Back to Journals » Open Access Emergency Medicine » Volume 12
Hypercapnia at Hospital Admission as a Predictor of Mortality
Authors Vonderbank S, Gibis N, Schulz A, Boyko M, Erbuth A, Gürleyen H, Bastian A
Received 12 December 2019
Accepted for publication 2 June 2020
Published 26 June 2020 Volume 2020:12 Pages 173—180
DOI https://doi.org/10.2147/OAEM.S242075
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Hans-Christoph Pape
Sandy Vonderbank, Natalie Gibis, Alina Schulz, Mariya Boyko, Annika Erbuth, Hakan Gürleyen, Andreas Bastian
Marienkrankenhaus Kassel, Kassel, Germany
Correspondence: Andreas Bastian
Marienkrankenhaus Kassel, Kassel, Germany
Tel +49 561 8073 1212
Fax +49 561 8073 4200
Email [email protected]
Introduction: Hypercapnia is an indicator of ventilatory exhaustion. There is some disagreement regarding whether hypercapnia is also a predictor of mortality. In this prospective study, we aimed to investigate whether hypercapnia can predict in-hospital and 1-year mortality rates in patients with dyspnea or pulmonary diseases.
Patients and Methods: All patients with dyspnea or pulmonary diseases underwent routine blood gas analysis at hospital admission. During the 12-month enrollment period, 2710 patients were enrolled, and 588 patients with hypercapnia at admission were identified. Of the 1626 normocapnic patients, 62 were randomly selected as controls. In-hospital and 1-year mortality rates were determined.
Results: There were significant increases in mortality rate between acute hypercapnic patients and both chronic hypercapnic patients and normocapnic controls. Their in-hospital mortality rates were 17%, 6.7% and 3.2%, respectively. Their 1-year mortality rates were 32%, 20.2% and 14.5%, respectively. The 1-year mortality rates of hypercapnic patients with different underlying diseases were 24.6% (chronic obstructive pulmonary disease), 28.4% (congestive heart disease), 1.6% (obstructive sleep apnea syndrome/obesity hypoventilation syndrome), 50.9% (pneumonia), 0% (suppressed central respiratory drive, primarily due to opiate abuse) and 22.8% (other conditions).
Discussion: The 1-year mortality rate of patients with acute hypercapnia at hospital admission was 32%, with significant differences compared to chronic hypercapnic patients (20.2%) and normocapnic patients (14.5%). There was a wide range of 1-year mortality rates between the hypercapnic patients with different underlying diseases.
Keywords: hypercapnia, mortality, COPD
Plain Language Summary
Hypercapnia is almost always an indicator of exhaustion of ventilatory force. This is why we explored whether hypercapnia might also be able to predict mortality, which we confirmed. In particular, acute hypercapnia (defined as pH <7.35) is a relevant predictor of mortality. Almost every third patient (32%) died 1 year after acute hypercapnia was detected at hospital admission. Patients with chronic hypercapnia had a slightly lower 1-year mortality rate (20%). Additionally, there were differences in the 1-year mortality rate depending on the underlying cause of the hypercapnia. Patients with obesity hypoventilation syndrome or hypercapnic obstructive sleep apnea syndrome almost never died when treated properly with noninvasive ventilation. In contrast, patients with pneumonia had a very high mortality rate (50%) and the mortality rates for chronic obstructive pulmonary disease and congestive heart disease were also considerable, at 24% and 28%, respectively.
Introduction
Hypercapnia, defined as a blood partial pressure of carbon dioxide (pCO2) ≥45 mmHg assessed in an arterial or capillary blood gas analysis, is almost always an indicator of ventilatory exhaustion. Only a minority of hypercapnic patients have a different underlying disease: their central respiratory drive is suppressed mainly due to the side effects of opiates or, extremely rarely, due to neurologic diseases such as undine syndrome, brain metastases or neurosarcoidosis.
