Back to Journals » Cancer Management and Research » Volume 12
Hypofractionated Radiotherapy for 35 Patients with Adrenal Metastases: A Single-Institution Experience
Authors Zhao R , Ma Y, Yang S , Liu Q, Tang Y, Wang K, Zhang Y, Bi N, Zhang H, Yi J, Li Y, Luo J, Xiao J
Received 27 August 2020
Accepted for publication 27 October 2020
Published 12 November 2020 Volume 2020:12 Pages 11563—11571
DOI https://doi.org/10.2147/CMAR.S278781
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Ruizhi Zhao,1 Yuchao Ma,1 Siran Yang,1 Qingfeng Liu,1 Yuan Tang,1 Kai Wang,1 Ye Zhang,1 Nan Bi,1 Hongmei Zhang,2 Junlin Yi,1 Yexiong Li,1 Jingwei Luo,1 Jianping Xiao1
1Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China; 2Department of Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, People’s Republic of China
Correspondence: Jianping Xiao; Jingwei Luo
National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academic of Medical Sciences, Peking Union Medical College, No. 17 Panjiayuan Nanli, Chaoyang District, Beijing 100021, People’s Republic of China
Tel +86-10-87788281
; Tel +86-10-87788288
Fax +86-10-67706153
Email [email protected]; [email protected]
Objective: To investigate the clinical outcomes of hypofractionated radiotherapy for adrenal metastases.
Materials and Methods: We retrospectively reviewed patients diagnosed with adrenal metastases and treated with hypofractionated radiotherapy, who did not receive adrenalectomy or have disease progression after chemotherapy, from 2007 to 2019. The Kaplan–Meier method was used to estimate local control rate (LCR), progression-free survival (PFS), and overall survival (OS). Univariate analysis was performed using Log rank test.
Results: Thirty-five patients with 42 lesions were enrolled, and the lung was the most common primary site (80.0%). The median follow-up time was 46.4 months. The median volume of GTV and PTV was 23.2 cm3 (range: 3.5– 97.8 cm3) and 38.3 cm3 (range: 10.2– 135.6 cm3), respectively. The main dose regimens were 60 Gy delivered in 4– 15 fractions, with the median dose of PTV being 60 Gy (range: 40– 66.3 Gy) and the biologically effective dose (BED) being 84 Gy (range: 56– 110 Gy). The 1-year and 2-year LCR, OS, and PFS were 92.7% and 88.1%, 76.9% and 45.4%, and 25.1% and 14.4%, respectively. Univariate analysis showed that chemotherapy, disease-free interval from primary disease diagnosis to adrenal metastases diagnosis, and age were significant factors for LCR, OS, and PFS, respectively (p=0.017, 0.049, and 0.004, respectively). No more than grade III toxicities were observed.
Conclusion: As a non-invasive approach, hypofractionated radiotherapy is safe and effective for metastatic adrenal lesions, without serious complications.
Keywords: adrenal metastases, hypofractionated radiotherapy, prognosis, toxicities
Introduction
Adrenal gland is a common metastatic site of primary cancers owing to rich blood sinus. The most common primary sites are lung, stomach, esophagus, and melanoma.1 Typically, most patients are asymptomatic. With advancements in diagnostic imaging, percutaneous biopsy, and fine-needle aspiration, the detection rate of adrenal metastases has increased.1,2 Adrenalectomy is considered the standard curative treatment in case of solitary metastasis. However, adrenalectomy is reserved for patients who met strict criteria, including well-controlled primary disease, solitary adrenal metastasis, without co-metastatic lesions in other organs, and good performance status.2 Therefore, more alternative approaches are required.
Radiotherapy is a non-invasive treatment and has been widely used to treat malignant tumors. However, traditional radiotherapy has been of limited use in metastatic adrenal disease because of the tolerance dose of organs at risk. Hypofractionated radiotherapy can deliver a higher biological equivalent dose to tumor targets than traditional radiotherapy, and has hence been used in many metastatic sites such as lung and liver3,4 with satisfying clinical outcomes. Thus far, except a Phase II, prospective stereotactic ablative body radiotherapy (SABR)-COMET study, most studies regarding adrenal metastases are retrospective analyses; the SABR-COMET trial demonstrated a survival benefit with SABR for all metastatic lesions, including adrenal gland, in various primary cancers.5
In our single-institution study, we aimed to analyze the clinical effects, failure patterns, and toxicities of hypofractionated radiotherapy for adrenal metastases.
