Back to Journals » OncoTargets and Therapy » Volume 12
Hydrocortisone Suppresses Early Paraneoplastic Inflammation And Angiogenesis To Attenuate Early Hepatocellular Carcinoma Progression In Rats
Authors Liu X, Cui H, Niu H, Wang L , Li X, Sun J, Wei Q, Dong J , Liu L, Xian CJ
Received 25 July 2019
Accepted for publication 28 September 2019
Published 8 November 2019 Volume 2019:12 Pages 9481—9493
DOI https://doi.org/10.2147/OTT.S224618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sanjay Singh
Xiaolong Liu,1,* Haiyan Cui,2,* Hongling Niu,1 Liping Wang,3 Xiangzhi Li,1 Jingbo Sun,1 Qingzhu Wei,4 Jianghui Dong,3 Lixin Liu,1 Cory J Xian3
1Department of General Surgery, The Third Affiliated Hospital of Southern Medical University, Guangzhou, Guangdong 510630, People’s Republic of China; 2Department of Internal Medicine, The Third Affiliated Hospital of Southern Medical University, Guangzhou, Guangdong 510630, People’s Republic of China; 3School of Pharmacy and Medical Sciences, and UniSA Cancer Research Institute, University of South Australia, Adelaide, SA 5001, Australia; 4Department of Pathology, The Third Affiliated Hospital of Southern Medical University, Guangzhou, Guangdong 510630, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lixin Liu
Department of General Surgery, The Third Affiliated Hospital of Southern Medical University, 183 Zhongshan Avenue West, Tianhe District, Guangzhou, Guangdong 510630, People’s Republic of China
Tel/fax +862062784437
Email [email protected]
Cory J Xian
School of Pharmacy and Medical Sciences, and UniSA Cancer Research Institute, University of South Australia, GPO Box 2471, Adelaide, SA 5001, Australia
Tel +618 8302 1944
Fax +618 8302 1087
Email [email protected]
Background: Inflammation is implicated in both hepatic cirrhosis development and hepatocellular carcinogenesis, and treatment with long-acting glucocorticoid dexamethasone prevented liver carcinogenesis in mice. However, it is unclear whether glucocorticoids have anti-inflammatory effect on hepatocellular carcinoma (HCC) and if short-acting glucocorticoids (with fewer adverse effects) inhibit paraneoplastic inflammation and HCC progression.
Methods: To investigate whether different types of anti-inflammatory agents attenuate HCC progression, the current study compared effects of treatments with hydrocortisone (a short-acting glucocorticoid) or aspirin on HCC progression. HCC was induced in diethylnitrosamine-treated rats which were randomly divided into 4 groups (n=8), respectively receiving orally once daily vehicle, glucuronolactone, glucuronolactone+hydrocortisone, and glucuronolactone+aspirin. Diethylnitrosamine (DEN) was given to rats in drinking water (100mg/L) to induce HCC. At weeks 12 and 16 post-induction, effects were compared on HCC nodule formation, microvessel density, and macrophage infiltration, and levels of paraneoplastic protein expression of tumor necrosis factor (TNF)-α, p38 mitogen-activated protein kinase (p38), phosphorylated p38 (p-p38), nuclear factor (NF)-κB, interleukin (IL)-10, hepatocyte growth factor (HGF), transforming growth factor (TGF)-β1 and vascular endothelial growth factor (VEGF).
Results: Compared to the model and glucuronolactone alone groups, HCC nodule number and microvessel density in the glucuronolactone+hydrocortisone group were significantly lower at week 12. At week 12 but not week 16, significantly lower levels of macrophages, TNF-α, p-p38, NF-κB, IL-10, HGF, TGF-β1 and VEGF were observed in the paraneoplastic tissue of the glucuronolactone+hydrocortisone group when compared with the control and glucuronolactone groups.
