Back to Journals » Journal of Inflammation Research » Volume 14
Hydrogen (H2) Alleviates Osteoarthritis by Inhibiting Apoptosis and Inflammation via the JNK Signaling Pathway
Authors Lu H, Wang W, Kang X, Lin Z, Pan J, Cheng S, Zhang J
Received 22 December 2020
Accepted for publication 18 March 2021
Published 13 April 2021 Volume 2021:14 Pages 1387—1402
DOI https://doi.org/10.2147/JIR.S297622
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Hongwei Lu,1– 3,* Wei Wang,1– 3,* Xiaodiao Kang,1– 3,* Zeng Lin,1– 3 Jun Pan,1– 3 Shaowen Cheng,4 Jingdong Zhang1– 3
1Department of Orthopaedics, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, 325000, People’s Republic of China; 2The Second School of Medicine, Wenzhou Medical University, Wenzhou, People’s Republic of China; 3Bone Research Institute, The Key Orthopaedic Laboratory of Zhejiang Province, Wenzhou, People’s Republic of China; 4Trauma Center, First Affiliated Hospital of Hainan Medical University, Haikou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shaowen Cheng
Trauma Center, First Affiliated Hospital of Hainan Medical University, 31 Long Hua Road, Haikou, 571100, People’s Republic of China
Email [email protected]
Jingdong Zhang
Department of Orthopaedics, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, 109 Xue Yuan Xi Road, Wenzhou, Zhejiang, 325000, People’s Republic of China
Tel +86-13587694869
Email [email protected]
Background: Osteoarthritis (OA) is a very common condition and leads to joint pain, disability, and price tag all over the world. Pathogenesis of OA is closely related to numerous inflammatory and apoptosis cytokines. Hydrogen (H2) reportedly exhibits a diversity of effects such as anti-apoptotic, anti-inflammatory, and anti-oxidative properties via the JNK pathway. However, it is unknown whether H2 has a protective effect against OA via the JNK signaling pathway. Therefore, the aim of this study was to figure out whether hydrogen has protective effect on chondrocyte and further explore the possible underlying mechanism.
Methods: The chondrocytes were obtained from the human cartilage tissues. Cells were stimulated by TBHP and treated with hydrogen. In vitro treatment effects were evaluated by Western blot assay, real-time PCR, immunofluorescence and TUNEL method. We conducted mice model of destabilization of the medial meniscus (DMM) and treated with hydrogen. In vivo treatment effects were evaluated by X-ray imaging assay, safranin O (SO) staining, TUNEL staining and immunohistochemical assay.
Results: Our results showed that hydrogen can inhibit inflammatory factors (ADAMTS5 and MMP13) and apoptosis factors (cleaved caspase-3, cytochrome c, and Bax) in TBHP-induced chondrocytes. Furthermore, hydrogen can suppress the activation of JNK signaling pathway, whereas the effect of hydrogen can be abolished by anisomycin (a JNK activator). In vivo results showed that hydrogen can down-regulate the expression of p-JNK and cleaved caspase-3 expression.
Conclusion: We uncovered that hydrogen (H2) could alleviate apoptosis response and ECM degradation in human chondrocytes via inhibiting the activation of the JNK signaling pathway. Meanwhile, in the surgically-induced DMM mice model, treatment with hydrogen (H2) performed a significant role in OA progression.
Keywords: hydrogen, osteoarthritis, apoptosis, inflammation, JNK pathway
Introduction
As an irreversible condition of arthritic degeneration that can result in seriously unsteady joints, osteoarthritis (OA) is pathologically characterized by degenerative articular cartilage, progressive subchondral sclerosis, genesis of osteophytes, as well as inflammatory synovium.1 Being the most widespread type of chronic arthritic condition, its impact on everyday activities is especially prominent in elderly populations.2,3 Plentiful studies have identified a range of influencing factors of OA development, including inflammation, aging, obesity, trauma, deformed joints, osteoporosis, among others.4 In spite of these, the pathogenesis of OA remains hardly understood. Oxidative stress and inflammation have been proven as crucial risk factors for the OA progression.5 Besides, a large number of OA factors are capable of causing oxidant-antioxidant level imbalance, which facilitates chondrocyte stimulation to result in inflammatory cytokine generation.6–8 The current clinical therapies for OA mainly aim at improving joint functions and relieving pain. However, this treatment regime does not alleviate the progression of OA. Therefore, it is essential to explore new strategies for OA treatment.