Ventilatory exhaustion results from a mismatch of ventilatory muscle load and capacity. In patients with neuromuscular diseases, for example amyotrophic lateral sclerosis, the ventilatory capacity is low and decreases over time, which results in an increased load/capacity mismatch even if the muscle load is normal. Chronic obstructive pulmonary disease (COPD) patients also have a lower ventilatory capacity, but to a much lesser degree than patients with neuromuscular diseases. The more important reason for their ventilatory muscle load/capacity mismatch is their increased ventilatory muscle load.
An increased ventilatory muscle load/capacity mismatch results in hypercapnia in most patients. Hypercapnia helps to reduce the ventilatory muscle load primarily by reducing the respiration rate. It is not clear what exactly causes the occurrence of hypercapnia. Abnormally high pCO2 levels have been identified as an indicator of severe respiratory fatigue and impending cardiopulmonary arrest.1 However, limited data are available on the value of abnormally high pCO2 as a predictor of mortality in hospitalized patients. We aimed to evaluate the potentially higher mortality of hypercapnic patients.
Patients and Methods
Study Design
This is an observational prospective study that involved enrollment from January to December 2015, with a follow-up period up to December 2016.
Ethics and Consent
The institutional ethics committee of Marienkrankenhaus Kassel, Germany, approved the study (reference no. MKH 07/2014). All patients gave written informed consent for the scientific use of the data acquired during hospitalization.
Definitions of Hypercapnia, Acute Hypercapnia and Chronic Hypercapnia
Hypercapnia was defined as a pCO2 ≥45 mmHg. Acute hypercapnia was defined as a pCO2 ≥45 mmHg with a pH <7.35. Chronic hypercapnia was defined as a pCO2 ≥45 mmHg with a pH ≥7.35.
Blood Gas Analysis
We preferred capillary blood gas analysis but also included patients with arterial blood gas analysis and some patients with only venous blood gas analysis. (Arterial blood gas analysis is the gold standard in the measurement of blood gases. However, the procedure to obtain arterial blood gas data is painful. Arterialized capillary gases sampled at the ear lobe give similar results for pH and pCO2.2 The interpretation of venous blood gas data is more difficult. The pH is slightly lower (0.02–0.04 pH units) and the pCO2 is slightly higher (3–8 mmHg). However, differences can be greater in patients with hypotension and they depend on local metabolism. We only used venous blood gases if the pCO2 was <45 mmHg and pH was >7.35, which allowed hypercapnia and acidosis to be excluded.3,4 If the pH was also >7.35 and oxygen saturation (measured by pulse oximetry) was normal, additional blood gases were unnecessary but, if not, arterial blood gas data were obtained.)
Study Protocol
The Marienkrankenhaus is a small general hospital with a focus on lung diseases. All patients with dyspnea or pulmonary diseases were included in the study. All these patients underwent routine blood gas analysis at hospital admission. The data in the patient records of the hypercapnic patients were used to identify the underlying diagnosis leading to hypercapnia and to identify the patients who died during their hospital stay. One year after hospital admission the patients or, if they were not available, their general practitioners were contacted to identify the patients who had died during the 1-year follow up.
Statistical Analysis
Data were analyzed using IBM®SPSS® version 2. To test for differences in mortality rates between acute hypercapnic patients and chronic hypercapnic patients or normocapnic controls, between acute and chronic hypercapnic COPD patients, and between hypercapnic patients with different underlying diseases and normocapnic controls, binary logistic regressions were applied. These multivariate models allow to test the mentioned differences adjusted for age and gender. So, all p-values reported in the results section are adjusted for age and gender. P values ≤0.05 were considered to be statistically significant.
Results
During the study period, 6750 patients were admitted to the medical department of the hospital. 2710 patients had dyspnea or were admitted for pulmonary diseases, and they were included in this study; 1626 were normocapnic, 496 were hypocapnic and 588 were hypercapnic. Additionally, 1-year mortality data were available for 583 of the hypercapnic patients (these data were unavailable for five hypercapnic patients). Hypercapnic patient details, including age, gender, diagnoses leading to hypercapnia, outcomes, and blood gas values, are given in the supplementary material file.