Materials and Methods
Inclusion Criteria
Patients aged ≥18 years with pathologically confirmed primary tumor and adrenal metastatic disease were included. All patients had inoperable tumors and/or refused surgery and had experienced failed therapy or were intolerant to systemic therapy. All patients had no history of irradiation to the treated sites. The Karnofsky Performance Scale (KPS) was ≥80 for all patients. All included patients signed an informed consent form before undergoing hypofractionated radiotherapy.
Radiation Procedures
Patients were positioned supinely with arms crossed over their foreheads and immobilized with a body mask. Four-dimensional enhanced computed tomography (CT) was performed from the carina to the fifth lumbar vertebra with a 3-mm slice thickness. The localization images were transferred to the Pinnacle planning system, version 9.10. Gross tumor volume (GTV) was delineated on 4-dimensional CT simulation images and expanded 3-dimensionally by 3 mm to generate planning target volume (PTV). Meanwhile, organs at risk (OAR) including liver, kidney, intestine, colon, stomach, and spinal cord were delineated. The spinal cord was expanded by 5 mm to obtain the planning organ–at–risk volume (PRV). The main prescribed dose was 60 Gy delivered in 4–15 fractions, and prescribed to the 95% isodose line. For lesions adjacent to vital normal tissues such as gastrointestinal tract, great vessels, and kidney, the GTV was contracted by 3 mm to create a simultaneously integrated boost region (Boost), and the prescribed doses were adjusted according to the actual dose distribution. Biological equivalent dose (BED) was calculated using the following formula: BED10=nd[1+d/(α/β)], assuming that α/β was 10. The radiation technology included intensity-modulated radiation therapy (IMRT), volumetric modulated arc therapy (VMAT), and tomotherapy (TOMO). Image-guided radiotherapy (IGRT) was applied during every treatment.
Efficacy Evaluation
An enhanced CT or MRI scan was performed after 2–3 months of radiotherapy and evaluated by clinicians and radiologists together. Tumor lesions were measured in the maximum diameter. Efficacy evaluation was defined as follows: complete response (CR): complete disappearance of the treated lesion; partial response (PR): reduction of the treated lesion by ≥30%; progressive disease (PD): enlargement of the treated lesion by ≥20%; and stable disease (SD): not met the definitions of CR, PR, or PD. Thereafter, a follow-up was planned every 3 months, including enhanced CT or MRI scan, complete blood count, biochemical and tumor marker tests. Local control was defined as the time from the beginning of radiotherapy to the progression of treated lesion; progression-free survival (PFS) was defined as time from the beginning of radiotherapy to progression of the treated lesion, new metastases to the adrenal gland, or other organs; and overall survival (OS) was defined as time from the beginning of radiotherapy to death or the end of follow-up. Failure patterns were recorded including the progression of treated lesion or primary disease and new metastases to the adrenal or other organs. Toxicities were evaluated according to the Common Terminology Criteria for Adverse Events (CTC AE, Version 4.0).
Statistical Analysis
Data analyses were performed using SPSS, version 20.0. The Kaplan–Meier method was used to estimate median LCR, PFS, and OS. Univariate analysis was performed using Log rank test. For variables with more than two categories, Log rank test was used for the overall test, followed by pairwise comparison with Bonferroni correction to adjust for multiple testing if the overall test is significant. P<0.05 was considered statistically significant.
Results
Clinical Characteristics
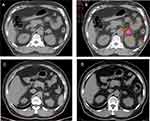
From September 2007 to May 2019, 35 patients with adrenal metastases were enrolled. All patients were diagnosed by CT or MRI. The median KPS was 80, and the median age was 59 years. Median disease-free interval from primary disease diagnosis to adrenal metastases diagnosis (DFI) was 12.6 months. Overall, 22 patients (62.9%) had distant metastases to other organs, and seven patients received prior target therapy. The clinical characteristics are summarized in Table 1. One patient diagnosed with adrenal metastasis and follow-up results were displayed in Figure 1.