Hydrogen (H2) is a colorless and odorless gas. It exhibits diverse biological activities, including anti-inflammatory and anti-oxidative properties.9–13 In 1975, Dole et al found that 97.5% H2 could be used as therapy for cancer.14 Ohta S found that a 2% concentration of H2 could ameliorate mitochondrial diseases due to its rapid diffusion into the tissues and cells.15 Ohsawa et al observed that the inhalation of 1–4% H2 could significantly alleviate cerebral ischemia-reperfusion injury by selectively reducing cytotoxic oxygen radicals.16
Previous studies revealed that the JNK signaling pathway can regulate inflammatory and apoptosis. When initiated by certain stimuli such as TBHP (tert-butyl hydroperoxide), the activation of JNK causes the phosphorylation of c-Jun. The c-Jun then decreases proteoglycan synthesis and enhances the production of matrix metalloproteinase-13 (MMP-13).17 Gao et al found that LncRNA MALAT-1 can suppress apoptosis and cartilage matrix degradation via the JNK signaling pathway.18 Jiang et al revealed that Nesfatin-1 inhibited the IL-1β-induced activation of NF-κB, the mitogen-activated protein kinase (MAPK), and the Bax/Bcl-2 signal pathway in chondrocytes.19 The activation of the JNK pathway stimulates the production of apoptosis mediators like Bax, Cytochrome c, cleaved caspase-3, etc.20,21 Therefore, inhibiting the activation of the JNK pathway might be a promising therapeutic strategy for the treatment of OA. A recent study indicated that H2 could suppress oxidative damage by decreasing MMP-13 and increasing collagenase type II (Col II) expression.22 However, it is unclear whether H2 mediates its protective effect in OA by utilizing the JNK pathway.
In this study, we found that H2 exerted a chondroprotective effect in OA and explored the potential mechanism under in vivo as well as in vitro conditions. Here, chondrocytes were exposed to tert-butyl hydroperoxide (TBHP) for the in vitro induction of oxidative stress. We found that H2 may suppress TBHP-induced apoptosis and inflammation in chondrocytes through the JNK pathway. Moreover, the activation of JNK by Anisomycin eliminated the anti-apoptotic and anti-inflammatory effects of H2. Thus, H2 suppressed apoptosis and inflammation and attenuated OA through the JNK signaling pathway. Our study highlighted the therapeutic potential of H2 in OA and explored the mechanism of anti-apoptotic and anti-inflammatory effects of H2 in the chondrocytes.
Materials and Methods
Ethics Statement and Experimental Animals
All Experimental procedures involving animal care strictly followed the guidelines for the Animal Care and Use outlined by the Committee of Wenzhou Medical University and approved by the Animal Care and Care Committee of Wenzhou Medical University. Tissue collection and experiments involving human OA were approved by the Second Affiliated Hospital of Ethics Committee of Wenzhou Medical University (ethic cord: LCKY-2019–67) and Yuying unknown Children’s Hospital and followed the guiding principles of the Helsinki Declaration. Informed consent was obtained from the human participants of this study.
Reagents
Anisomycin, Collagenase-II, Safranin-O/Fast Green, and dimethylsulfoxide (DMSO) were obtained from Solarbio Science & Technology Co., Ltd. (Beijing, China). Primary antibodies of Collagenase II, Aggrecan, ADAMTS-5, and MMP-13 were gained from Abcam (Cambridge, MA, USA). Primary antibodies directed against P-JNK and JNK were procured from Cell Signaling Technology (MA, USA). Primary antibodies against Bcl-2 and GAPDH were procured from ProteinTech Group (Wuhan, China). Primary antibodies against cleaved caspase-3 and Bax were procured from Affinity Biosciences (Cincinnati, OH, USA). Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM)/F12 were obtained from Gibco (Grand Island, NY, USA). Tert-Butyl hydroperoxide solution (TBHP) was procured from Sigma-Aldrich (St Louis, MO, USA). The secondary antibodies of Goat Anti-Mouse IgG, Goat Anti-Rabbit IgG, Alexa Fluor®488 and labeled Alexa Fluor®594 were obtained from bioWORLD (OH, USA). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). The BCA protein assay kit was procured from Beyotime Biotechnology (Shanghai, China). Cell Counting Kit-8 (CCK-8) was obtained from Dojindo Laboratories (Kumamoto, Japan).The In Situ Cell Death Detection Kit was purchased from Roche (San Francisco, CA, USA). Caspase-3 colorimetric assay kit was obtained from Keygen Biotech Co., Ltd. (Nanjing, China).
Development of Mice OA Models
For the experiment, forty-five 10-week-old C57BL/6 female mice were obtained from the Animal Center of Chinese Academy of Sciences Shanghai. All mice procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Wenzhou Medical University and approved by the Animal Ethics Committee of Wenzhou Medical University (ethics code: wydw2019–0808). After injection with 2% pentobarbital sodium (40 mg kg−1) intraperitoneally for anesthesia, we established OA model by the method described in the previous study.23 The tibial ligament of the medial meniscus was cut with microsurgical scissors. An operation of arthrotomy without the transaction of medial meniscus ligament was also performed in the left knee joint of the mice in the sham operation group. After the operation, the mice were randomly divided into three groups (n = 15): sham group, an OA group (DMM), and an OA group treated with H2 (DMM + 75% H2). The gas was inhaled for 1 h per day.