Of the 583 hypercapnic patients, 300 were males (51.5%). The age range was 18–96 with a median of 67.5 years. Regarding the underlying diseases, 50.8% (296 patients) had COPD, 11.5% (67) had congestive heart disease (CHD), 10.8% (63) had obstructive sleep apnea syndrome (OSAS)/obesity hypoventilation syndrome (OHS), 9.8% (57) had pneumonia (community-acquired pneumonia or pneumonic sepsis), 3.6% (21) had suppressed central respiratory drive (primarily due to opiate abuse) and 13.5% (79) had other underlying diseases such as pulmonary fibrosis, kyphoscoliosis, postpolio syndrome or neuromuscular diseases (Figure 1).
Mortality Rates of Hypercapnic Patients
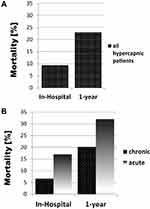
Of the 583 hypercapnia patients, 55 died in hospital (9.4%) and 136 died after 1 year (23.3%). The mortality rates were higher when the patients were acutely hypercapnic at hospital admission. Of the 153 acutely hypercapnic patients, 26 died in hospital (17%) and 49 died after 1 year (32%). There were significant differences in the mortality rates between the chronic and acute hypercapnic patients regarding both the in-hospital mortality rate (6.7% vs 17%; p=0.001) and the 1-year mortality rate (20.2% vs 32%; p=0.019) (Figure 2A and B).
Mortality Rates of Acute vs Chronic Hypercapnic COPD Patients
COPD patients with acute hypercapnia at hospital admission had a significantly higher in-hospital mortality rate (p=0.042) and a non-significantly higher 1-year mortality rate (p=0.288) than chronic hypercapnic COPD patients (Figure 3).
1-Year Mortality Rates of Hypercapnic Patients, by Underlying Disease, vs Normocapnic Controls
In the acute hypercapnic group, pneumonia patients had the highest 1-year mortality rate, with CHD and COPD patients following. The difference between acute hypercapnic pneumonia and COPD patients was significant (p=0.036).
In the chronic hypercapnic group, pneumonia patients had the highest 1-year mortality rate, with COPD and CHD patients following. These differences were not significant.
The 1-year mortality rate of the normocapnic controls was 14.5%. Regarding the differences in the 1-year mortality rates of the hypercapnic patients with different underlying diseases compared to the normocapnic controls, there were significant differences (p=0.002), Acute hypercapnic pneumonia patients had a significantly higher 1-year mortality rate. There were no significant differences in the 1-year mortality between normocapnic controls and any disease group for chronic hypercapnic patients (Figure 4).
Mortality Rates of All Hypercapnic Patients, by Underlying Disease, vs Normocapnic Controls
In-hospital and 1-year mortality rates of the patients with any type of hypercapnia at hospital admission were different between the chronic hypercapnic patients with different underlying diseases. Regarding the 1-year mortality rates compared to the normocapnic controls, there was a significant difference only for the pneumonia patients, with the pneumonia patients having a significantly higher 1-year mortality rate (p=0.004) and OHS having a significantly lower 1-year mortality rate (p=0.039). In-hospital mortality rates were significantly higher in hypercapnic pneumonia patients (p=0.004) and CHD patients (p=0.013) compared to the normocapnic controls (Figure 5).
Discussion
Overview of Hypercapnia and Mortality Rates
The acute hypercapnic patients (defined by a pCO2 ≥45 mmHg and a pH <7.35) at hospital admission had very poor outcomes. Their 1-year mortality rate was 32%, which was significantly higher than the 1-year mortality rates of chronic hypercapnic patients (20.2%) and normocapnic patients (14.5%). This is even more relevant considering that almost all acute hypercapnic patients were initially admitted to our intensive care unit and treated with noninvasive ventilation (NIV).
