Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Hypertension and Its Associated Factors Among Type 2 Diabetes Mellitus Patients at Debre Tabor General Hospital, Northwest Ethiopia
Received 19 March 2020
Accepted for publication 30 April 2020
Published 13 May 2020 Volume 2020:13 Pages 1621—1631
DOI https://doi.org/10.2147/DMSO.S254537
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Yonas Akalu, Yitayeh Belsti
Department of Human Physiology, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Yonas Akalu
Department of Human Physiology, College of Medicine and Health Sciences, University of Gondar, Gondar, P.O. Box 196, Ethiopia
Tel +251 918318230
Email [email protected]
Background: Type 2 diabetes mellitus (T2DM) is associated with a high risk of early mortality and morbidity from hypertension. Even though Ethiopia is Africa’s first country among the top five in the prevalence of DM, there is a paucity of data on hypertension and its associated factors among patients with type 2 diabetes mellitus. Therefore, this study aimed to determine the prevalence and associated factors of hypertension among type 2 diabetes mellitus patients at Debre Tabor General Hospital, 2019.
Methods and Materials: An institution-based cross-sectional study was employed on 378 T2DM patients. Data were collected using an interviewer-administered questionnaire and analyzed by Stata 14. A multivariable logistic regression model was used to identify associated factors of hypertension among T2DM patients. Associated factors were declared at p < 0.05.
Results: The prevalence of hypertension among T2DM patients was 59.5% (95% CI: 54.5– 64.5). Stage 1 hypertension was the most common (30.95%). The odds of hypertension was higher among age group of 50– 60 years (adjusted odds ratio (AOR)=2.5, 95% confidence interval (CI) (1.27– 4.90)), patients from urban area (AOR = 2.8, 95% CI (1.08– 7.18)), with longer duration of T2DM (AOR =1.16, 95% CI (1.08– 1.25)), with BMI ≥ 25 kg/m2 (AOR = 3.2, 95% CI (1.71– 5.96)), with poor glycemic control (AOR = 3.0, 95% CI (1.75– 5.19)), and patients who were current cigarette smokers (AOR = 3.8, 95% CI (1.98– 14.96)).
Conclusion: The prevalence of hypertension is high and the majority have poor blood pressure control. Hence, DM care providers and other health sector stakeholders have to work in collaboration to prevent it through designing appropriate strategies especially for those at higher risk of developing hypertension.
Keywords: hypertension, associated factors, type 2 diabetes mellitus, Ethiopia
Background
Type 2 diabetes mellitus (T2DM) is associated with a high risk of early mortality and morbidity due to cardiovascular diseases (CVD) such as hypertension (HTN), stroke, and end-stage renal disease. Hypertension is the leading CVD-attributable cause of morbidity and mortality among T2DM patients.1–3 The comorbidity of DM and hypertension is increasing globally.4 Hypertension with diabetes increases mortality risk by 7.2 times with a higher risk of death in developing countries5 like Ethiopia, Africa’s first ranked country with the number of diabetes mellitus patients.6 Hypertension contributes to the development and progression of microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular (atherosclerotic) complications of diabetes.7 It is a major risk factor for cardiovascular mortality and morbidity through its effects on target organs like the brain, heart, eye, and kidney due to structural alterations in the microcirculation secondary to oxidative stress, inflammation, or endothelial dysfunction.8 Uncontrolled hypertension leads to heart attack, stroke, kidney disease or failure, vision loss, sexual dysfunction, and peripheral arterial disease (PAD).8 Hypertension induced organ damage is manifested by elevations in albumin excretion (proteinuria),9 and left ventricular hypertrophy and presence of left ventricular strain pattern in ECG.10 Moreover, detection of hard exudates, cotton wool spots, papilledema, retinal hemorrhages, and microaneurysms in fundoscopy, and presence of ischaemic or hemorrhagic brain injury in brain imaging by computed tomography (CT) are other manifestations of hypertension induced end organ damage.11,12
Eighty percent of diabetes patients die from CVD, especially HTN and stroke.13,14 Moreover, HTN exacerbates diabetic cardiomyopathy, accelerates the progression of diabetic renal disease and CVD.15 A study on DM patients showed that the coexistence of hypertension in diabetes mellitus is attributed to the risk of death and cardiovascular events by 44% and 41%, respectively, as compared to 7% and 9% of risks in people with diabetes alone.2 Hypertension is also the largest contributor to the high costs of DM patients.3 The prevalence of HTN among DM patients is higher than in non-DM patients. The higher percentage of hypertension among diabetes patients is attributed by hyperglycemia, insulin resistance, and dyslipidemia. All of these factors induce the development and progression of atherosclerosis by disrupting the blood vessel wall through the promotion of vascular inflammation and endothelial cell dysfunction, derangements of various cell types like platelets and promotion of coagulation.16 These all lead to narrowing of blood vessels and an increment of total peripheral arterial resistance to causes hypertension. Hyperinsulinemia and insulin resistance contribute to elevated blood pressure because insulin is known to promote sodium retention and enhances sympathetic nervous