Back to Journals » Biologics: Targets and Therapy » Volume 17
Hypereosinophilic Dermatitis: Successful Treatment with Dupilumab
Authors Wu C , Zhang J, Zhao Y
Received 11 January 2023
Accepted for publication 4 April 2023
Published 20 April 2023 Volume 2023:17 Pages 57—60
DOI https://doi.org/10.2147/BTT.S400073
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shein-Chung Chow
Chenyu Wu, Jianzhong Zhang, Yan Zhao
Department of Dermatology, People’s Hospital of Peking University, Beijing, People’s Republic of China
Correspondence: Yan Zhao, Email [email protected]
Abstract: Hypereosinophilic dermatitis (HED) is a subtype of hypereosinophilic syndrome. HED is characterized by eosinophilic granulocytes increased in peripheral blood and bone marrow and infiltrated in skin. The clinical manifestations of HED are diffussed by erythema, papule and maculopapule with severe itching. The etiology of HED is unknown. At present, in addition to HED with FIP1L1-PDGFRA fusion gene positive, whose treatment is tyrosine kinase inhibitor, other types of HED first-line treatment are oral glucocorticoids, supplemented by antihistamines and immunosuppressants. Dupilumab is a human monoclonal antibody, which inhibits the IL-4 and IL-13 signaling by binding to the IL-4R-α and IL-13R-α-1 subunits of the receptor. We report a 76-year-old male patient with HED whose peripheral blood eosinophils decreased from 20.7% to 4.1% after 8 weeks of dupilumab, and his pruritus was completely relieved. Dupilumab was discontinued after 6 months of treatment. It is exciting that the patient has not experienced relapse for 17 months after the discontinuation. No adverse event was reported.
Keywords: hypereosinophilic dermatitis, hypereosinophilic syndrome, dupilumab
Introduction
Hypereosinophilic dermatitis (HED) is a special subtype of Hypereosinophilic syndrome (HES) which affect skin instead of other organs. The clinical manifestations of HED is generalized, pleomorphic, pruritic rash. Current therapies include corticosteroids, hydroxyurea, interferon alfa, and imatinib mesate, also immunosuppressants. Dupilumab is a human monoclonal antibody, which inhibits the IL-4 and IL-13 signaling by binding to the IL-4R-α and IL-13R-α-1 subunits of the receptor.1 Dupilumab is currently widely used in atopic dermatitis effectively. Here, we present a patient with HED who was treated successfully with IL-4R blockade, and sustained response in the 17 months follow-up after dupilumab discontinuation.
Case Report
A 76-year-old man presented with chronic erythema and papule with pruritus for 6 years. The treatment before included antihistamines, doxepin, tripterygium glycosides, and topical corticosteroids. Only methylprednisolone (60 mg) is effective, but the patient’s condition worsened during reduction. Physical examination showed diffused erythema, eczematous lesions of the trunk and severe pruritus in the extremities (Figure 1A). There was no evidence of parasitic infection. The proportion of eosinophils in the peripheral blood was 20.7%, the absolute number was 1.74×109/L, and the serum IgE level was 28400IU/mL. The skin biopsy showed infiltration of neutrophils, lymphocytes, and eosinophils in the dermis (Figure 2A and B). B-ultrasound of the abdomen and pelvis and electrocardiogram showed no obvious abnormality. Computed tomography of chest showed emphysema, and bilateral axillary and mediastinal lymphadenopathy. Based on the clinical and histopathological findings, HED was diagnosed. Dupilumab was initiated with the recommended dose. There was a significant improvement of the skin lesions after 2 weeks from the first injection of dupilumab. The lesions had completely resolved after 8 weeks (Figure 1B). Pruritus was assessed with visual rating scale (VRS, 0–10). After a week of dupilumab treatment, pruritus score was reduced to 4 from 10 and to 0 after 8 weeks. The proportion and absolute value of eosinophils in the peripheral blood was reduced to 4.1% and 0.3×109 /L after a week. The patient discontinued dupilumab after 6 months of treatment. The patient reports that there was no recurrence of rash or pruritus up to 17 months after discontinuing dupilumab. No adverse event was reported.