The in-hospital mortality of the patients with acute hypercapnia at hospital admission was 17%, which was significantly higher than for chronic hypercapnic patients (6.7%) and normocapnic patients (3.2%). A French study5 investigated the mortality rate of elderly patients (> 65 years of age) with acute respiratory failure, including a subgroup of hypercapnic patients (n=119) that was somewhat comparable to our study patients. Their hypercapnic patients’ in-hospital mortality was 24.4%, while our hypercapnic patients’ in-hospital mortality rate was 9.5%. This difference may be due to differences in the patient populations. While the French group included only patients with acute respiratory failure, we also included hypercapnic patients admitted without acute respiratory problems (ie, patients with conditions that did not have a large influence on ventilation, such as urinary tract infections, gastric ulcers or thyroid problems).
Mortality Rates of Hypercapnic Patients with Different Underlying Diseases
There was a wide range of 1-year mortality rates between the hypercapnic patients with different underlying diseases. Hypercapnic patients with OHS/OSAS with daytime hypercapnia or patients with suppressed central respiratory drive (primarily due to opiate abuse) had almost no risk of death if properly treated. In contrast, the 1-year mortality rate of the hypercapnic pneumonia patients was very high (50.9%); these patients’ outcomes were negatively influenced by the inclusion of patients with dementia and metastasized cancer (who were treated with best supportive care only). The 1-year mortality rates of the hypercapnic patients with CHD or COPD were similar (28.4% and 24.6%, respectively), which were non-significantly higher rates than in the normocapnic controls (14.5%). Interestingly, the in-hospital mortality was significantly higher for the hypercapnic CHD patients than the hypercapnic COPD patients, at 19.4% and 7.5%, respectively.
Pneumonia
Several studies have investigated the relationship between hypercapnia and mortality in community-acquired pneumonia patients, but they have reported contradictory results. A study of invasively ventilated patients with community-acquired pneumonia demonstrated a 4.73-fold decreased relative risk of mortality for the hypercapnic patients compared to those with a pCO2 <45 mmHg.6 This much improved outcome is probably a result of the control group selection, as normocapnic and hypocapnic patients combined served as the control group, and hypocapnic patients have a higher mortality rate. A study including only patients with pneumococcal bacteremia reported a higher mortality in patients with a pCO2 >45 mmHg compared to normocapnic patients.7 Laserna and colleagues8 investigated pneumonia patients (n=453) and found that hypercapnia (n=70; 15%) and hypocapnia were both associated with a higher 30-day mortality (odds ratio for hypocapnia patients: 2.84, with 20% mortality rate) compared to normocapnic patients. This was also applicable when COPD patients were excluded, as hypercapnia was associated with an increased mortality of nearly threefold overall but a nearly ninefold if COPD patients were excluded. These results are consistent with data published by Sin and colleagues,9 which showed that hospitalized community-acquired pneumonia patients with hypercapnia had a threefold increased in-hospital mortality rate compared to patients with a pCO2 of 40–44 mmHg.
Our study included 57 hypercapnic patients with pneumonia (CAP or pneumonic sepsis). These patients had high in-hospital and 1-year mortality rates (28% and 50.9%, respectively). Of the 16 patients who died in hospital, seven were not admitted to the intensive care unit, and the medical care for these patients was not intended to cure them but to provide the best supportive care.
COPD
Various studies have reported contradictory results regarding hypercapnia in COPD patients: hypercapnia has led to increased mortality,10 decreased mortality11 and no association.12,13 Additionally, Ahmadi and colleagues14 found that pCO2 predicted in-hospital mortality in a U-shaped manner. Hypocapnic patients with a pCO2 <33.75 mmHg had a 17% increased mortality compared to normocapnic patients, while severely hypercapnic patients with a pCO2 >60 mmHg had a 15% increased mortality compared to normocapnic patients. In contrast, Solar-Cataluna and colleagues15 investigated various factors that might influence COPD outcome, the most important one being severe acute COPD exacerbations, and they found that hypercapnia did not influence the 1-year mortality rate.
Budweiser and colleagues16 investigated 98 chronic hypercapnic patients with COPD treated with NIV at home, and their mortality rate during the 28.9 ± 8.8 months of follow up was 31.6%. Our study included 296 hypercapnic patients with COPD, and their 1-year mortality rate was 24.6%. The 218 chronic hypercapnic COPD patients had a 1-year mortality rate of 23.1%. The 78 acute hypercapnic COPD patients had a 1-year mortality rate of 28.8% and an in-hospital mortality rate of 12.5%. In contrast, an important older case series published by Connors and colleagues17 reported a lower in-hospital mortality rate of 11% in COPD patients with acute hypercapnic exacerbations. Their 1-year mortality rate was 49%, in contrast to 28.8% for our acute hypercapnic patients.