X-Ray Imaging Assay
The mice were subjected to radiographic assessment at 8 weeks after surgery. Kubtec Model XPERT.8 X-ray machine (KUB Technologies Inc.) was utilized to determine the formation of osteophyte, joint clearance, and calcification of the cartilage surface. The settings of the machine were as follows: 160 µA and 50 kV.
Histopathologic Analysis
Safranin-O/Fast Green was used to measure the articular cartilage destruction. A light microscope was utilized to assess the morphological changes in the mice chondrocytes and the surrounding tissues. The destruction of articular cartilage was evaluated by the Osteoarthritis Research Society International (OARSI) scoring system for the medial tibial condyle and medial femoral plateau.
TUNEL Staining
After fixing the chondrocytes or cartilage sections, TUNEL staining was performed in a dark, humidified room using an In Situ Cell Death Detection kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Next, DAPI (4′,6-diamidino-2-phenylindole) was used to stain the cell nuclei. Positive staining of DNA strand breaks in the apoptotic cells was detected under a fluorescence microscope.
Immunohistochemical Assay
As a first step, the knee joints were subjected to 4% paraformaldehyde (PFA) fixation, decalcification, paraffin-embedment, as well as cutting into 7 µm sections for deparaffinization and rehydration processes. Then, 30 min treatment of histological sections was carried out at 37 °C using hydrogen peroxide (3% v/v) and trypsin-EDTA solution (0.25%). After a further 60 min incubation of these sections at 37 °C in BSA (10%), they were treated with the primary antibodies at 4 °C against the cleaved caspase-3 and P-JNK for a 24 h duration. Afterwards, 1 h section incubation was performed on the 2nd d, at 4 °C using an HRP-conjugated second antibody. Aided by the Image-Pro Plus version 6.0 (Media Cybernetics, MD, USA), image analysis was accomplished, while in the quantitative analysis, five sections from every group were used.
Primary Human Chondrocyte Isolation and Culture
Initially, from 10 patients with OA (half males and half females, age of 54 ± 8 years), who had received complete knee arthroplasty at the Second Hospital Affiliated to Wenzhou Medical University, we collected the human cartilage tissues, which were cut into pieces (1×1×1 mm3) and thrice washed using PBS. Then, 4 h incubation of the cut pieces was performed at 37 °C using collagenase II (2 mg/mL). After 6 min centrifugation at 800 rpm, suspension of the digested cartilage tissues was carried out, which was followed by plating into flasks for tissue cultures. At 37 °C, chondrocyte incubation was achieved under a 5% CO2 atmosphere using DMEM/F12 medium involving 10% FBS. Medium replacement was implemented every alternate day. We utilized trypsin-EDTA (0.25%) to subculture the human cells till reaching 80 to 90% confluences.
The Application of H2
H2 was stored in gas cylinders before the experiment. Then, hydrogen, oxygen, and nitrogen were mixed, and the H2 gas concentration (6.25, 12.5, 25, 50, and 75% (vol/vol)) was adjusted through a three-way connection and measured with TRACE GC Ultra gas chromatography (Thermo Fisher, MA, USA). After a volume of H2 was removed in each group, the remaining volume was mixed according to the ratio of nitrogen and oxygen in the air, that is, 0.78:0.21. Chondrocytes were pretreated with 25 µM TBHP for 24 h, and H2 was introduced into the cultured cells for 4 h.
CCK-8 Assay
The cytotoxicity of H2 on chondrocytes was detected with CCK-8 kits by following the manufacturer’s instruction. Firstly, cells were plated in 96-well plates at a density of 50,000 cell per cm2 for 24 h and incubated with various concentrations of H2 (0, 12.5, 25, 50, and 75%) for 4 h. Later, the chondrocytes were rinsed thrice in PBS. Finally, 10 mol/L CCK-8 solution was added to each well for 2 h, and the optical density was observed at 450 nm with a spectrophotometer (Thermo Fisher Scientific).
qRT-PCR
After stimulation with TBHP (25 µM) and treatment with H2 (75%), total RNA was extracted with the aid of TRIzol (Invitrogen) from the human chondrocytes. Regarding the RT-qPCR procedure, the CFX96 PCR System (Bio-Rad Laboratories, California, USA) was utilized under the following PCR cycling conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The reaction mixture comprised, in a 10 µL total volume, 5 µL of 2 X SYBR Master Mix, 4.5 µL of diluted cDNA, as well as each 0.25 µL of primer. Collection and normalization of cycle threshold (Ct) values were carried out towards the expression levels of GAPDH. To achieve computation of relative mRNA levels for every target gene, the 2−ΔΔCt method was employed. The primer creation for Bax, Bcl-2 and GAPDH was accomplished via the NCBI Primer-Blast program. In Table 1, the sequences of forward and reverse primers are detailed.