system activity.17 Furthermore, insulin resistance is associated with the inappropriate activation of the Renin-Angiotensin-Aldosterone System (RAAS). Once RAAS is activated, multiple mechanisms that increase BP will get activated. For instance, angiotensin II, the product of activation of RAAS, stimulates vasoconstriction and production of aldosterone, a hormone responsible for retention of salt and water in the kidney to cause hypertension.18 Apart from this, the presence of renal insufficiency secondary to diabetes may impair the ability to excrete water and solutes, thus perpetuating the volume expansion that was induced by different factors.17 In addition to T2DM, a persistent high salt diet is also ascribed to the development of high blood pressure (BP). A high salt diet raises the amount of sodium in the blood which then reduces the ability of the kidney to eliminate water from the body due to its osmotic effect. This excess sodium in the blood provokes excess water retention and hence high BP which in turn puts extra strain on the walls of the arteries. To cope up with this high BP, the muscular layer of the artery thickened and raises the BP by reducing the space in the vessel.18 Moreover, high salt intake causes microvascular endothelial inflammation,18 an increase in systemic peripheral resistance, alterations in the endothelial function and modification of sympathetic activity to end up with hypertension.18
The incidence of hypertension in T2DM patients is related to the degree of obesity, advanced age, and extensive atherosclerosis.19 A study in Iraq revealed 89.6% prevalence of hypertension among DM patients which was associated with age, BMI, insulin use, and duration of diabetes.20 There were 85.6%, 54.2%, and 56.3% prevalence of hypertension among DM patients in Benghazi,4 Nigeria (54.2%),19 and Adama, Ethiopia,21 respectively. A study conducted in Benghazi DM patients showed that hypertension was associated with older age, male sex, DM duration, and overweight.4 The prevalence of HTN depends on the type and duration of diabetes, age, sex, race/ethnicity, BMI, and history of glycemic control.22
Although hypertension is a significant and controllable risk factor for many diseases, its asymptomatic nature makes many people to get life-threatening complications.23 Different studies revealed that 50% of the total HTN cases were diagnosed during the study which indicates that HTN is an underdiagnosed comorbid disease in DM patients.20,24,25 Information about the prevalence of hypertension in different countries of the world is important to design appropriate policies to tackle the impact of hypertension on DM.25 Furthermore, recognizing associated factors of hypertension among diabetic patients is important for both healthcare professionals, to successfully minimize its impact on patients, and policy-makers for designing appropriate strategies to avoid such factors. However, such data are scanty in Ethiopia, particularly in Debre Tabor. Therefore, this study aimed to determine the prevalence and associated factors of hypertension among T2DM patients at Debre Tabor General Hospital.
Methods and Materials
Study Design, Area, and Period
We conducted an institution-based cross-sectional study at Debre Tabor General Hospital, Debre Tabor, Northwest Ethiopia which is located 667 km away from Addis Ababa, the capital city of Ethiopia. The actual data collection period was from February 1 to May 30, 2019.
Source and Study Population
All T2DM patients of Debre Tabor General Hospital were the source population and all T2DM patients who came to the diabetic clinic during the data collection period were the study population.
Sample Size Determination and Sampling Technique
The required sample size (n) was estimated using a single population proportion formula proposed by Cochran14 by taking a 56% proportion of hypertension among T2DM patients of another study,26 0.05 level of significance (α), and 5% margin of error.
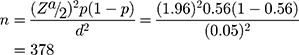
Where n = the desirable sample size, (α/2) = the critical value at 95% level of significance (1.96), p = proportion of hypertension among T2DM (0.56), and d = precision of measurement (acceptable marginal error) (0.05). Therefore, the final sample size for this study was 378. Computer-generated simple random sampling technique by using their medical registration number was employed to select the study participants.
Inclusion and Exclusion Criteria
We included adult T2DM patients who were present during the study period. Type 2 DM patients who were severely ill, with a history of hypertension before the diagnosis of T2DM, and pregnant women were excluded from the study.
Variables of the Study
Dependent variable: Hypertension
Independent variables: Age, sex, residence, occupation, monthly income, educational level, cigarette smoking, treatment options for diabetes, BMI, duration of T2DM, fasting blood glucose (FBG), and glycated hemoglobin (HbA1c).
Operational Definitions
Hypertension (HTN)
Patients with Systolic Blood Pressure (SBP) and/or Diastolic Blood Pressure (DBP) of 140/90 mmHg or greater, respectively,21 or patients on antihypertensive therapy were taken as hypertensive.
Staging of Hypertension
Blood pressure of 120/80 to 139/89 is prehypertension; BP of 140/90 to 159/99 is Stage 1 HTN; 160/100 to 179/109 is Stage 2 HTN, and BP ≥180/110 is hypertensive crisis.21
Current Smokers
A person who smoked a cigarette at least once in the last month before the study.