 |
Table 1 Clinical Characteristics and Univariate Analysis of 35 Adrenal Metastatic Patients |
Clinical Results
In all, 42 lesions were treated. The largest diameter was 2.9 cm (range: 1.3–4.6 cm). The median volume of GTV and PTV were 23.2 cm3 (range: 3.5–97.8 cm3) and 38.3 cm3 (range: 10.2–135.6 cm3), respectively. Dose regimens included 60 Gy in 15 fractions (40.0%), 60 Gy in 12 fractions (14.3%), and 60 Gy in 20 fractions (28.6%). The median dose of PTV and BED10 was 60 Gy (range: 40–66.3 Gy) and 84 Gy (range: 56–110 Gy). Twenty-three lesions had Boost regions. The median volume of Boost was 10.5 cm3 (range: 1.5–33.2 cm3), and the median dose was 69 Gy (range: 52.5–75 Gy). The dosimetric characteristics are shown in Table 2.
 |
Table 2 The Dosimetric Characteristics of 42 Lesions and Univariate Analysis of LCR |
At the end of follow-up, three cases showed progression from treated lesions, and the local control duration was 17.0 months, 14.9 months, and 1.1 months, respectively. The first patient received another round of radiotherapy at a local hospital with unclear treatment information, the second patient received atezolizumab and ipilimumab for 8 weeks, and the last patient who was diagnosed with lung sarcomatoid carcinoma with synchronous metastasis, died with a short time. The proportions of CR, PR, SD, and PD were 42.9%, 25.7%, 28.6%, and 2.9%, respectively. The 1- and 2-year LCR were 92.7% and 88.1%, respectively. The median local control time could not be calculated because of limited progression events of treated lesions. The results of the univariate analysis are listed in Tables 1 and 2. Prior chemotherapy was a significant factor for LCR (p=0.017). The 1- and 2-year OS and PFS were 76.9% and 45.4%, and 25.1% and 14.4%, respectively. The median survival time (MST) was 22.7 months, and the median PFS was 3.4 months. Univariate analysis showed that DFI was a significant factor for OS (p=0.049), and age for PFS (p=0.004). The originally planned multivariate analysis was not performed because of the limited sample size. The survival curves are shown in Figure 2.
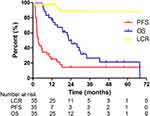 |
Figure 2 The survival curve of LCR, OS, and PFS in the 35 patients with adrenal metastases by Kaplan–Meier method. |
Failure Patterns
Thirty-three of 35 patients had progressed finally; three cases had progressed from only primary disease, 22 had progressed from metastasis to other organs, three cases had progressed from both treated lesions and other organs, and five cases had progressed from new metastasis to the contralateral adrenal gland and other organs. Of these progressed patients, eight received systemic therapy, and one received another round of radiotherapy; the remaining patients did not undergo any treatment.
Toxicities
The median V20 of the left and right kidneys were 8.3% (range: 0–63.2%) and 7.3 (range: 0–27.8%), respectively. The median V20 and mean dose of the liver were 2.3% (range: 0–37.8%) and 5.0 Gy (range: 0–17.4 Gy), respectively. The maximum dose of the stomach, intestine, and colon was 29.2 Gy (range: 5.1–65.9 Gy), 49.4 Gy (range: 0–70.2 Gy), and 29.1 Gy (range: 1.3–65.5 Gy), respectively. The maximum dose of the spinal cord PRV was 26.0 Gy (range: 12.4–49.3 Gy). All patients tolerated the treatment well, as no greater than grade 3 toxicities were observed during follow-up. Overall, 23 of 35 patients died. The reasons for death included systemic failure (n=17), progression of primary disease (n=3), brain metastasis (n=2), and other reasons (n=1). No death due to treatment-related toxicities was recorded.
Discussion
Adrenal metastasis is usually associated with poor prognosis.1 Surgery has been considered a curative treatment for cancers with metastasis to the lung and liver.3,4 A meta-analysis regarding adrenalectomy for adrenal metastasis originating from non-small cell lung cancer (NSCLC) showed that the median survival time was 31 months for metachronous metastasis, and 12 months for synchronous metastasis, with a 5-year OS of 25–26%.6 Lam1 summarized that patients with adrenal metastasis undergoing surgical resection had better survival when than those without surgical resection over a 30-year period (p=0.03). However, many patients had metastases to other organs concurrently with poor medical conditions, which likely made them unsuitable for adrenalectomy. In our study, 20% patients were diagnosed with bilateral adrenal metastasis, and 62.9% had metastasis to other organs. These patients were not suitable for curative adrenalectomy.
