two groups of three normoglycemic young female rats (8–12 weeks old) were randomly constituted of a population of 25 rats. Each rat has fasted for 6 hours, weighed, dosed orally once with 2g/kg b.w of aqueous and methanolic extracts dissolved in CMC 1%, and watched for up to 14 days, recording abnormal signs of toxicity and mortality according to the Up and Down test procedure.
Statistical Analysis
Values were represented as mean ± S.E.M of five samples. Statistical analysis was conducted using one-way analysis of variance (ANOVA) by Tukey’s multiple comparison tests using GraphPad Prism (version 8.0.1) between the “test” and “control” group data. P < 0.05 was considered to be statistically different.
Results
Hypoglycemic Assessment and GTT Assessment
All animals involved were divided into 8 groups: (1) CMC1% (1 mL/100g b.w), (2) glibenclamide reference (5 mg/kg b.w), (3), AEPP (100 mg/kg b.w), (4) AEPP (200 mg/kg b.w), (5) AEPP (400 mg/kg b.w), (6) MEPP (100 mg/kg b.w), (7) MEPP (200 mg/kg b.w), (8) MEPP (400 mg/kg b.w). The Evolution of mean blood glucose concentrations and measured at different time in both hypoglycemic and anti-hyperglycemic assessments are presented in Tables 1 and 2.
 |
Table 1 Time-Dependent Glycemic Values (Mg/dL) in Hypoglycemic Test |
 |
Table 2 Time-Dependent Glycemic Values (Mg/dL) in Antihyperglycemic Test |
In the hypoglycemic assessment model, the fasting control glycemia (CMC1%) in the rats used remained almost unchanged compared to baseline (93.45 ± 2.19). After administration of reference and plant extracts, the baseline was reduced significantly at any measuring times compared to negative control CMC 1%. Glibenclamide and extracts reduced glycemia in a dose-dependent manner.
In the antihyperglycemic assessment model, the glycemia reached peak concentration at 30 minutes and then decreased up to 120 minutes, i.p., glucose load. Thirty minutes after glucose-loading, the fasting blood glucose increased significantly (p < 0.0001) in all experimental groups compared to the control and reference groups, which received CMC 1% (1 mL/100 g b.w) and glibenclamide (5 mg/kg b.w), respectively.
Figure 1 shows the graphical evolution of mean blood glucose concentrations measured at different times in hypoglycemic and antihyperglycemic assessments and the percentage of reduction of mean glycemia concentration at times 30 min and 120 min. Figure 2 shows the graphical effect change caused per each treatment calculated as differences against control values CMC 1%.
In general, no clear statistical difference was observed between the aqueous and methanolic extracts. Typically, all differences were expected to be negative, meaning lower blood glucose levels than the control. However, the negative values were more pronounced at times 60, 90, and 120 min. In comparison, at 30 min, there were some positive differences in most groups, signifying an increase in blood glucose rather than a decrease. However, this value increased significantly (p < 0.01) in the group receiving AEPP100 and MEPP400.
On the other side, in the hypoglycemic test, the reduction of T120-T30 of the group treated with glibenclamide was more potent (36.68%), followed by MEPP100 (23.88%), AEPP100 (19.37%), MEPP400 (16.39%), and AEPP400 (14.17%) compared to other groups. Nonetheless, in an antihyperglycemic study, AEPP100 reduced blood glucose levels (56.24%) better than all treated and reference groups (54.66%). Treatment groups under MEPP400 and MEPP200 lowered notably BGL, 51.82%, and 46.50%, respectively.