Conclusion: The results suggest that hydrocortisone treatment reduces macrophage polarization, expression of inflammatory and anti-inflammatory cytokines, and angiogenesis in paraneoplastic tissue, and attenuates early HCC progression. Although hydrocortisone did not have attenuation effect on advanced solid tumor, the current study shows the potential benefits and supports potential clinical use of hydrocortisone in attenuating early progression of HCC, which is through suppressing paraneoplastic inflammation and angiogenesis.
Keywords: hepatocellular carcinoma, inflammation, macrophage, angiogenesis, steroid, aspirin
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequent malignant cancers which has a high rate of mortality and is a huge economic burden worldwide.1 Although the treatment of liver cancer has improved rapidly, the 5-year survival rate of HCC is still lower than 20%.2 In addition, the mortality of HCC has increased annually in developed and developing countries.3,4 Although further studies are required to fully understand the pathogenesis of HCC, chronic inflammation has been considered to be an important factor in the pathogenesis process of liver cancer, especially HCC.5–8 In patients with virus infection or alcohol addiction, chronic hepatitis induces liver fibrosis and cirrhosis of stellate cells, resulting in HCC.9
In the past few decades, the modulatory role of the immune system in tissue homeostasis has been widely reported, and different previous studies have focused on the impact of the inflammatory microenvironment on tumor progression.10,11 However, the effect of macrophage-regulated inflammation in the paraneoplastic liver tissue on hepatic carcinogenesis was still underestimated. Among all the effective immune cell types, the monocyte-macrophage lineage has been considered an important moderator of local paraneoplastic inflammation in carcinogenesis. Ohtsuki et al.12 reported that, when compared to M1 polarization, M2 polarization of macrophages may accelerate HCC progression in mice infected with hepatitis C virus. Apart from their role in local inflammatory mediation, the polarization of macrophages in paraneoplastic tissue is now known to contribute to carcinogenesis. We previously reported that the increase in the total number of macrophages in the paraneoplastic tissue correlated with the poor pathological grade in human HCC.11,13 In 2016, Mendes et al.14 found that both M1 and M2 polarized macrophages were present at higher numbers in fibrotic and cirrhotic tissues of HCC compared to healthy liver. In mouse models, inhibition of the inflammatory microenvironment within tumors has been implicated as a therapeutic target for inflammation-associated cancers such as HCC.17–19
In addition, tumorous angiogenesis was promoted by cytokines in inflammation,15,16 and microvessel density was found positively associated with the presence of macrophages.16 Furthermore, cytokines derived from macrophage-regulated inflammation are known to also contribute to cancer progression by promoting angiogenesis.20 Although the mechanism of how the increased neo-angiogenesis in paraneoplastic tissue contributes to cancer progression is still unclear, it was regarded as an initial transformation in carcinogenesis.21 Thus, due to the important role of inflammation in cancer initiation and progression, controlling the inflammation in paraneoplastic tissue would potentially reduce the angiogenesis in paraneoplastic tissue and even limit the carcinogenesis.
While two major groups of anti-inflammatory agents, namely the non-steroid anti-inflammatory drugs like aspirin and the steroids known as glucocorticoids (GCs), have been reported to be potential adjuvant medications for cancer,27–29 more research effort should be made to investigate their economic oral or intravenous medications as a therapy to control the HCC by inhibiting the macrophage-regulated inflammation in paraneoplastic liver tissues. Clinically, because of their significant immunosuppression properties, GCs are the most commonly used steroids in the treatment of autoimmune diseases and infections. Exogenous GCs block the antigen presentation of antigen-presenting cells, and downstream, inhibit the release of inflammatory cytokines by immune cells such as T cells and macrophages.30–32 The long-acting GC, dexamethasone, can prevent liver carcinogenesis in mice through inducing a switch from glycolysis to gluconeogenesis.33 In clinical practice, long-acting GCs have more side effects on the hypothalamic-pituitary-adrenal axis than short-acting GCs.34 However, it is unclear whether the short-acting GCs (with fewer adverse effects) would have any inhibitory effects on chronic inflammation in paraneoplastic tissue and on HCC progression. On the other hand, aspirin has been more widely accepted than GCs to be used to prevent cancers by inhibiting the platelet-dependent pathway. A mouse model of chronic hepatitis B has shown that aspirin prevents HCC development by inhibiting inflammatory events that are independent of platelets.35,36 However, the anti-inflammatory effect of GCs and aspirin on HCC was not studied in previous investigations, and the exact effect of aspirin on the macrophage-moderated paraneoplastic inflammation and HCC progression remains unclear.