 |
Table 1 Primers Used in the Studies |
Western Blotting
The expression level of the proteins was measured by Western blotting. The proteins were separated with the RIPA lysis buffer, sonicated on ice for 10 min, and then centrifuged at 12,000 rpm for 15 min at 4 °C. The BCA protein detection kit was used to estimate the protein concentration. After protein isolation by gel electrophoresis with sodium dodecyl sulfate-polyacrylamide (SDS-PAGE), the isolated 40 mg of proteins were shifted to the polyvinylidene difluoride (PVDF) membranes (Millipore), and subjected to 3 h blockage using non-fat milk (5%). Incubation of the resulting membranes was performed against such primary antibodies as JNK (1:2000), P-JNK (1:2000), Bax (1:2000), Bcl-2 (1:2000), GAPDH (1:2000), cleaved caspase-3 (1:2000), MMP-13 (1:2000), ADAMTS-5 (1:2000), Collagenase II (1:2000), as well as Aggrecan (1:2000). Next, an additional 2.5 h incubation was carried out at room temperatures against secondary antibodies. For blots visualization, the Image Lab Touch 3.0 (Bio-Rad, Hercules, CA, USA) was utilized, followed by washing thrice using TBST.
Analysis of Immunofluorescence
The cells were rinsed with PBS and fixed with 4% paraformaldehyde for 15 min. Next, the chondrocytes were incubated with 0.1% Triton X-100 at room temperature. Further, 10% goat serum solution was used to block in 37 °C water bath for 30 minutes and incubated with primary antibodies against Collagenase II (1:300) for the entire night at 4 °C. The next day, the cells were exposed to Alexa Fluor® 488-labeled conjugated secondary antibodies (1:400) for 1.5 h. Finally, the cells were exposed to DAPI (Beyotime) for 1 min. Ultimately, the cell samples were detected on the Olympus fluorescence microscope (Tokyo, Japan). The fluorescence intensity was observed by using the Image J software.
Statistical Analysis
The experiments were performed at least three times. The data obtained were expressed as the mean ±SEM. The data were analyzed via GraphPad Prism (United States). Inter-group comparisons were performed using a one-way ANOVA followed by the Tukey’s test. Probability values of P < 0.05 were considered statistically significant.
Results
H2 Inhibits the Cartilage Deterioration in a Murine DMM Model
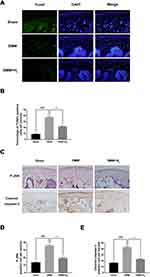
A murine OA model was created through surgical operation, which was achieved by making the medial meniscus unstable In order to figure out whether H2 exerted protective functions on the progression of OA in vivo, we examined the cartilage histology in OA mice based on Safranin–O (S–O) staining in conjunction with X-radioscopy. As a result of the surgery, the density of cartilage surface was elevated and the joint space was narrowed. In the H2 group, on the contrary, improvements in the above impairments were noticed (Figure 1A). Based on the S–O staining outcomes presented in Figure 1B, the H2 therapy reduced the erosion of superficial cartilage and the substantial loss of proteoglycan. Plus, both the synovitis and OARSI scores agreed with the S–O staining outcomes. As shown in Figure 1C and D, the two types of scores were lower in the group treated with H2 than the untreated OA group.
H2 Attenuates the Expression of Cytokines and Apoptosis-Related Proteins Following DMM in Mice
The apoptosis of chondrocytes was measured by TUNEL staining and cleaved caspase-3 immunohistochemical staining. As shown in Figure 2A and B, a higher proportion of cellular apoptosis was observed in the DMM group than in the sham group. However, the H2 group significantly reversed this pathological phenomenon. Besides, immunohistochemical staining of cleaved caspase-3 was performed to detect the in vivo protective effect of H2. As shown in Figure 2C and E, H2 decreased the expression of cleaved caspase-3 in mouse articular cartilage. Furthermore, immunohistochemical staining of P-JNK demonstrated increased levels of P-JNK in the DMM group than in the sham group. Again, the H2 treatment significantly reduced the expression of P-JNK (Figure 2C and D). These data indicated that H2 was a potential agent of treatment of OA under in vivo conditions.