 |
Figure 2 Effect of different treatments given on the baseline glycemia measured at different times in both hypoglycemic and antihyperglycemic assessments. |
The baseline values are put to zero. The differences are positive or negative accordingly. Positive and negative values represent the increase and decrease in BGL compared to the control group. The highest negative values are observed (High percentage of reduction) at T-90, followed by T-120, T-60, and T-30. Between different groups, BGL lowered the group treated with glibenclamide T-30, T-60, T-90, and T-120, with percentages of −18.23, 33.47, 46.92, and 46.91, respectively. In the treatment group, AEPP200 produced a reduction in BGL also from T-30 to T-120. Hence, 9.5% (T-30), 16.74% (T-60), 26.19% (T-90) and 25.46 reduced BGL (T-120). It is the only plant extract that has lowered BGL since T0 (7.27%). In other treated groups, BGL increased at T0. On the other hand, MEPP100 lowered BGL by 8.74%, 20.01%, and 19.64% at T-60, T-90, and T-120, respectively. However, all plant extracts decreased BGL variably from T-30 to T-120. In the antihyperglycemic study, as previously values in glibenclamide-treated (reference) group decreased (p< 0.0001, after glucose loading to the end of the study. Thus, the percentages of reduction were 6.5%, 33.1%, 43.65% and 51.99% at T-30, T-60, T-90 and T-120, respectively. Values of BGL decreased variably in treatment groups. In methanolic extract treated, BGL lowered significantly (p< 0.0001) at T-90 with percentages of reduction of 48.37% (MEPP200), 36.37% (MEPP100), and 30.6% (MEPP400). The same tendency was noted at T-120 with the same plant extracts. Thus, MEPP100 and MEPP200 reduced significantly (p < 0.0001) at 37.08%, followed by MEPP400 (31.26%). Furthermore, at T-90 and T-120, AEPP100 reduced significantly (p < 0.01) at 21.5% and 24.09%, respectively. Overall, the other plant extract reduced BGL differently.
Acute Oral Study
No signs of diarrhea, anogenital stains, piloerection, lethargy, salivation, excessive sweating, central nervous system activities, vomiting, gait, posture, or mortality were noted. Based on acute toxicity results, 100, 200, and 400 mg/kg doses were selected for antidiabetic test in vivo.
Discussion
Antidiabetic Assessment
The present study results in experimental rats show that the aqueous and methanolic leaf extracts of P. peruviana possess may have antidiabetic activity. The morning fasting (6 hours) rats had significantly suppressed basal plasma levels such that there were no differences between treated and control groups. At 100 mg/kg dose, both aqueous and methanolic extract produced significant glucose-lowering at the end of the study (T-120) in hypoglycemic rats. A similar previous study also reported a hypoglycemic effect in guinea pigs. Furthermore, under OGTT conditions, Physalis extract significantly reduced the peak concentration (p < 0.05) compared to both control and reference at the corresponding given doses. The slope of the extract was smaller than that obtained from glibenclamide.9 The changes (mg/dL) in blood glucose in alloxan-induced diabetic guinea pigs treated for 28 days significantly lowered in the group treated with leaf aqueous of P. peruviana and in the reference group treated with glibenclamide. On days 14 and 28, P. peruviana extracts presented the highest mean change percentages. Moreover, at the end of the experiment, all rats treated with P. peruviana extract survived compared to the control group. All animals died and only 83.3%; stayed in the glibenclamide-treated group.10 The literature reports that several mechanisms can be involved in decreasing blood glucose levels in normoglycemic rats when using plant extracts and derivatives. Among those mechanisms is the stimulation of the residual pancreatic, increased peripheral utilization of glucose, converted glucose to glycogen, and promoted glycogen storage in the liver and skeletal muscles.22