Endogenous glucocorticoids (GCs) modulate cell growth, differentiation, and metabolism, and therefore, they are essential for mammalian homeostasis.28
Therefore, the current study investigated the potential effect of a short-acting GC, hydrocortisone, on paraneoplastic inflammation and HCC progression as well as the correlation between paraneoplastic inflammation and HCC progression. In addition, this study compared its treatment effect with that of aspirin on HCC progression.
Materials And Methods
Animals And Treatments
A rat model of diethylnitrosamine (DEN)-induced HCC was established according to previous studies.37,38 Thirty-two male adult Wistar rats (180–220 g) were purchased from the Experimental Animal Center of the Southern Medical University (Guangzhou, Guangdong, China). Rats were housed individually, provided with a standard rat laboratory diet and drinking water ad libitum, and maintained in a room with a constant temperature of 23 ± 1°C and humidity of 55 ± 5% and with a 12-h light/12-h dark cycle. The rats were randomly divided into four groups of eight animals. The model control (CON) group was administered DEN (ToYongBio, Shanghai, China) and saline; the GLU group was administered DEN and glucuronolactone (GLU) (ToYongBio); the GLU+HYD group was administered DEN, GLU, and hydrocortisone (HYD) (ToYongBio); and the GLU+ASP group was administered DEN, GLU, and aspirin (ASP) (ToYongBio). In this study, 0.01% DEN was administered in drinking water (100mg/L), and the other agents (GLU at 10 mg/kg, HYD at 6 mg/kg, and ASP at 10 mg/kg) were administered by gavage once daily from the study commencement until the animal was sacrificed (week 12 or week 16). The rats were examined periodically, and their body weights were recorded every other week. All animal procedures were carried out according to the Guidelines for the Welfare and Use of Animals in Cancer Research and were approved by the Animal Care and Use Committee of Southern Medical University.
Three animals in each group were sacrificed by cervical dislocation under deep anesthesia with 2% pentobarbital sodium (3 mL/kg i.p.) at the end of week 12. Since one animal was lost from the CON, GLU and ASP+GLU groups, respectively, before the end of week 16, four animals in the CON, GLU and ASP+GLU groups and five in the GLU+HYD group were sacrificed by cervical dislocation under deep anesthesia with 2% pentobarbital sodium (3 mL/kg i.p.) at week 16. Liver specimens were removed carefully after the rats were sacrificed. The liver surface was observed macroscopically for obvious nodules (see below). Some hepatic nodules on the liver surface and paraneoplastic tissue were fixed in 4% paraformaldehyde solution for histopathological and immunohistochemical examinations. The remaining liver tissue was quickly frozen in liquid nitrogen before storing at −80°C for Western blot assays.