Poor Glycemic Control
A poor glycemic control was considered when a patient had HbA1c >7%.27
Data Collection Instrument and Procedure
We used pre-tested interviewer-administered questionnaire to collect the data. The questionnaire were adapted from relevant literatures19,20,24,25,28-31 and reviewed by clinicians in diabetes care to ensure its content validity. It was initially prepared in English by language experts, and translated into the local language (Amharic) and then retranslated to English by another expert to ensure its consistency. Data were collected by 4 nurses (BSc) and one supervisor (MSc). Type 2 DM patients were identified by checking the physician diagnosis on their chart/medical record. A person is considered as having T2DM when he/she has a FBG of ≥126 mg/dl, HbA1C ≥ 6.5%, or on medication for T2DM.32,33
Data on socio-demographic characteristics (age, sex, residence, educational status, occupation, monthly income, and current cigarette smoking status) were collected from participants by interview. Age was categorized as <50, 50–59, and ≥ 60 years and educational status was categorized as cannot read and write, read and write, elementary (1–8), and secondary education and above while income was categorized by interquartile range. The clinical data like duration of T2DM and type of anti-diabetic drug on use were extracted from patients’ medical records. Body weight (kg) and height (cm) were measured to the nearest 0.1 unit in barefoot subjects with light clothing. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). BMI was classified into two categories (< 25 and ≥25) according to their risk of hypertension.34 Blood pressure (BP) was recorded using a mercury sphygmomanometer BP cuff with the appropriate size that covers two-thirds of the arm with the subject in the sitting position. The arm which was used for BP measurement was supported on a flat table. Participants were asked to rest for at least 5 minutes and if they were taking any caffeinated beverages they were rested for 30 minutes before measurement. Two consecutive measurements were taken 5 minutes apart, and the mean value was taken. All patients were advised to fast on the morning of their clinic appointment to allow for the measurement of their FBG using a glucometer. Patients underwent venous sampling for FBG and HbA1c, and the blood sent to the laboratory. Glycated hemoglobin was measured using a 902 Automatic Analyzer with Roche/Hitachi kit.
Data Quality Control
To assure data quality, the questionnaire were pre-tested on 19 T2DM patients at another diabetic center (Addis Zemen District Hospital). The training was given for data collectors. Data were also appropriately entered and coded before analysis.
Data Processing and Analysis
Data were checked for completeness, entered into Epi Data-V.4.6, and analyzed using Stata-14. Bivariable logistic regression analysis was performed to determine the association of each independent variable with hypertension. All variables with a p < 0.25 in the bivariable analysis were entered into multivariable logistic regression. In multivariable logistic regression, a p <0.05 was considered statistically significant. The strength of association of factors, with hypertension was demonstrated by computing the adjusted odds ratio (AOR) and its 95% confidence interval (CI).
Results
Socio-Demographic and Clinical Characteristics of Study Participants
A total of 378 T2DM patients with a mean age of 56.0 years (SD ±12.0) participated in the study. One hundred and fifty (39.7%) of the study participants were 60 years old and above. Majority of the study participants were males (225 (59.5%)), urban residents (250 (66.1 %)), unable to read and write (145 (38.4%)), and government employees (115 (30.4%)). One hundred and eleven (29.9%) study participants earned a monthly income of 92.1–153.4 USD (United States dollar). The mean duration of T2DM since diagnosis was 11.8 (SD ± 5.0, range: 2–28) years. Most (75.6%) of the study participants had a BMI of 25 kg/m2 and above. The mean glycated hemoglobin was 6.9% (SD± 1.2) and 224 (59.3%) had poor glycemic control. The mean FBG was 152.1 mg/dl (SD± 20.1), and the mean systolic and diastolic blood pressure of T2DM patients were 134.6 mmHg (SD ± 26.1) and 86.4 mmHg (SD± 14.3) respectively. Two hundred and ninety-three (77.5%) patients relied on oral hypoglycemic agents as treatment option for DM. Twenty-six (6.9%) study participants were current cigarette smokers (Table 1).
 |  |  |  |
Table 1 Socio-Demographic and Clinical Characteristics of Type 2 Diabetes Mellitus Patients, Debre Tabor, Northwest Ethiopia, 2019 |
Prevalence and Patterns of Hypertension
The prevalence of hypertension was 59.5% (225/378) (95% CI: 54.5–64.5). Of these, 65 (17.2%) were newly diagnosed at the time of data collection. Only 41 (25.6%) of previously diagnosed hypertensive patients properly controlled their blood pressure. The majority (30.95%) of hypertensive patients were stage I. Forty-five (11.9%) of study participants were stage 2; 22 (5.8%) on hypertensive crisis; and 86 (22.7%) were pre-hypertensive (Figure 1). Hypertension was predominant among ≥60 years age group (106 (70.7%)), males (146 (64.9%)), urban dwellers (177 (70.8%)), overweight participants (192 (67.1%)), patients who attained elementary school (41 (74.6%)), merchants (75 (79.8%)), insulin users (24 (64.9%)), those with poor glycemic control (160 (71.4%)), and current cigarette smokers (21 (80.8%)) (Table 2).