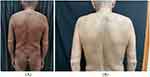 |
Figure 1 Erythema and eczematous lesions diffused in extremities before dupilumab (A); lesions resolved after dupilumab in 8 weeks (B). |
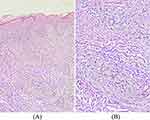 |
Figure 2 Infiltration of neutrophils, lymphocytes and eosinophils in dermis. HE stain, ×100 (A); HE stain, ×200 (B). |
Discussion
The treatment of HED is challenging. Glucocorticoid is effective in our patient, but adverse reactions and rebound are frustrating, especially in elderly people. Dupilumab has good effect and safety in the treatment of moderate to severe AD. In clinical trials, a decreased tendencies in eosinophils count was observed. Benzecry et al2 described a case of HES treated with Dupilumab successfully. So our patient was given Dupilumab. Fortunately, our patient tolerated Dupilumab well and was responsed excellent to dupilumab.
Dupilumab was approved by the FDA in April 2017 for the treatment of adult patients with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.3 It is also reported to treat prurigo nodosa,4,5 bullous pemphigoid,6 eosinophilic esophagitis,7,8 asthma,9 etc.
The possible mechanisms of dupilumab in HED presumably is reduction of Th2 cytokine production. Both IL-4 and IL-13 can activate and promote survival of Th2 cells, including IL-5, which is a critical factor of differentiation, activation and survival of eosinophils.10 IL-4 promotes endothelial cells to express vascular cell adhesion molecules, which is important for the chemotaxis of eosinophils to skin lesions.11 Thus, it is speculated that dupilumab can prevent the migration of eosinophils to the skin by inhibiting IL-4 signal transduction. More evidence is needed.
Dupilumab, a biologic for atopic dermatitis, has shown promising results in HED. It is exciting that the patient has not experienced relapse since discontinuing dupilumab. There is no evidence of clonal T cells or eosinophilic leukemia in our patient. However, longer-term follow-up is still necessary.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Patient Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Institutional approval is not required for this case study.
Funding
This work was not supported.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Regeneron. Dupixent® (dupilumab) prescribing information. Important legal document for physicians prescribing dupilumab; 2017.
2. Wieser JK, Kuehn GJ, Prezzano JC, et al. Improvement in a patient with hypereosinophilic syndrome after initiation of dupilumab treatment. JAAD Case Rep. 2020;6(4):292–2951. doi:10.1016/j.jdcr.2020.02.030
3. Regeneron Pharmaceuticals Inc. Regeneron and sanofi announce FDA approval of DUPIXENT® (dupilumab), the first targeted biologic therapy for adults with moderate-to-severe atopic dermatitis. PR Newswire.2017.
4. Beck KM, Yang EJ, Sekhon S, Bhutani T, and Liao W. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155(1):118–120. doi:10.1001/jamadermatol.2018.3912
5. Holm JG, Agner T, Sand C, Thomsen SF. Dupilumab for prurigo nodularis: Case series and review of the literature. Dermatol Ther. 2020;33(2):e13222. doi:10.1111/dth.13222.
6. Seidman JS, Eichenfied DZ, Orme CM, et al. Dupilumab for bullous pemphigoid with intracrable pruritus. Dermatol Online. 2019;25(11):13222.
7. Dellon ES, Rothenberg ME, Collins MH, et al. Dupilumab in adults and adolescents with eosinophilic esophagitis. N Engl J Med. 2022;387(25):2317–2330. doi:10.1056/NEJMoa2205982
8. Hirano I, Dellon ES, Hamilton JD, et al. Efficacy of dupilumab in a Phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology. 2020;158(1):111–122. doi:10.1053/j.gastro.2019.09.042
9. Santini G, Mores N, Malerba M, et al. Dupilumab for the treatment of asthma. Expert Opin Investig Drugs. 2017;26(3):357–366. doi:10.1080/13543784.2017.1282458
10. Seegräber M, Srour J, Walter A, Knop M, and Wollenberg A. Dupilumab for treatment of atopic dermatitis. Expert Rev Clin Pharmacol. 2018;11(5):467–474. doi:10.1080/17512433.2018.1449642
11. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immun. 2010;125(2):S73–S80. doi:10.1016/j.jaci.2009.11.017
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