In the glucose tolerance test, the antihyperglycemic effect of the methanolic extracts (100, 200, and 400 mg/kg b.w.) was found to be similar to in-group treated with glibenclamide (5 mg/kg b.w). They significantly reduced blood glucose levels in a dose-dependent manner by 39.78%, 46.50%, and 54.88, respectively. Although potent in normoglycemic rats, administration of P. peruviana extracts exhibited more pronounced blood glucose lowering in intraperitoneal glucose-loaded rats. At 30 minutes after the glucose load, the blood glucose level was significantly increased in almost all experimental groups compared to the control and reference groups. Aqueous extract at the dose of 100 mg/kg showed the highest percentage reduction in the blood glucose level at 38.97% (vs 24.66% for glibenclamide) and 56.18% (55.66% for glibenclamide), respectively, at the 60th and 120th minute. When using intraperitoneal routes, the glucose tolerance test causes the elevation of glucose excursion and a largely absent insulin excursion compared to oral administration, which leads to the incretin potency potentiating glucose-induced insulin release. Accordingly, glucose handling during the glucose tolerance test depends on some factors, including insulin secretion and action and the ability of blood glucose to regulate glucose uptake.23–25
Many primary and secondary metabolites with antidiabetic potential have been isolated and characterized in different plant species. Thus, the antidiabetic potential of P. peruviana is due to the presence of phytocompounds, which are extractable by water and methanol. These phytoconstituents include alkaloids, amino acids, amides, carbohydrates, glycosides, polyphenols, saponins, sucrose esters, terpenes, terpenoids, and others.6
The current literature shows that only one study has established the antidiabetic property of compounds isolated (Sucrose esters named peruvioses) from the exudate of P. peruviana that showed an alpha-amylase inhibition in vitro.26
Toxicity
The findings from this study indicated the safety of aqueous and methanolic leaf extracts of P. peruviana at 2000 mg/kg b.w. The method for determining LD50 values at two doses resulted in higher than 0% and lower than 100%.27 Therefore, no mortality observed at the dose suggests that the LD50 of aqueous and methanolic extracts of P. peruviana was more remarkable than 2000 mg/kg. In the same way, a recent toxicological evaluation of the aqueous and methanolic bark extracts of the P. peruviana did not show any observable signs of acute oral toxicity effects, with an LD50 value estimated to be more than 2000 mg/kg in Wistar rats.28 Similarly, another study29 using lyophilized fruit juice reported LD50 > 5000 mg/kg b.w. in rats. However, a previous study on guinea pigs found that the leaf LD50 aqueous extract was 1300 mg/kg.9 Forty percent of mortality in rats was obtained at 1500 mg/kg of leaf extract.30 Solanine is a toxic glycoalkaloid that may cause hemorrhagic gastroenteritis, weakness, excessive salivation, dyspnea, tremors, progressive paralysis, prostration, and death.31 The concentration of solanine is variable according to plant species; likely, P. peruviana, investigated in the present study, is at infra toxic levels to explain its safety.
Conclusion
The present antidiabetic investigation found that P. peruviana aqueous and methanolic leaf extracts possess the potential to control blood glucose levels. Moreover, they are safe at a dose of 2000 mg/kg, which supports their use in local traditional medicine. Nonetheless, in-depth preclinical investigations are needed to meet scientific requirements for introducing the plant into the national pharmacopeia.
Ethical Approval
The Research Ethical Committee of Mbarara University of Science and Technology (REC-MUST) and Uganda National Council for Science and Technology (UNCST) ethically cleared the research under registration number MUST-REC 25/01-19 NS440ES, respectively.
Acknowledgments
We are thankful to the Pharm-Bio Technology and Traditional Medicine Center (PHARMBIOTRAC) and Mbarara University of Science and Technology for funding this study. The authors are grateful to Dr. Clement Ajayi Olusoji, Mr. Bright James Rujumba, and Mr. Ivan Twinamatsiko, a Bachelor’s student at Mbarara University of Science and Technology, for their support during this research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. International Diabetes Federation. IDF diabetes atlas; 2021. Available from: www.diabetesatlas.org.