Hypercapnic COPD patients have a better outcome when they are obese. In many of them, the main reason for hypercapnia is probably OHS. Patients with OHS/OSAS with daytime hypercapnia who were treated properly with NIV had a very low mortality rate in our study and in various others. For example, a French multicenter study demonstrated a much lower mortality rate over an almost 4-year period (47.7 months) for obese (32.4%) compared to non-obese (57.7%) NIV-treated COPD patients.18
OHS/OSAS with Daytime Hypercapnia
Regarding the mortality rates of OHS patients, it is necessary to distinguish between the era before and after home mechanical ventilation became a relevant treatment option for these patients. Patients with untreated OHS have a significantly higher risk of mortality, as shown in a retrospective study that reported that 7 out of 15 OHS patients (46%) who refused long-term NIV died during the mean 50-month follow-up period.19 Additionally, a prospective study by Nowbar and colleagues20 explored the 18-month mortality rate of patients with severe obesity and OHS (with daytime hypercapnia) (n=47) after hospital discharge, only six (13%) of whom were treated for OHS by NIV after hospital discharge. They found that the overall group of 47 had a significantly higher mortality rate (23%) than the control patients (n=103) with severe obesity alone (9%), who were well-matched for BMI, age, and a number of comorbidities. A disadvantage of this study was that hypercapnia was defined as pCO2 ≥43 mmHg.
The situation is quite different if the patients are treated with NIV at home. Budweiser and colleagues21 conducted a retrospective analysis of 126 OHS patients. Their 1, 2 and 5-year mortality rates were 3%, 8%, and 30%, respectively. pCO2 was not a significant outcome predictor, but reduction of pCO2 during NIV therapy was. The 1- and 2-year mortality rates of NIV-treated OHS patients in a study by Blankenburg and colleagues22 were higher (14.9% and 27.9%, respectively). We included 63 hypercapnic patients with OHS/OSAS with daytime hypercapnia (all our OHS patients were treated with NIV at home). None of these died in-hospital and their 1-year mortality rate was 1.5% (1 patient died). These mortality rates are comparable to the results of Budweiser and colleagues.
Availability of data and material
All data relevant to the study are in the supplementary material file.
Abbreviations
CHD, congestive heart disease; COPD, chronic obstructive pulmonary disease; OHS, obesity hypoventilation syndrome; OSAS, obstructive sleep apnea syndrome; pCO2, partial pressure of carbon dioxide; NIV, noninvasive ventilation.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki. The institutional ethic committee of the Marienkrankenhaus approved the study (reference no. MKH 07/2014). All patients gave consent for the scientific use of the data acquired during hospitalization.
Acknowledgments
All authors were working at the Marienkrankenhaus Kassel at the time of the study.
Disclosure
None of the authors have any competing interests or financial interests in this study.