 |
Table 2 Factors Associated with Hypertension Among Type 2 Diabetes Mellitus Patients, Debre Tabor, Northwest Ethiopia, 2019 |
 |
Figure 1 Blood pressure levels of type 2 DM patients, Debre Tabor, northwest Ethiopia, 2019. |
Factors Associated with Hypertension Among T2DM Patients
Crude association of all independent variables with the dependent variable (HTN) was checked by binary logistic regression. Age, sex, residence, monthly income, occupation, educational status, T2DM duration, BMI, and cigarette smoking were a candidate for the final model. In the multivariable analysis; age, residence, duration of T2DM, BMI, poor glycemic control, and current cigarette smoking were significantly associated with HTN.
The odds of acquiring HTN was 2.5 times higher in the age group of 50–59 (AOR=2.5, 95% CI (1.27–4.90)) than those below 50 years old. Patients from urban areas were 2.8 times (AOR = 2.8, 95% CI (1.08–7.18)) more likely to be hypertensive than their counterpart. A one year increase in the duration of T2DM increases likelihood of developing HTN by 16% (AOR =1.16, 95% CI (1.08–1.25)). Those patients with BMI ≥ 25 kg/m2 were 3.2 times more likely to develop hypertension (AOR = 3.2, 95% CI (1.71–5.96)) than those with BMI < 25 kg/m2. Patients with poor glycemic control had 3 (AOR = 3.0, 95% CI (1.75–5.19)) times likelihood to develop HTN as compared to those with good glycemic control. Type 2 DM patients who were current cigarette smokers were 3.9 times (AOR = 3.8, 95% CI (1.98–14.96)) more likely to develop HTN than those who were not (Table 2).
Discussion
This study revealed the prevalence and associated factors of HTN among T2DM patients at Debre Tabor General Hospital. The prevalence of hypertension in this study was 59.5% which is in line with studies in Hosanna (55%),25 Adama, Ethiopia (56.3%),21 Botswana (63.1%),30 and Israel (60.2%).24 However, the current finding is higher than the prevalence reported at Jimma, Ethiopia (46.5%).35 The possible reason for this could be due to the presence of age difference between the two study populations. The age of study participants in a study conducted at Jimma was < 45 years among 48.9% patients,35 resulting in a lower prevalence of hypertension, whereas only 22.5% of the study population in our study were below 50 years old with a large proportion of >50 years old population. This may be the possible cause of a higher prevalence of hypertension due to the fact that age increment is associated with a higher prevalence of hypertension.36 The prevalence of hypertension in this study is also higher than that of Nigeria (54.2%).19 This difference could be due to the higher mean duration of T2DM (11.8 years) in our study than the study in Nigeria (6.6 years). Different studies showed that an increase in the duration of T2 DM is associated with an increased prevalence of hypertension.20,28,37,38 On the contrary, the prevalence of HTN in this study is lower than the findings reported in Jordan (72.4%)38 and Iraq (89.6%).20 The higher prevalence in these studies is most likely due to the cut point used for the diagnosis of hypertension, which was defined at a lower level of blood pressure (>130/80 mmHg). The other possible difference might be due to a difference in BMI of the study populations. Greater than 91% of the Jordan study population had a BMI of ≥ 25kg/m2, which is higher than the proportion of our study population whose BMI was ≥ 25kg/m2. So the higher BMI in Jordan might be the possible cause for the higher prevalence of hypertension because high BMI is associated with increased risk of hypertension.20,28,38-42 The higher prevalence of hypertension reported in three Moroccan regions (70.4%)29 is perhaps related to the fact that most diabetics were aged 60 years old and over, having a high risk of hypertension.29,43,44
The odds of getting HTN among T2DM patients was higher in the age group of 50–59 years than the age group of <50 years. This was supported by the study done in Israel,24 Iraq,20 Botswana,30 and Jordan38 which reported that the prevalence of HTN was increased with age. It could be attributable to the vascular changes during aging. As age increases, arterial stiffening and thickening will be triggered by a complex change in each layer of blood vessels.27 Aging induced thickening of the intima compromises endothelium integrity and decreases the availability of vasodilators like nitric oxide.45 Stiffening of the arterial walls disturbs the normal blood flow, creating favorable conditions for calcium and fatty deposits to accumulate on the inside of the arteries to narrow the artery further and cause hypertension.45–47 Patients from urban areas were more likely to have hypertension than those who were from rural areas. This is consistent with the finding of other studies.25,48-50 A sedentary lifestyle and changes in dietary habits following increasing urbanization are the main contributors to the rise of hypertension in urban areas51,52 especially in Africa.50 An increase in the duration of T2DM was associated with an increase in the likelihood of developing HTN. Consistently, other studies reported that the duration of T2DM has a direct relationship with the prevalence of hypertension.20,28,38 This might be because as the duration of T2DM increases the effect of hyperglycemia, dyslipidemia, and insulin resistance will be more pronounced.53 As the duration gets longer, changes caused by diabetes mellitus, such as micro-vascular damage, sympathetic damage, an enhanced renin-angiotensin