As another effective local treatment, hypofractionated radiotherapy has been increasingly used in adrenal metastasis. Our study showed the 1- and 2-year LCR and OS were 92.7% and 88.1%, and 76.9% and 45.4%, respectively, comparable with previous literature. Several published studies were summarized in Table 3. In the literature,5,7–21 studies were mostly retrospective with a small number of patients. The primary disease was multiple including lung, breast, and colorectal cancer. The dose regimens varied with different BED10. The one-year LCR ranged from 55% to 100%, and the 1-year OS, from 44% to 90%. The MST ranged from 7.2 to 41 months, and the median PFS ranged from 3.5 to 12.0 months. SABR-COMET5 was a prospective Phase 2 study, aimed to compare SABR with standard therapy for patients with oligometastatic cancers. A dosage of 60 Gy in eight fractions was delivered for adrenal metastases. The MST and PFS in the SABR group were 41 months and 12 months, respectively, significantly longer than the standard therapy group.
 |
Table 3 Published Studies About Radiotherapy in the Treatment of Adrenal Metastases |
Our study did not find significant differences between LCR and volume or dose of PTV or between LCR and BED10. The significant factors for LCR differed in published studies. Zhao13 reported that BED≥85.5 Gy and a volume of GTV<30 cm3 were correlated with LCR in univariate analysis. Konig et al22 found a superior local control if BED10 was ≥75 Gy or the volume of PTV was <100 mL, but these findings were not significant. Buergy et al12 reported that neither lesion size nor radiation dose were significant factors for local control. While the increased dose of adrenal metastases might improve LCR, considering the unique location of the adrenal gland, it would be hard to deliver a higher radiation dose with respect to the surrounding normal organs. Although the SABR-COMET study5 reported improved PFS and OS rates, toxicities of > grade 2 had increased from 9% to 29% (p=0.026), and three patients died due to treatment-related causes. Therefore, BED10 of adrenal metastases was often lower than lung or liver metastases.3,23 The range of BED varied from 30 Gy9 to 151 Gy22 in published studies about adrenal metastasis, and the median BED ranged from 60 Gy24 to 112.5 Gy.14 In our study, a portion of the lesions was given lower BED to protect the adjacent vital organs; the median BED was 84 Gy, and hence consistent with other studies.
In the current study, DFI was the unique significant factor for OS (p=0.049). OS was longer if DFI was ≥12.6 months, consistent with the study from Howell,25 which reported that DFI<12 months was associated with worse survival (p=0.038) in surgically resected patients. DFI represents the disease-free interval from primary disease to diagnosis of adrenal metastases, and was different from oligometastatic (OM) or oligoprogressive (OP) disease, which required consideration of more complicated metastatic burden and progression of malignancy. In our study, the MST was 24.5 months in the OM patients and 10.5 months in the OP patients, but without significant statistical differences. Similar survival results were shown in the metachronous or synchronous metastases. Figura et al15 also showed that there were no significant differences between OS and OM or OP and between metachronous and synchronous metastases. However, Buergy12 reported that patients with OM had a longer OS (33 months) than those with OP disease (6.5 months). We should note that accurate clinical definitions of OM or OP are still unclear and differ across research studies. Besides, most reports, including the prospective SABR-COMET study, enrolled various primary diseases, which represented different prognosis and treatment options. Therefore, the true relationship between survival with metastatic status may require further detailed prospective studies.
Our study showed that age was a significant factor for PFS (p=0.004) in the univariate analysis. In the database, there were more cases diagnosed with SCLC (9/16 vs 5/19), more patients with extra-adrenal metastases (13/16 vs 9/19), and fewer cases of oligometastases (7/16 vs 14/19) in the age ≤59 years group. These factors might have led to positive results in univariate analysis.
The failure patterns revealed that failure was mainly due to new metastases or progression to other organs. Therefore, subsequent regular imaging examinations for early detection and diagnosis of progression, and timely systemic therapy may be beneficial for metastatic patients.