References
1. Caruana Montaldo B, Gleeson K, Zwillich CW. The control of breathing in clinical practice. Chest. 2000;117(1):205–225. doi:10.1378/chest.117.1.205
2. Zavorsky GS, Cao J, Mayo NE, Gabbay R, Murias JM. Arterial versus capillary blood gases: a meta-analysis. Respir Physiol Neurobiol. 2007;155(3):268–279. doi:10.1016/j.resp.2006.07.002
3. Kelly AM, Kyle E, Mc Alpine A. Venous pCO2 and pH can be used to screen for significant hypercarbia in emergency patients with acute respiratory disease. J Emerg Med. 2002;22(1):15–19. doi:10.1016/S0736-4679(01)00431-0
4. McKeever TM, Hearson G, Housley G, et al. Using venous blood gas analysis in the assessment of COPD exacerbations: a prospective cohort study. Thorax. 2016;71(3):210–215. doi:10.1136/thoraxjnl-2015-207573
5. Ray P, Birolleau S, Lefort Y, et al. Acute respiratory failure in the elderly: etiology, emergency diagnosis and prognosis. Crit Care. 2006;10(3):R82. doi:10.1186/cc4926
6. Lee JH, Ryu YJ, Chun EM, Chang JH. Outcomes and prognostic factors for severe community-acquired pneumonia that requires mechanical ventilation. Korean J Intern Med. 2007;22(3):157–163. doi:10.3904/kjim.2007.22.3.157
7. Afessa B, Greaves WL, Frederick WR. Pneumococcal bacteremia in adults: a 14-year experience in an inner-city university hospital. Clin Infect Dis. 1995;21(2):345–351. doi:10.1093/clinids/21.2.345
8. Laserna E, Sibila O, Aguilar PR, et al. Hypocapnia and hypercapnia are predictors for ICU admission and mortality in hospitalized patients with community-acquired pneumonia. Chest. 2012;142(5):1193–1199. doi:10.1378/chest.12-0576
9. Sin DD, Man SF, Marrie TJ. Arterial carbon dioxide tension on admission as a marker of in-hospital mortality in community-acquired pneumonia. Am J Med. 2005;118(2):145–150. doi:10.1016/j.amjmed.2004.10.014
10. Chailleux E, Fauroux B, Binet F, Dautzenberg B, Polu JM. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. A 10-year analysis of ANTADIR observatory. Chest. 1996;109(3):741–749. doi:10.1378/chest.109.3.741
11. Dubois P, Jamart J, Machiels J, Smeets F, Lulling J. Prognosis of severely hypoxemic patients receiving long-term oxygen therapy. Chest. 1994;105(2):469–474. doi:10.1378/chest.105.2.469
12. Crockett AJ, Cranston JM, Moss JR, Alpers JH. Survival on long-term oxygen therapy in chronic airflow limitation: from evidence to outcomes in the routine clinical setting. Intern Med J. 2001;31(8):448–454. doi:10.1046/j.1445-5994.2001.00103.x
13. Aida A, Miyamoto K, Nishimura M, Aiba M, Kira S, Kawakami Y. Prognostic value of hypercapnia in patients with chronic respiratory failure during long-term oxygen therapy. Am J Respir Crit Care Med. 1998;158(1):188–193. doi:10.1164/ajrccm.158.1.9703092
14. Ahmadi Z, Bornefalk-Hermansson A, Franklin KA, Midgren B, Ekström MP. Hypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: a population-based prospective study. Respir Res. 2014;15(1):30. doi:10.1186/1465-9921-15-30
15. Solar-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
16. Budweiser S, Hitzl AP, Jörres RA, Schmidbauer K, Heinemann F, Pfeifer M. Health-related quality of life and long-term prognosis in chronic hypercapnic respiratory failure: a prospective survival analysis. Respir Res. 2007;8(1):92. doi:10.1186/1465-9921-8-92
17. Connors AF, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med. 1996;154(4):959–967. doi:10.1164/ajrccm.154.4.8887592
18. Borel JC, Pepin JL, Pison C, et al. Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients. Respirology. 2014;19(6):857–865. doi:10.1111/resp.12327
19. Perez de Llano LD, Golpe R, Piquer M, et al. Short-term effects and long-term effects of NIPPV in patients with obesity hypoventilation syndrome. Chest. 2005;128(2):587–594. doi:10.1378/chest.128.2.587
20. Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116(1):1–7. doi:10.1016/j.amjmed.2003.08.022
21. Budweiser S, Riedl SG, Jörres RA, Heinemann F, Pfeifer M. Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med. 2007;261(4):375–383. doi:10.1111/j.1365-2796.2007.01765.x
22. Blankenburg T, Benthin C, Pohl S, et al. Survival of hypercapnic patients with COPD and obesity hypoventilation syndrome treated with high intensity non-invasive ventilation in the daily routine care. Open Respir Med J. 2017;11(1):31–40. doi:10.2174/1874306401711010031
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.