Liver Morphology And Histopathology
Macroscopically visible hepatic nodules on the liver surface were recorded. For histological examination, fixed liver samples were processed and embedded in paraffin blocks. Tissue block sections of 5μm were cut using a paraffin microtome (RM2125 RTS, Leica, Wetzlar, Germany) and processed routinely for staining with hematoxylin and eosin. The liver pathological changes including pathological grade (Edmondson-Steiner grade), tumor maximum diameter and nodule number on liver surface were measured by three independent pathologists in a double-blind manner.39
Immunohistochemistry
Numbers of macrophages and M2 polarized macrophages and density of microvessels (MVD) in the paraneoplastic tissue microenvironment were evaluated using immunohistochemistry and image analyses, with CD68-positive cells being counted as macrophages, CD206-positive cells as M2 polarized macrophages, and CD34-positive vessels being measured for MVD. For immunostaining, paraffin sections of 3-μm thick were deparaffinized and rehydrated and were boiled in 0.01 M sodium citrate buffer (pH 6.0) for 3 min (CD206) or 8 min (CD68 and CD34) in a microwave oven for antigen retrieval. After blocking endogenous peroxidase with 0.3% H2O2 in methanol, the sections were incubated with anti-CD68 antibody (Abcam, Cambridge, UK; 1:1500), anti-CD206 antibody (Abcam; 1:1000), and anti-CD34 antibody (Abcam; 1:1500) at 4°C overnight. After incubation with a horseradish-peroxidase-conjugated secondary antibody (ZhongShanJinQiao, Beijing, China) at 37°C for 2 h, the immunostaining signal was developed with 3, 3′-diaminobenzidine tetrachloride (ZhongShanJinQiao) for 5 min. The sections were counterstained with hematoxylin, and the stained tissue sections were reviewed under a light microscope (Nikon ECLIPSE Ni-U, Tokyo, Japan).
Macrophages and M2 polarized macrophages were counted in 10 random fields (400×) of paraneoplastic tissue of each liver, respectively. The mean value of CD68-positive cells per field (400×) was recorded as macrophage density, and CD206-positive cells per field (400×) as M2 polarized macrophage density.16 To analyze MVD, five representative fields (200×) in paraneoplastic tissue from two random sections of each liver were selected. CD34-positive vessels were counted with a magnification of 200 in the representative fields and MVD was assessed as the mean value of CD34-positive vessels per measured field (200×).40
Western Blotting
To examine treatment effects on expression of inflammatory and anti-inflammatory cytokines, inflammation regulatory kinase/transcription factor(s), growth factors and the major angiogenic factor, protein expression levels of tumor necrosis factor (TNF)-α, p38 mitogen-activated protein kinase (p38 MAPK, p38), phosphorylated p38 MAPK (p-p38), interleukin (IL)-10, nuclear factor (NF)-κB, hepatocyte growth factor (HGF), and transforming growth factor (TGF)-β1, and vascular endothelial growth factor (VEGF) in the precancerous hepatic tissue were analysed using Western blotting after 12 and 16 weeks of HCC induction. The precancerous hepatic tissue was homogenized using SDS-PAGE lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 0.3% Triton X-100, 0.03% SDS, 1 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 mM PMSF) at 4°C. The homogenates were centrifuged at 12 000 g for 30 min. The protein extracts were quantified using a bicinchoninic acid assay kit (Pierce Biotechnology, Rockford, IL, USA).
For Western blotting analyses, 50 μg (for p38, p-p38, NF-κB, IL-10, HGF, TGF-β1 and VEGF) and 75 μg (for TNF-α) of protein lysates were resolved on 10% (p38, p-p38, NF-κB, IL-10, HGF, TGF-β and VEGF) and 15% (TNF-α) SDS-PAGE, respectively, and electrotransferred to polyvinylidene fluoride membranes (Immobilon P; Millipore, Bedford, MA). After being blocked in 5% nonfat dry milk in tris-buffered saline (pH 7.5), the membranes were immunoblotted overnight at 4°C with anti-TNF-α (1:1500; Abcam), anti-p38 (1:500; Cell Signalling, Danvers, MA), anti-p-p38 (1:500; Cell Signalling), anti-NF-κB (1:3000; Proteintech, Chicago, IL), anti-IL-10 (1:800; Abcam), anti-HGF (1:2000; Abcam), anti-TGF-β1 (1:2000) and anti-VEGF (1:250; Cell Signalling) followed by their respective secondary antibodies. Signals were detected using enhanced chemiluminescence (Pierce, Rockford, IL). The integral optical density of the bands was determined using Gel-Pro Analyzer 4.0 software (Media Cybernetics, Rockville, MD). The association between levels of these cytokines/factors in the hepatic microenvironment and HCC was analyzed.