We did not observe severe toxicities or deaths due to radiotherapy in our study. The previously reported toxicities included nausea, fatigue, diarrhea, and digestive ulcers. Except for the SABR-COMET5 study discussed in the previous section, Casamassima26 reported one patient with grade 2 adrenal insufficiency, and Zhao13 reported one patient with grade 3 diarrhea. In our study, other approaches to reduce radiation dose to OAR included a 4-dimensional CT scan, a smaller expansion margin from GTV to PTV, Boost regions, and IGRT to ensure accuracy during every treatment.
Our study has some limitations. First, it was a retrospective study in a single institution, with only 35 patients, similar to other studies with limited sample size. Second, we were unable to carry out multivariate statistical analyses to identify risk factors of local control and survival. Furthermore, adrenal metastases from various primary cancers were included, similar to previous literature. These limitations might influence the results of different therapeutic options. A large-sized randomized control trial should be designed in future to validate these findings.
Conclusions
It is usually difficult to deliver higher radiation doses to adrenal metastases considering surrounding organs at risk. This retrospective study indicates that hypofractionated radiotherapy for adrenal metastases is effective and safe without severe toxicities or treatment-related deaths. More strategies are needed to deliver higher BED with minimal toxicities. Further prospective randomized controlled trials are required to investigate the role of hypofractionated radiotherapy for adrenal metastases.
Abbreviations
GTV, gross tumor volume; PTV, planning target volume; OAR, organs at risk; PRV, planning organ-at-risk volume; BED, biological equivalent dose; Boost, simultaneously integrated boost region; IMRT, intensity-modulated radiation therapy; VMAT, volumetric modulated arc therapy; TOMO, tomotherapy; CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease; LCR, local control rate; OS, overall survival; PFS, progression-free survival; MST, median survival time. DFI: disease-free interval from primary disease diagnosis to adrenal metastases diagnosis; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; OM, oligometastatic disease; OP, oligoprogressive disease; IGRT, image-guided radiotherapy.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author (Jianping Xiao) upon request.
Ethics Approval and Consent to Participate
This retrospective research has been reviewed, and approved to publish by the ethics committee of National Cancer Center/National Cancer Clinical Medical Research Center/Chinese Academy of Medical Sciences, Peking Union Medical College, Cancer Hospital, Beijing, China. The consents of all the patients was waived due to the retrospective nature of the review, and all the data were anonymized to maintain confidentiality and in compliance with the Declaration of Helsinki.
Acknowledgments
This work was supported by National Major Special Project of China [2016YFC0904600] and Wu Jieping Medical Foundation [320.6750.1208].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Lam K-Y, Lo C-Y. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf). 2002;56(1):95–101. doi:10.1046/j.0300-0664.2001.01435.x
2. Spartalis E, Drikos I, Ioannidis A, et al. Metastatic Carcinomas of the Adrenal Glands: from Diagnosis to Treatment. Anticancer Res. 2019;39(6):2699–2710. doi:10.21873/anticanres.13395
3. Scorsetti M, Comito T, Clerici E, et al. Phase II trial on SBRT for unresectable liver metastases: long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat Oncol. 2018;13(1):234. doi:10.1186/s13014-018-1185-9
4. Nuyttens JJ, van der Voort VZN, Verhoef C, et al. Stereotactic body radiation therapy for oligometastases to the lung: a phase 2 study. Int J Radiat Oncol Biol Phys. 2015;91(2):337–343. doi:10.1016/j.ijrobp.2014.10.021
5. Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058. doi:10.1016/S0140-6736(18)32487-5