system, and decreased insulin sensitivity will get worse and aggravate hypertension.37 Similarly, age is also increasing, thus blood vessels become more stiffened, which in turn are highly related to the development of hypertension.54 Those patients with high BMI were many times more likely to develop hypertension than those with normal BMI. This finding is in agreement with different studies in different countries.20,28,38-41 The possible justification for the association between BMI and hypertension is due to the reason that obesity increases salt and water reabsorption in the kidney by directly activating mineralocorticoid receptors. This leads to an increase in blood volume and finally hypertension.42 Furthermore, obesity generally decreases parasympathetic tone and increases sympathetic activity resulting in increased cardiac output and vasoconstriction followed by hypertension.42 Sympathetic stimulation and compression of the kidney by visceral obesity lead to activation of RAAS, resulting in the rise of the blood level of angiotensinogen II and aldosterone, which increase blood volume by different mechanisms.55 At the same time, angiotensinogen II is a potent vasoconstrictor.42 These effects together lead to hypertension. Patients with poor glycemic control had higher odds of hypertension than patients with good glycemic control. This finding is in agreement with the finding of many other studies.28,56-58 This association is attributable to persistent hyperglycemia. Excess glucose chemically attaches to free amino groups of proteins, collagen, and other long-lived proteins in blood vessel walls which, in turn, may trap circulating low-density lipoprotein (LDL) that promotes the deposition of cholesterol in the intima, thus accelerates atherogenesis and subsequent hypertension.59 Also, hyperglycemia increases the osmolality of the extracellular fluid, triggering water to shifts from the intracellular to extracellular space and cause volume expansion and high BP.60
Diabetes mellitus patients who were current cigarette smokers were more likely to develop HTN than those who were not. This finding is consistent with other studies.61,62 It could be as a result of the unscrupulous effect of nicotine and other ingredients of cigarettes on blood vessels.55 Nicotine inhibits vasodilation by impairing nitric oxide production.63,64 It also stimulates the release of catecholamine, which increases platelet aggregation, thrombosis, and vasoconstriction. Moreover, nicotine induces insulin resistance, dyslipidemia, vascular inflammation, and abnormal vascular growth.63,64 Activation of endogenous free radicals by nicotine or the free radicals directly from cigarette smoke leads to oxidative stress which is a major contributor to atherosclerosis.65 All these effects narrow blood vessels and predispose to hypertension.
Age, current cigarette smoking, and high BMI (≥25kg/m2) which were significantly associated with hypertension in T2DM patients in the current study were also found significantly associated with hypertension in non-diabetic patients in other studies.61,62,66-69 But the degree of association of the aforementioned factors with hypertension in the two sets of the population is different. They have a stronger association with hypertension in the diabetic population. The odds of hypertension (3.9) in the currently smoking diabetic population of the current study is higher than that of the currently smoking non-diabetic population in Nepal (2.0),66 Jordan (1.8),62 Jilin Province, China (1.2),61 and Kenya (1.1).68 The odds of hypertension (3.7) in diabetic populations with high BMI were higher than that of the non-diabetic population with the same high BMI in Nepal (1.2),66 Ethiopia (2),41 Kenya (2.4),68 and Greek 2.6.67 Similarly, the likelihood of hypertension in elder diabetic patients is higher than the elder non-diabetic population in different studies.68,69 These results imply that these factors on hypertension are worse for people with T2DM than non-DM patients.
Conclusions
The prevalence of hypertension was high. Only 25.6% of previously diagnosed hypertensive patients were normotensive. Stage 1 hypertension was the most common. Age, residence, duration of T2DM, BMI, cigarette smoking, and glycemic control were significantly associated with hypertension. Diabetes care providers should work coordinately with other health sector stakeholders to prevent hypertension by designing preventive strategies especially for those at higher risk of developing HTN. Future research using a larger sample size should be carried out to investigate the causes of uncontrolled blood pressure among diabetic patients.
Limitations of the Study
Since the study was cross-sectional, it cannot show the cause-effect relationship. Small sample size and the missing of some important variables like the history of medication of hypertensive patients were other limitations of this study.
Abbreviations
BMI, body mass index; BP, blood pressure; CVD, cardiovascular diseases; FBG, fasting Blood glucose; HbA1c, glycated hemoglobin (HbA1c); T2DM, type 2 diabetes mellitus.
Authors’ Information
Yonas Akalu (MSc, Lecturer of Medical Physiology), Department of Physiology, College of Medicine and Health Sciences, University of Gondar, Ethiopia. Yitayeh Belsti (BSc, Assistant Lecturer of Medical Physiology), Department of Physiology, College of Medicine and Health Sciences, University of Gondar, Ethiopia.
Data Sharing Statement
The data will be available from the corresponding author upon request.