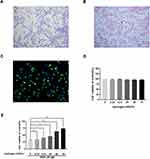
H2 Protects Chondrocytes from TBHP Treatment
We used toluidine blue, immunofluorescence, and Safranin O staining to characterize the isolated human chondrocytes. (Figure 3A). The chondrocytes were stained red by Safranin O staining, while their cytoplasm was stained purple by toluidine blue. The collagen II in the cytoplasm of human chondrocytes was stained green by immunofluorescence without any positive staining in the nucleus (Figure 3B and C). Both stains demonstrated that the cells extracted from the articular cartilage were chondrocytes. The cytotoxic effects of H2 on human chondrocytes were detected by the CCK-8 assay. The cells were treated with a concentration gradient (0,12.5, 25, 50, and 75%) of H2 for 4 h. The CCK-8 analysis revealed that H2 did not show any obvious cytotoxicity toward human chondrocytes (Figure 3D). Furthermore, the chondrocytes were treated with different concentrations of H2 (0,12.5, 25, 50, and 75%) for 4 h after stimulation with TBHP for 24 h. As shown in Figure 3E, the viability of the chondrocytes was reduced in the TBHP group whereas H2 significantly increased the viability of the cells in a dose-dependent manner. Thus, we adopted 75% H2 as the concentration to conduct subsequent experiments.
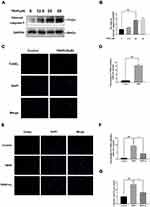
H2 Exerts Anti‑Apoptotic Effects in TBHP-Induced Human Chondrocytes
To identify the effects of TBHP on the human chondrocytes, different concentrations (0, 12.5, 25, and 50 µM) were used for stimulation. As pictured in Figure 4A and B, the apoptosis-related protein expression of cleaved-caspase 3 upregulated with increasing concentrations of TBHP. Moreover, TBHP induced the apoptosis of chondrocytes (Figure 4C and D). Therefore, the TUNEL assay was used to detect the anti-apoptotic effect of H2 on human chondrocytes. As shown in Figure 4E and F, a greater incidence of apoptosis was recorded in the chondrocytes in the TBHP (25 µM) group compared with the control group; the H2 treatment significantly reversed this trend. The activation of caspase-3 was evaluated by the caspase colorimetric assay kit. As shown in Figure 4G, the activation of caspase-3 was significantly upregulated after stimulation with TBHP. However, H2 treatment downregulated this activation of caspase-3.
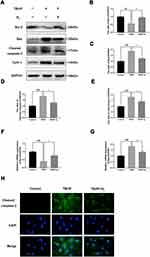
H2 Decreases the Expression of Cytokines and Apoptosis‑Related Proteins in TBHP-Induced Human Chondrocytes
To determine the effect of H2 on apoptosis in TBHP-induced human chondrocytes, the expression of cleaved caspase-3, Bcl-2, and Bax was evaluated by Western blot. As pictured in Figure 5A–E, TBHP increased the expression of Bax, cytochrome c, and cleaved caspase-3 and downregulated the expression of Bcl-2. On the contrary, H2 suppressed the expression of Bax, cytochrome c, and cleaved caspase-3 and improved the Bcl-2 expression. In addition, the results of qRT-PCR showed that H2 suppressed the generation of Bax, which had increased after TBHP stimulation (Figure 5F and G). Moreover, the immunofluorescence of cleaved caspase-3 also indicated that H2 suppressed the expression of cleaved caspase-3 in TBHP-induced chondrocytes (Figure 5H). Therefore, these data demonstrated that H2 was capable of preventing the TBHP-induced apoptosis of chondrocytes.
H2 Exerts Anti‑Inflammatory Effects in TBHP-Induced Human Chondrocytes
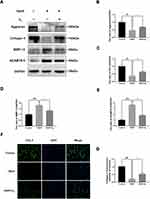
To investigate the role of H2 in inhibiting the degradation of extracellular matrix (ECM) in TBHP-induced human chondrocytes, Western blotting was utilized. We, respectively, assessed the protein levels of Collagen II, Aggrecan, ADAMTS-5, and MMP-13 in the chondrocytes. As pictured in Figure 6A–E, chondrocytes exerted an apparent downregulation of the expression of collagen II and Aggrecan following TBHP-induction. In contrast, an upregulation of the expression of ADAMTS-5 and MMP-13 was observed. Moreover, H2 treatment could reverse this trend. Further, the immunofluorescence outcomes demonstrated that H2 attenuated the degradation of collagen II (Figure 6F and G). Therefore, these results indicated that H2 can inhibit inflammation induced by TBHP in human chondrocytes.
H2 Exerts Anti-Apoptotic and Anti‑Inflammatory Effects in the TBHP-Induced Human Chondrocytes by Suppressing the JNK Pathway
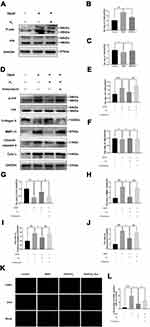
Western blot was used to investigate the effect of H2 on the JNK signaling pathway. The expression of P-JNK significantly increased in the chondrocytes after stimulation with TBHP. Conversely, H2 notably suppressed the TBHP-induced activation of JNK in the human chondrocytes (Figure 7A–C).