6. Tanvetyanon T, Robinson LA, Schell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol. 2008;26(7):1142–1147. doi:10.1200/JCO.2007.14.2091
7. Chawla S, Chen Y, Katz AW, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys. 2009;75(1):71–75. doi:10.1016/j.ijrobp.2008.10.079
8. Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187(4):245–251. doi:10.1007/s00066-011-2192-z
9. Scorsetti M, Alongi F, Filippi AR, et al. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: a retrospective analysis of 34 patients. Acta Oncol. 2012;51(5):618–623. doi:10.3109/0284186X.2011.652738
10. Ahmed KA, Barney BM, Macdonald OK, et al. Stereotactic body radiotherapy in the treatment of adrenal metastases. Am J Clin Oncol. 2013;36(5):509–513. doi:10.1097/COC.0b013e3182569189
11. Franzese C, Franceschini D, Cozzi L, et al. Minimally Invasive Stereotactical Radio-ablation of Adrenal Metastases as an Alternative to Surgery. Cancer Res Treat. 2017;49(1):20–28. doi:10.4143/crt.2016.057
12. Buergy D, Rabe L, Siebenlist K, et al. Treatment of Adrenal Metastases with Conventional or Hypofractionated Image-guided Radiation Therapy – patterns and Outcomes. Anticancer Res. 2018;38(8):4789–4796. doi:10.21873/anticanres.12788
13. Zhao X, Zhu X, Fei J, et al. Short-term outcomes and clinical efficacy of stereotactic body radiation therapy (SBRT) in treatment of adrenal gland metastases from lung cancer. Radiat Oncol. 2018;13(1):205. doi:10.1186/s13014-018-1152-5
14. Scouarnec C, Pasquier D, Luu J, et al. Usefulness of Stereotactic Body Radiation Therapy for Treatment of Adrenal Gland Metastases. Front Oncol. 2019;9:732. doi:10.3389/fonc.2019.00732
15. Figura NB, Oliver DE, Mohammadi H, et al. Novel Dose Escalation Approaches for Stereotactic Body Radiotherapy to Adrenal Oligometastases: A Single-Institution Experience. Am J Clin Oncol. 2020;43(2):107–114. doi:10.1097/COC.0000000000000634
16. Li J, Shi Z, Wang Z, et al. Treating adrenal tumors in 26 patients with CyberKnife: a mono-institutional experience. PLoS One. 2013;8(11):e80654. doi:10.1371/journal.pone.0080654
17. Gamsiz H, Beyzadeoglu M, Sager O, et al. Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori. 2015;101(1):98–103. doi:10.5301/tj.5000222
18. Haidenberger A, Heidorn SC, Kremer N, Muacevic A, Furweger C. Robotic Radiosurgery for Adrenal Gland Metastases. Cureus. 2017;9(3):e1120. doi:10.7759/cureus.1120
19. Celik E, Semrau R, Baues C, Trommer-Nestler M, Baus W, Robot-assisted Extracranial MS. Stereotactic Radiotherapy of Adrenal Metastases in Oligometastatic Non-small Cell Lung Cancer. Anticancer Res. 2017;37(9):5285–5291. doi:10.21873/anticanres.11954
20. Chance WW, Nguyen QN, Mehran R, et al. Stereotactic ablative radiotherapy for adrenal gland metastases: factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract Radiat Oncol. 2017;7(3):e195–e203. doi:10.1016/j.prro.2016.09.005
21. Toesca D, Koong AJ, von Eyben R, Koong AC, Chang DT. Stereotactic body radiation therapy for adrenal gland metastases: outcomes and toxicity. Adv Radiat Oncol. 2018;3(4):621–629. doi:10.1016/j.adro.2018.05.006
22. Konig L, Hafner MF, Katayama S, et al. Stereotactic body radiotherapy (SBRT) for adrenal metastases of oligometastatic or oligoprogressive tumor patients. Radiat Oncol. 2020;15(1):30. doi:10.1186/s13014-020-1480-0
23. Ricco A, Davis J, Rate W, et al. Lung metastases treated with stereotactic body radiotherapy: the RSSearch(R) patient Registry’s experience. Radiat Oncol. 2017;12(1):35. doi:10.1186/s13014-017-0773-4
24. Rudra S, Malik R, Ranck MC, et al. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol Cancer Res Treat. 2013;12(3):217–224. doi:10.7785/tcrt.2012.500320
25. Howell GM, Carty SE, Armstrong MJ, et al. Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann Surg Oncol. 2013;20(11):3491–3496. doi:10.1245/s10434-013-3050-2
26. Casamassima F, Livi L, Masciullo S, et al. Stereotactic radiotherapy for adrenal gland metastases: university of Florence experience. Int J Radiat Oncol Biol Phys. 2012;82(2):919–923. doi:10.1016/j.ijrobp.2010.11.060
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.