Ethics and Consent Statement
Ethical approval for the study was obtained from the Institute of Public Health College of Medicine and Health Sciences, University of Gondar. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study participants and confidentiality was kept. All the study participants answered the administered pre-tested questionnaires voluntarily and confidently.
Acknowledgments
We would like to thank Debre Tabor General Hospital staff at the diabetic clinic for their support. The study participants are duly acknowledged for kindly giving the required data.
Author Contributions
Both authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Eren NK, Harman E, Dolek D, Levent F. Rate of blood pressure control and antihypertensive treatment approaches in diabetic patients with hypertension. Arch Turk Soc Cardiol. 2014;42(8):733–740. doi:10.5543/tkda.2014.53384
2. Emdin CA, KazemRahimi B, Neal TC, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes a systematic review and meta-analysis. JAMA. 2015;313(6):603–615. doi:10.1001/jama.2014.18574
3. Chen G, Mcalister FA, Walker RL, Hemmelgarn BR, Campbell NRC, Commentary SE. Population science/epidemiology cardiovascular outcomes in Framingham participants the importance of blood pressure. Hypertension. 2011;57(5):891–897. doi:10.1161/HYPERTENSIONAHA.110.162446
4. Nouh F, Omar M, Younis M. Prevalence of hypertension among diabetic patients in Benghazi: a study of associated factors. Asian J Med Heal. 2017;6(4):1–11. doi:10.9734/AJMAH/2017/35830
5. Vargas-uricoechea H, Cáceres-acosta MF. Control of blood pressure and cardiovascular outcomes in type 2 diabetes. Open Med. 2018;13:304–323. doi:10.1515/med-2018-0048
6. International Diabetes Federation. Eighth Edition 2017. 2017. doi:10.1016/S0140-6736(16)31679-8
7. Ward JD, Manes C, Ionescu-tirgoviste C, Witte DR, Fuller JH. Vascular risk factors and diabetic neuropathy. New Engl J Med Orig. 2005;352(4):341–350. doi:10.1056/NEJMoa032782
8. Cohuet GS-B, Struijker-Boudier H. Mechanisms of target organ damage caused by hypertension: therapeutic potential. Pharmacol Ther. 2006;111(2006):81–98. doi:10.1016/j.pharmthera.2005.09.002
9. Forlemu AAMGASK, Menanga A, Ashuntantang G, Kingue S. Urinary protein excretion is associated with left ventricular hypertrophy in treatment- naïve hypertensive patients in an African hospital setting. Cardiorenal Med. 2013;3(3):57–62. doi:10.1159/000349938
10. Rodrigues JCL, Amadu AM, Dastidar AG, et al. ECG strain pattern in hypertension is associated with myocardial cellular expansion and diffuse interstitial fibrosis: a multi-parametric cardiac magnetic resonance study. Eur Hear J Cardiovasc Imaging. 2017;2017(18):441–450. doi:10.1093/ehjci/jew117
11. Siddiqui MA, Mittal PK, Brent P, et al. Secondary hypertension and complications: diagnosis and role of imaging. RadioGraphics. 2019;39(4):1036–1055. doi:10.1148/rg.2019180184
12. Esh H, Agabiti E, France MA, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Hear J. 2018;2018(00):1–98. doi:10.1097/HJH
13. Nithish C, Prasad RB, Reddy MRRM. Estimation of risk factors for cardio vascular diseases in urban & semi-urban population: a prospective observational study. International J Med Heal Res. 2019;2019(5):84–86.