Statistics
The in vivo data were from n=3–5 rats/group. SPSS 19.0 (IBM SPSS Inc. Chicago, IL, USA) was used to evaluate data. One-way analysis of variance followed by LSD t-test were used to analyze differences between groups, and 2-tailed significance was determined. Results are presented as the mean ±standard deviation (SD) for all parameters measured. P<0.05 was considered statistically significant.
Results
Hydrocortisone Or Aspirin Exposure Does Not Affect Body Weight Changes Of HCC Rats
The body weights of each group were measured at 12- and 16-weeks post-HCC induction (Suppl file 1). No significant differences of body weight were seen among groups at each time point. At the end of Week 12, the body weight of Control was 287.13±24.53 g, GLU 293.77±23.01 g, GLU+HYD 285.14±13.88 g, and GLU+ASP 293.06±23.01 g. By the end of the study (Week 16), the body weight of Control was 290.07±23.38 g, GLU 305.15±18.37 g, GLU+HYD 294.74±6.29 g, and GLU+ASP 305.40±19.46 g. These data suggested that neither low dose hydrocortisone exposure nor aspirin exposure influenced the growth and development of rats with HCC.
Hydrocortisone Treatment Suppresses Morphological And Pathological Changes After HCC Induction
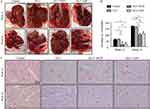
To assess the effect of each intervention on HCC progression, by Week 12 and Week 16 the numbers of HCC nodules and liver cirrhosis levels were measured macroscopically and histopathologically (Suppl file 2). Liver cirrhosis and HCC nodules were obvious in both Week 12 and Week 16 (Figure 1A). The numbers of HCC nodules in the GLU+HYD and GLU+ASP groups showed a marked decline when compared with those in the Control and GLU groups at each time post-HCC induction (Figure 1B). All groups developed HCC in Week 12 after HCC induction (Figure 1C). While Grade II HCC was seen in the Control and GLU groups in Week 12 (Table 1), only Grade I HCC was found in the GLU+HYD and GLU+ASP groups in Week 12 (Table 1). In Week 16, all groups developed both Grade I and Grade II HCC (Table 1). The maximum diameters of hepatic nodules in all groups were also measured at different weeks, and the maximum diameter in GLU+HYD was reduced when compared to other groups (Table 1).
 |
Table 1 Pathological Results Of Liver In Each Group At 12 And 16 Weeks Post-HCC Induction |
Hydrocortisone Treatment Reduces Density Of Microvessels And Expression Of Angiogenic Growth Factor VEGF At Week 12 After HCC Induction
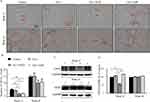
The local CD34 positive vessels were measured to analyze the effect of each intervention on angiogenesis in paraneoplastic tissue (Suppl file 3). Vessels formed by CD34 positive endothelial cells were found increased with time (Figure 2A), and the MVDs were found reduced in the GLU+HYD and GLU+ASP groups when compared to GLU and Control groups in the paraneoplastic tissue in Week 12, although the reductions in the two treatment groups at Week 16 were not statistically significant (Figure 2B). These data indicate that aspirin or hydrocortisone treatment inhibits early paraneoplastic angiogenesis in HCC carcinogenesis.
Interestingly, at Week 12, the protein expression level of the major angiogenic growth factor, VEGF, was found to be lower in the GLU+HYD group when compared to the other groups as shown by Western blotting analyses (Figure 2C and D). At Week 16, no differences of VEGF expression were found among the groups.