2. Tripathy JP, Thakur JS, Jeet G, et al. Prevalence and risk factors of diabetes in a large community-based study in North India: results from a STEPS survey in Punjab, India. Diabetol Metab Syndr. 2017;9:8. doi:10.1186/s13098-017-0207-3
3. Kasole R, Martin HD, Kimiywe J. Traditional medicine and its role in the management of diabetes mellitus: “Patients’ and herbalists’ perspectives”. Evid-Based Complement Altern Med. 2019;2019:1–12. doi:10.1155/2019/2835691
4. Jawla S, Kumar Y, Khan M. Hypoglycemic activity of Bougainvillea spectabilis stem bark in normal and alloxan-induced diabetic rats. Asian Pac J Trop Biomed. 2012;2(2 SUPPL.):919–923. doi:10.1016/S2221-1691(12)60337-2
5. Muñiz-Ramirez A, Garcia-Campoy AH, Pérez Gutiérrez RM, Garcia Báez EV, Flores JMM. Evaluation of the antidiabetic and antihyperlipidemic activity of spondias purpurea seeds in a diabetic zebrafish model. Plants. 2021;10:1417. doi:10.3390/plants10071417
6. Kasali FM, Kadima JN, Peter EL, et al. Antidiabetic medicinal plants used in Democratic Republic of Congo: a critical review of ethnopharmacology and bioactivity data. Front Pharmacol. 2021;12:7579090. doi:10.3389/fphar.2021.757090
7. Kasali FM, Ali MS, Tusiimire J, et al. Phytochemical constituents found in Physalis peruviana L. leaf extracts and their ability to inhibit alpha-glucosidase and scavenge DPPH free radicals in vitro. Trends Phytochem Res. 2022;6(1):3–10. doi:10.30495/tpr.2022.1946170.1230
8. Kasali FM, Tusiimire J, Kadima JN, Tolo CU, Weisheit A, Agaba AG. Ethnotherapeutic uses and phytochemical composition of Physalis peruviana L.: an overview. Sci World J. 2021;2021:1–22. doi:10.1155/2021/5212348
9. Kasali F, Kadima J, Mpiana P. Assessment of antidiabetic activity and acute toxicity of leaf extracts from Physalis peruviana L. in Guinea-pig. Asian Pacific J. 2013;4(6):14–19.
10. Kadima J, Kasali F, Bavhure B, Mahano A, Bwironde F. Comparative antidiabetic potential and survival function of Harungana madagascariensis, Physalis peruviana, Solanum americanum and Tithonia diversifolia extracts on alloxan-induced diabetes in Guinea-pigs. Int J Pharm Pharm Res. 2016;5(3):196–206.
11. Kasali FM, Fokunang CN, Ngoupayo J, et al. Evaluation of the antidiabetic properties of hydro-alcoholic extract and its fractions from Physalis peruviana L. leaves on streptozotocin-induced diabetic Wistar rats. J Dis Med Plants. 2016;2(6):67–73. doi:10.11648/j.jdmp.20160206.12
12. Senhaji S, Lamchouri F, Toufik H. Phytochemical content, antibacterial and antioxidant potential of endemic plant anabasis aretioïdes Coss. & Moq. (Chenopodiaceae). Biomed Res Int. 2020;2020:1–16. doi:10.1155/2020/6152932
13. NRC National Reasearch Council. Nutrient requirements of the laboratory rat; 1995. doi:10.17226/4758
14. Peter EL, Nagendrappa PB, Ajayi CO, Sesaazi CD, Šiler BT. Total polyphenols and antihyperglycemic activity of aqueous fruits extract of Abelmoschus esculentus: modeling and optimization of extraction conditions. PLoS One. 2021;16(4):e0250405. doi:10.1371/journal.pone.0250405
15. Belayneh YM, Birru EM. Antidiabetic activities of hydromethanolic leaf extract of Calpurnia aurea (Ait.) Benth. Subspecies aurea (Fabaceae) in mice. Evid Based Complement Altern Med. 2018;2018:1–9. doi:10.1155/2018/3509073