However, treatment with Anisomycin (a JNK activator) inhibited the dephosphorylation of JNK. Moreover, H2 mediated a reduction in the expression of MMP-13, cytochrome c, and cleaved caspase-3, and this reduction was abolished by the addition of Anisomycin in the H2+Anisomycin group (Figure 7D–J). Moreover, the TUNEL assay showed that H2 decreased the incidence of apoptosis in TBHP-induced chondrocytes compared to the TBHP group, whereas the Anisomycin treatment could significantly reverse this trend (Figure 7K and L). These results revealed that H2 suppressed the apoptosis induced by TBHP in human chondrocytes via the JNK signaling pathway.
Discussion
Osteoarthritis (OA) is a chronic joint degradation disease that is characterized by long-term pain and joint limitation.2 The current options for the treatment of OA are an early application of non-steroidal anti-inflammatory drugs (NSAIDs), but they only relieve the clinical symptoms.24,25 Moreover, they also result in a series of side effects such as heart attack and stroke.26 Artificial joint replacement remains the only choice for the treatment of the final stages of OA.27 Therefore, an agent that prevents the progress of OA and is accompanied by fewer side effects is a potential therapeutic strategy for OA. Recently, increasing attention has been focused on anti-apoptosis compounds that may be capable of treating OA in the absence of harmful side effects.28 Biochemical and biomechanical events induce apoptosis and imbalance between the catabolism and anabolism of the ECM in the chondrocytes.29 Several pharmacological treatments for the genetic regulation of apoptosis have shown therapeutic effects against OA development in cellular and animal models.30 Therefore, studying the underlying mechanism of apoptosis in the chondrocytes may result in a potential therapy for OA.
Oxidative stress is the main process in chondrocyte apoptosis. When chondrocytes are stimulated with TBHP, they generate ROS like superoxide anion (O2−), H2O2, and hydroxyl radical (OH); this leads to mitochondrial damage and eventually apoptosis.31 An increasing number of studies have found that excessive ROS leads to the degradation of cartilage and apoptosis of chondrocytes.32,33 Therefore, decreasing the production of ROS may be an effective strategy for OA treatment. Interestingly, hydrogen is an anti-oxidative compound.
H2 is the lightest element and characteristic of the simplest and abundant elements in nature.16 Many studies have reported the anti-inflammatory, anti-apoptotic, and anti-oxidative effects of H2 in several diseases.10,34 They demonstrated that inhalation of 4% H2 was a safe and efficient treatment for human diseases. Hydrogen has aroused the attention of researchers due to its simple preparation, high safety, strong permeability, and ease of carrying it.16 Peng Guan et al found that H2 improved chronic intermittent hypoxia (CIH)-induced kidney injury via the JNK signaling pathway.35 Zhao et al revealed that H2 alleviated ER stress and apoptosis of cardiac myocytes by blocking the c-Jun N-terminal kinase(JNK)-MAPK.36 Wu et al uncovered that H2 attenuated the hypoxic-ischemic brain damage by suppressing apoptosis and inflammation in neonatal rats.37 Chen et al found that Inhalation of hydrogen could ameliorate apoptosis and oxidative stress of spinal cord neurons in spinal cord injury in mice.38 The JNK pathway is a classical pro-apoptosis pathway and has been demonstrated to play a pivotal role in the regulation of apoptosis of chondrocytes with OA development. Previous studies have demonstrated that some drugs such as Urolithin A, Kinsenoside, and Luteolin protected the chondrocytes by inhibiting the activation of the JNK signaling pathway.39–41 Hence, targeted downregulation of phosphorylation of JNK may be deemed to cure OA effectively.
Therefore, in the present study, we explored the anti-apoptotic capability of H2 on human chondrocytes with the JNK signaling pathway. In the TBHP-treated chondrocytes of humans, remarkable inhibition of JNK phosphorylation by H2 was demonstrated. Due to the inhibited activity of JNK pathway, the production of such apoptosis factors as Bax and cleaved caspase-3 was downregulated, which in turn led to improvements in the OA progression. Collectively, our data somewhat suggested that the H2 possessed an anti-apoptotic activity during OA development, which was demonstrated on THBP-treated chondrocytes, and that the JNK pathway might be involved in its mechanism of action. The potential value of H2 was thus apparent. Moreover, shrinking space of joint, degradative ECM, seriously eroded cartilage and vastly deficient proteoglycan were noticed in the DMM groups in contrast to the sham group. Upon the H2 therapy, the aforementioned symptoms all got improved, and the murine DMM model also exhibited lower grading score of OARSI. According to the TUNEL stain of cartilage and IHC stain of cleaved caspase-3, the apoptosis of cartilage was mitigated by H2. Furthermore, the OA progression got improved by H2, which involved the signaling pathway of JNKs. Collectively, these outcomes and the preparatory findings suggest the ameliorating activity of H2 against the OA development, which was achieved by suppressing the JNK pathway activation (Figure 8).