14. Cochran WG. Sampling techniques, 2nd edn. JBiometrische Zeitschrift. 1965;7(3):203.
15. Voulgari C, Papadogiannis D, Tentolouris N. Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc Health Risk Manag. 2010;6(1):883–903. doi:10.2147/VHRM.S11681
16. Thiruvoipati T. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6(7):961. doi:10.4239/wjd.v6.i7.961
17. Zhou M, Wang A, Yu H. Link between insulin resistance and hypertension: what is the evidence from evolutionary biology? Diabetol Metab Syndr. 2014;6(12):1–8. doi:10.1186/1758-5996-6-12
18. Grillo A, Salvi L, Coruzzi P, Paolo Salv GP. Sodium intake and hypertension. Nutrients. 2019;11(1970):1–16. doi:10.3390/nu11091970
19. Unadike BC, Eregie A, Ohwovoriole AE. Prevalence of hypertension amongst persons with diabetes mellitus in Benin City, Nigeria. Niger J Clin Pract. 2011;14(3):300–302. doi:10.4103/1119-3077.86772
20. Mansour AA. Prevalence and control of hypertension in Iraqi diabetic patients: a prospective cohort study. Open Cardiovasc Med J. 2012;6(1):68–71. doi:10.2174/1874192401206010068
21. Williams B, Esh GM, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertension. 2018;36(10):1953–2041. doi:10.1093/eurheartj/ehy339
22. Benetos A, Davis AM, Michos ED, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273–1284. doi:10.2337/dci17-0026
23. Owolabi EO, Ter GD, Adeniyi OV, Seekoe E. Social epidemiology of hypertension in Buffalo City Metropolitan Municipality (BCMM): cross-sectional study of determinants of prevalence, awareness, treatment and control among South African adults. BMJ Open. 2017;7(6):1–12. doi:10.1136/bmjopen-2016-014349
24. Kabakov E, Koffle M, Karen Tordjman CN. Prevalence of hypertension in type 2 diabetes mellitus: impact of the tightening definition of high blood pressure and association with confounding risk factors. J Cardiometabolic Syndr. 2006;1(2):95–101. doi:10.1111/j.1559-4564.2006.05513.x
25. Tadesse K, Amare H, Hailemariam T, Gebremariam T. Prevalence of hypertension among patients with type 2 diabetes mellitus and its socio demographic factors in Nigist Ellen Mohamed Memorial Hospital. J Diabetes Metab. 2018;9(1):4. doi:10.4172/2155-6156.1000792
26. Dedefo A, Galgalo A, Jarso G, Mohammed A. Prevalence of hypertension and its management pattern among type 2 diabetic patients attending, Adama Hospital Medical College, Adama. J Diabetes Metab. 2018;9(10):1–8. doi:10.4172/2155-6156.1000808
27. Alice YY. Canadian diabetes association clinical practice guidelines expert committee. Can J Diabetes. 2019;37(2013):1–3. doi:10.1016/j.jcjd.2013.01.009
28. Mariye T, Girmay A, Tasew H, Teklay G. Determinants of hypertension among diabetic patients in public hospitals of the Central Zone, Tigray, Ethiopia 2018: unmatched case- control study. Pan Afr Med J. 2019;8688:1–12. doi:10.11604/pamj.2019.33.100.17094
29. Berraho M, Achhab YE, Benslimane A, Rhazi KEL, Chikr M. Open access. Pan Afr Med J. 2012;8688(1–9).
30. Mengesha AY. Hypertension and related risk factors in type 2 diabetes mellitus (DM) patients in Gaborone City Council (GCC) clinics, Gaborone. Afr Health Sci. 2007;7(4):244–245.
31. Giday A, Wolde M, Yihdego D. Hypertension, obesity and central obesity in diabetics and non diabetics in Southern Ethiopia. Ethiop J Heal Dev. 2010;24(2):2–4.
32. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes d 2019. Diabetes Care. 2019;42(1):13–28. doi: 10.2337/dc19-S002.
33. Noh J, Chang Y, Park M, Kwon YD, Ryu S. Self-rated health and the risk of incident type 2 diabetes mellitus: A cohort study. Sci Rep Open. 2019;9(3697):1–8. doi:10.1038/s41598-019-40090-y
34. Nuttall FQ. Body mass index. Nutr Res. 2015;50(3):117–128. doi:10.1097/NT.0000000000000092
35. Solomon T, Tamiru S, Alemseged F. Risk factors for cardiovascular diseases among diabetic patients in southwest Ethiopia. Ethiop J Health Sci. 2010;20(2):121–128. doi:10.4314/ejhs.v20i2.69438
36. Waly EH, Hamed MS. Hypertension and dyslipidemia among type II diabetic patients and related risk factors and complications methods. Egypt J Community Med. 2018;36(1):31–43.
37. Tatsumi Y, Ohkubo T. Hypertension with diabetes mellitus: signi fi cance from an epidemiological perspective for Japanese. Hypertens Res. 2017;(March):1–12. doi:10.1038/hr.2017.67
38. Mubarak FM, Froelicher ES, Jaddou HY, Ajlouni M. original article. Ann Saudi Med. 2008;28(5):346–351. doi:10.5144/0256-4947.2008.346
39. Demarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Endocrinology. 2014;10(6):1–13. doi:10.1038/nrendo.2014.44
40. Humayun A, Shah AS, Sultana R. Relation of hypertension with body mass index and age in male and female population of Peshawar, Pakistan. J Ayub Med Coll Abbottabad. 2009;21(3):63–65.
41. Asresahegn H, Tadesse F, Beyene E. Prevalence and associated factors of hypertension among adults in Ethiopia: a community based cross ‑ sectional study. BMC Res Notes. 2017;2017(10:629):1–8. doi:10.1186/s13104-017-2966-1
42. Hall JE, Do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2016;116(6):991–1006. doi:10.1161/CIRCRESAHA.116.305697.OBESITY-INDUCED
43. Liew SJ, Lee JT, Tan CS, Huat C, Koh G, Müller-Riemenschneider F. Sociodemographic factors in relation to hypertension prevalence, awareness, treatment and control in a multi-ethnic Asian population: a cross- sectional study. BMJ Open. 2019;9(5):1–10. doi:10.1136/bmjopen-2018-025869
44. Khanam R, Ahmed S, Rahman S, et al. Prevalence and factors associated with hypertension among adults in rural Sylhet district of Bangladesh: a cross- sectional study. BMJ Open. 2019;9(10):1–9. doi:10.1136/bmjopen-2018-026722
45. Head T, Daunert S, Goldschmidt-Clermont PJ. The aging risk and atherosclerosis: a fresh look at arterial homeostasis. Front Genet. 2017;8(12):1–11. doi:10.3389/fgene.2017.00216