16. Ojewole JAO, Adewunmi CO. Anti-inflammatory and hypoglycaemic effects of Tetrapleura tetraptera (Taub) [Fabaceae] fruit aqueous extract in rats. J Ethnopharmacol. 2004;95(2–3):177–182. doi:10.1016/j.jep.2004.06.026
17. Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A, Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. DARU J Pharm Sci. 2012;20:56. doi:10.1186/2008-2231-20-56
18. Benedé-Ubieto R, Estévez-Vázquez O, Ramadori P, Cubero FJ, Nevzorova YA. Guidelines and considerations for metabolic tolerance tests in mice. Diabetes Metab Syndr Obes Targets Ther. 2020;13:439–450. doi:10.2147/DMSO.S234665
19. Kihdze TJ, Mayowa AA, Joseph O, et al. Phytochemical and antidiabetic evaluation of the methanolic stem bark extract of Spathodea campanulata (P. Beauv.) Bignoniaceae. Pharmacogn J. 2016;8:243–249. doi:10.5530/pj.2016.3.12
20. Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacol. 2006;105(3):374–379. doi:10.1016/j.jep.2005.11.027
21. Kale OE, Awodele O, Akindele AJ. Subacute and subchronic oral toxicity assessments of Acridocarpus smeathmannii (DC.) Guill. & Perr. root in Wistar rats. Toxicol Rep. 2019;6:161–175. doi:10.1016/j.toxrep.2019.01.005
22. Hashim MA, Yam MF, Hor SY, Lim CP, Asmawi MZ, Sadikun A. Anti-hyperglycaemic activity of Swietenia macrophylla king (Meliaceae) seed extracts in normoglycaemic rats undergoing glucose tolerance tests. Chin Med. 2013;8(1):11. doi:10.1186/1749-8546-8-11
23. Small L, Ehrlich A, Iversen J, et al. Comparative analysis of oral and intraperitoneal glucose tolerance tests in mice. Mol Metab. 2022;57:4–11. doi:10.1016/j.molmet.2022.101440
24. Bowe JE, Franklin ZJ, Hauge-Evans AC, King AJ, Persaud SJ, Jones PM. Assessing glucose homeostasis in rodent models. J Endocrinol. 2014;222(3):13–25. doi:10.1530/JOE-14-0182
25. Bruce CR, Hamley S, Ang T, Howlett KF, Shaw CS, Kowalski GM. Translating glucose tolerance data from mice to humans: insights from stable isotope labelled glucose tolerance tests. Mol Metab. 2021;53:101281. doi:10.1016/j.molmet.2021.101281
26. Bernal C-A, Castellanos L, Aragón DM, et al. Peruvioses A to F, sucrose esters from the exudate of Physalis peruviana fruit as α-amylase inhibitors. Carbohydr Res. 2018;461:4–10. doi:10.1016/j.carres.2018.03.003
27. Simon-delso N, Martin GS, Bruneau E, Hautier L. Time-to-death approach to reveal chronic and cumulative toxicity of a fungicide for honeybees not revealed with the standard ten-day test. Sci Rep. 2018;8:7241. doi:10.1038/s41598-018-24746-9
28. Kathare JM, Mbaria JM, Nguta JM, Moriasi GA. Antimicrobial, cytotoxicity, acute oral toxicity and qualitative phytochemical screening of the aqueous and methanolic extracts of Physalis peruviana L (Solanaceae). Appl Microbiol Open Access. 2021;7:189.
29. Perk BO, Ilgin S, Atli O, Duymus HG, Sirmagul B. Acute and subchronic toxic effects of the fruits of physalis peruviana L. Evid Based Complement Altern Med. 2013;2013:1–10. doi:10.1155/2013/707285
30. Aerm K-A, El-Gengaihi SE, Hamed MA, Zahran HG, Mohammed MA. Chemical composition of golden berry leaves against hepato-renal fibrosis. J Diet Suppl. 2016;13(4):378–392. doi:10.3109/19390211.2015.1099584
31. Pomilio AB, Falzoni EM, Vitale AA. Toxic chemical compounds of the Solanaceae. Nat Prod Commun. 2008;3(4):593–628. doi:10.1177/1934578x0800300420
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.