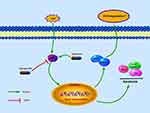 |
Figure 8 Schematic reveals the involvement of H2 via the JNK pathway and the potential protective effects in the progression of osteoarthritis. |
Interestingly, hydrogen has been studied for its therapeutic effects on various diseases at different concentrations. In this study, high H2 concentration (6.23–75%) was used to study the protective effect of H2 on osteoarthritis. However, the role of H2 in osteoarthritis still needs to be investigated as this study has some limitations. For example, we do not know whether a high concentration of H2 has adverse effects on other systems of the body. Moreover, the effects of long-term low concentration of hydrogen on osteoarthritis have not been studied; these need to be further studied in the future.
In conclusion, we uncovered that H2 alleviates the apoptotic reaction and degradative ECM by deactivating the signaling pathway of JNKs in chondrocytes of humans. In the meantime, a vital function of H2 therapy is also found against the OA development in the murine model of surgically-induced DMM. Our data collectively imply an anti-OA protective activity exerted by H2.
Funding
This study is supported by Zhejiang Provincial Natural Science Foundation of China (LY16H060010).
Disclosure
The authors have declared that no competing interests exists.
References
1. FrenchH P, Galvin R, Horgan NF, et al. Prevalence and burden of osteoarthritis amongst older people in Ireland: findings from The Irish Longitudinal Study on Ageing (TILDA). Osteoarthr Cartil. 2015;23(1):A188. doi:10.1016/j.joca.2015.02.967
2. Thysen S, Luyten FP, Lories RJ. Targets, models and challenges in osteoarthritis research. Dis Model Mech. 2015;8(1):17–30. doi:10.1242/dmm.016881
3. Aicher WK, Rolauffs B. The spatial organization of joint surface chondrocytes: a review of its potential roles in tissue functioning, disease and early, preclinical diagnosis of osteoarthritis. Ann Rheum Dis. 2013.
4. Shen CL, Smith BJ, Lo DF. Dietary polyphenols and mechanisms of osteoarthritis. J Nutr Biochem. 2012;23(11):1367–1377. doi:10.1016/j.jnutbio.2012.04.001
5. Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332:639–642. doi:10.1136/bmj.332.7542.639
6. Santangelo KS, Nuovo GJ, Bertone AL. In vivo reduction or blockade of interleukin-1beta in primary osteoarthritis influences the expression of mediators implicated in pathogenesis. Osteoarthr Cartil. 2012;20:1610–1618. doi:10.1016/j.joca.2012.08.011
7. Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28:5–15. doi:10.1016/j.berh.2014.01.004
8. Goldring MB, Fukuo K, Birkhead JR, et al. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. Cell Biochem. 1994;54:85–99. doi:10.1002/jcb.240540110
9. Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol. 2011;148:91–95. doi:10.1016/j.ijcard.2010.08.058
10. Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi:10.1016/j.bbrc.2007.07.088
11. Wang F, Yu G, Liu S, Li J. Hydrogen-rich saline protects against renal ischemia/reperfusion injury in rats. J Surg Res. 2011;167:339–344. doi:10.1016/j.jss.2010.11.005
12. Chen H, Sun Y, Hu P, et al. The effects of hydrogen-rich saline on the contractile and structural changes of intestine induced by ischemia-reperfusion in rats. J Surg Res. 2011;167:316–322. doi:10.1016/j.jss.2009.07.045
13. Huang Y, Xie K, Li J, et al. Beneficial effects of hydrogen gas against spinal cord ischemia-reperfusion injury in rabbits. Brain Res. 2011;1378:125–136. doi:10.1016/j.brainres.2010.12.071
14. Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190(4210):152–154. doi:10.1126/science.1166304
15. Ohta S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim Biophys Acta. 2012;1820(5):586–594. doi:10.1016/j.bbagen.2011.05.006
16. Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi:10.1038/nm1577
17. Ge H-X, Zou F-M, Li Y, et al. JNK pathway in osteoarthritis: pathological and therapeutic aspects. J Recept Signal Transduct Res. 2017;37(5):431–436. doi:10.1080/10799893.2017.1360353
18. Gao GC, Cheng XG, Wei QQ, Chen WC, Huang W-Z. Long noncoding RNA MALAT-1 inhibits apoptosis and matrix metabolism disorder in interleukin-1β-induced inflammation in articular chondrocytes via the JNK signaling pathway. J Cell Biochem. 2019;120(10):17167–17179. doi:10.1002/jcb.28977
19. Jiang L, Kai X, Jin L, et al. Nesfatin-1 suppresses interleukin-1β-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes and ameliorates osteoarthritis in rats. Aging. 2020;12(2):1760–1777. doi:10.18632/aging.102711
20. Dai X, Li Y, Zhang R, et al. Effects of sodium selenite on c-Jun N-terminal kinase signalling pathway induced by oxidative stress in human chondrocytes and c-Jun N-terminal kinase expression in patients with Kashin-Beck disease, an endemic osteoarthritis. Br J Nutr. 2016;115(9):1547–1555. doi:10.1017/S0007114516000362
21. Zhang H-B, Zhang Y, Chen C, et al. Pioglitazone inhibits advanced glycation end product-induced matrix metalloproteinases and apoptosis by suppressing the activation of MAPK and NF-κB. Apoptosis. 2016;21(10):1082–1093. doi:10.1007/s10495-016-1280-z
22. Cheng S, Peng L, Xu B, et al. Protective effects of hydrogen-rich water against cartilage damage in a rat model of osteoarthritis by inhibiting oxidative stress, matrix catabolism, and apoptosis. Med Sci Monit. 2020;12(26):e920211.
23. Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the mouse. Osteoarthr Cartil. 2007;15:1061–1069. doi:10.1016/j.joca.2007.03.006
24. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390(10090):e21–e33. doi:10.1016/S0140-6736(17)31744-0
25. Sun MM, Beier F, Pest MA. Recent developments in emerging therapeutic targets of osteoarthritis. Curr Opin Rheumatol. 2017;29:96–102. doi:10.1097/BOR.0000000000000351
26. Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17(8):971–979. doi:10.1016/j.joca.2009.03.002
27. Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016;28:8–13. doi:10.1016/j.coph.2016.02.009
28. Asada S, Fukuda K, Nishisaka F, Matsukawa M, Hamanisi C. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res. 2001;50:19–23. doi:10.1007/s000110050719
29. Sutipornpalangkul W, Morales NP, Harnroongroj T. Free radicals in primary knee osteoarthritis. J Med Assoc Thai. 2009;92(Suppl. 6):S268eS274.
30. Hadjigogos K. The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med. 2003;45:7–13.
31. Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035–26054. doi:10.3390/ijms161125943
32. Hosseinzadeh A, Kamrava SK, Joghataei MT. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J Pineal Res. 2016;61(4):411–425. doi:10.1111/jpi.12362
33. Ansari MY, Khan NM, Ahmad I, et al. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26(8):1087–1097. doi:10.1016/j.joca.2017.07.020
34. Ohta S. Recent progress toward hydrogen medicine: the potential of molecular hydrogen for preventive and therapeutic applications. Curr Pharm Des. 2011;17(22):2241–2252. doi:10.2174/138161211797052664
35. Guan P, Sun Z-M, Luo L-F, et al. Hydrogen protects against chronic intermittent hypoxia-induced renal dysfunction by promoting autophagy and alleviating apoptosis. Life Sci. 2019;225:46–54. doi:10.1016/j.lfs.2019.04.005
36. Zhao Y-S, Ji-Ren A, Yang S, et al. Hydrogen and oxygen mixture to improve cardiac dysfunction and myocardial pathological changes induced by intermittent hypoxia in rats. Oxid Med Cell Longev. 2019;2019:7415212. doi:10.1155/2019/7415212
37. Wu G, Chen Z, Wang P, et al. Hydrogen inhalation protects hypoxic-ischemic brain damage by attenuating inflammation and apoptosis in neonatal rats. Exp Biol Med. 2019;244(12):1017–1027. doi:10.1177/1535370219855399
38. Chen X, Cui J, Zhai X, et al. Inhalation of hydrogen of different concentrations ameliorates spinal cord injury in mice by protecting spinal cord neurons from apoptosis, oxidative injury and mitochondrial structure damages. Cell Physiol Biochem. 2018;47(1):176–190. doi:10.1159/000489764
39. Ding S-L, Pang Z-Y, Chen X-M, et al. Urolithin a attenuates IL-1β-induced inflammatory responses and cartilage degradation via inhibiting the MAPK/NF-κB signaling pathways in rat articular chondrocytes. J Inflamm. 2020;17:13. doi:10.1186/s12950-020-00242-8
40. Zhou F, Mei J, Han X, et al. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF- κ B/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B. 2019;9(5):973–985. doi:10.1016/j.apsb.2019.01.015
41. Xue J, Ye J, Xia Z, et al. Effect of luteolin on apoptosis, MAPK and JNK signaling pathways in guinea pig chondrocyte with osteoarthritis. Cell Mol Biol. 2019;65(6):91–95. doi:10.14715/cmb/2019.65.6.15
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.