46. National Institute of Aging. Aging Hearts and Arteries a Scientific Quest. 2005.
47. Kohn JC, Lampi MC, Reinhart-king CA. Age-related vascular stiffening: causes and consequences. Front Genet. 2015;6(3):1–18. doi:10.3389/fgene.2015.00112
48. Tesfaye F, Nawi NG, Van MH, et al. Association between body mass index and blood pressure across three populations in Africa and Asia. J Hum Hypertens. 2007;21(1):28–37. doi:10.1038/sj.jhh.1002104
49. Agyemang C. Rural and urban differences in blood pressure and hypertension in Ghana, West Africa. J R Inst Public Heal. 2006;120(6):525–533. doi:10.1016/j.puhe.2006.02.002
50. Taylor BC, Wilt TJ, Welch HG. Impact of diastolic and systolic blood pressure on mortality: implications for the definition of normal. J Gen Intern Med. 2011;26(7):685–690. doi:10.1007/s11606-011-1660-6
51. Van de Poel E, O’Donnell O, Van Doorslaer E. Urbanization and the spread of diseases of affluence in China. Econ Hum Biol. 2009;7(2):200–216. doi:10.1016/j.ehb.2009.05.004
52. Gong P, Liang S, Carlton EJ, et al. Urbanisation and health in China. Lancet. 2012;379(9818):843–852. doi:10.1016/S0140-6736(11)61878-3
53. Patel TP, Rawal K, Bagchi AK, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016;21(1):11–23. doi:10.1007/s10741-015-9515-6
54. Gates PE, Strain WD, Shore AC. Human endothelial function and microvascular ageing. Exp Physiol. 2009;94(3):311–316. doi:10.1113/expphysiol.2008.043349
55. Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. 2019;33(2):363–382. doi:10.1111/jvim.15454
56. Heianza Y, Arase Y, Kodama S, et al. Fasting glucose and HbA1c levels as risk factors for the development of hypertension in Japanese individuals: toranomon hospital health management center study 16. J Hum Hypertens. 2014;29(4):254–259. DOI:10.1038/jhh.2014.77
57. Ray KK, Rao S, Seshasai K, et al. Eff ect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373(9677):1765–1772. doi:10.1016/S0140-6736(09)60697-8
58. Stettler C, Allemann S, Ju P. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J. 2006;151(1):27–38. doi:10.1016/j.ahj.2005.09.015
59. Katakami N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J Atheroscler Thromb. 2018;25(1):27–39. doi:10.5551/jat.RV17014
60. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD clinical practice consensus guidelines ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(10):155–177. doi:10.1111/pedi.12701
61. Wu J, Li T, Song X, Sun W, Zhang Y, Liu Y. Prevalence and distribution of hypertension and related risk factors in Jilin Province, China 2015: a cross- sectional study. BMJ Open. 2018;8(3):1–10. doi:10.1136/bmjopen-2017-020126
62. Khader Y, Batieha A, Jaddou H, et al. Hypertension in Jordan: prevalence, awareness, control, and its associated factors. Int J Hypertens. 2019;2019:1–9. doi:10.1155/2019/3210617
63. Andreas FA, Flouris D, Constantine I, Vardavas GS, Metsios AM. Tsatsakis and yiannis koutedakis. Am J Physiol Lung Cell Mol Physiol. 2010;298(1):L3–L2. doi:10.1152/ajplung.00215.2009
64. Lee J, Cooke JP. The role of nicotine in the pathogenesis of atherosclerosis. Atherosclerosis. 2013;215(2):281–283. doi:10.1016/j.atherosclerosis.2011.01.003.The
65. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi:10.1097/WOX.0b013e3182439613
66. Dhungana RR, Pandey AR, Bista B, Joshi S, Devkota S. Prevalence and associated factors of hypertension: a community-based cross-sectional study in municipalities of Kathmandu, Nepal. Int J Hypertens. 2016;2016:1–11. doi:10.1155/2016/1656938
67. Papathanasiou G, Zerva E, Zacharis I, et al. Association of high blood pressure with body mass index, smoking and physical activity in healthy young adults. Open Cardiovasc Med J. 2015;9(1):5–17. doi:10.2174/1874192401509010005
68. Olack B, Wabwire-mangen F, Smeeth L, Montgomery JM, Kiwanuka N, Breiman RF. Risk factors of hypertension among adults aged 35 – 64 years living in an urban slum Nairobi, Kenya. BMC Public Health. 2015;2015(1251):1–9. doi:10.1186/s12889-015-2610-8
69. Wang J, Sun W, Wells GA, et al. Differences in prevalence of hypertension and associated risk factors in urban and rural residents of the northeastern region of the People ’ s Republic of China: a cross-sectional study. Plos One. 2018;13(4):1–14.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