Hydrocortisone Treatment Reduces Paraneoplastic Macrophage Presence, Inflammatory And Anti-Inflammatory Cytokine And NF-B Expression, And P38 Activation At Week 12 After HCC Induction
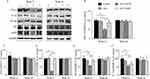
The paraneoplastic CD68-positive cells were measured by immunohistochemistry as total macrophages after HCC induction (Figure 3A). At Week 12, densities of the CD68-positive cells (macrophages) were similar in Control, GLU and GLU+ASP treatment groups, but comparatively were decreased significantly in the GLU+HYD group (Figure 3A). At Week 16, densities of CD68-positive cells were not different among all groups.
The paraneoplastic CD206-positive cells were measured as M2 polarized macrophages (Figure 3B). The treatment effects on densities of CD206-positive cells had the similar trends as for CD68 positive cells. At Week 12, densities of the CD206-positive cells were decreased significantly in the GLU+HYD group compared to the Control, GLU and GLU+ASP groups (Figure 3B). At Week 16, densities of CD206-positive cells were similar among all groups.
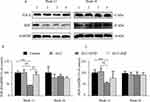
Western blotting analyses (Figure 4A) showed that, at Week 12 (Suppl file 4), levels of paraneoplastic TNF-α (Figure 4B), phosphorylated p38 (p-p38) (Figure 4D), NF-κB (Figure 4E) and IL-10 (Figure 4F) were reduced in the GLU+HYD group when compared to the Control, GLU and GLU+ASP groups, although levels of total p38 (Figure 4C) were not significantly different between all groups. At Week 16 (Suppl file 5), no significant differences in levels of paraneoplastic TNF-α, p38, p-p38, NF-κB and IL-10 were seen among all groups. These results indicate that hydrocortisone treatment inhibits the inflammation in paraneoplastic tissue sufficiently during the first 12 weeks of HCC induction.
Hydrocortisone Treatment Reduces Paraneoplastic Expression Of TGF-1 And HGF At Week 12 After HCC Induction
Furthermore, Western blotting analyses were carried out to examine treatment effects on expression of TGF-β1 and HGF, two paraneoplastic growth factors induced by chronic inflammation and known to be involved in HCC progression (Figure 5). At Week 12 (Suppl file 4), levels of the paraneoplastic TGF-β1 (Figure 5A and B) and HGF (Figure 5A and C) were reduced in the GLU+HYD group when compared to the Control, GLU and GLU+ASP groups. At Week 16 (Suppl file 5), no significant differences in levels of paraneoplastic TGF-β1 and HGF were seen among all groups. These results indicate that hydrocortisone treatment inhibits expression of growth factors TGF-β1 and HGF in paraneoplastic tissue sufficiently during the first 12 weeks of HCC induction.
Discussion
Previously, the contribution of local chronic inflammation to carcinogenesis has been noticed clinically, as chronic hepatitis virus infection was found correlated with HCC progression,41 anti-inflammatory treatment was reported to attenuate carcinogenesis35,36,42 and aspirin treatment can reduce the risk of developing HCC.36 However, the potential of chronic inflammation as a therapeutic target to halt the HCC progression is still obscure. Moreover, while glucocorticoids (GCs) have been shown to enhance cell proliferation and inhibit apoptosis for most mammalian cell types,43,44 there have not been sufficient in vitro tests of GCs on cancer cells to indicate the potential benefit of GCs on carcinogenesis. Furthermore, while the use of GCs may be harmful for skin cancer, lymphoma and breast cancer,45,46 effects of GCs on the progression of HCC have not been fully investigated by in vivo studies. Therefore, in the current study, to investigate whether different types of anti-inflammatory agents attenuate HCC progression, treatment effects of hydrocortisone vs aspirin in conjunction with the commonly used hepatoprotective medication, glucuronolactone, on inflammation and angiogenesis in hepatic paraneoplastic tissue were measured at different time points in a rat HCC model. We demonstrated that hydrocortisone treatment can reduce macrophage polarization, expression of inflammatory and anti-inflammatory cytokines, activation of inflammation mediator p38, and angiogenesis in paraneoplastic tissue, and attenuate early HCC progression. This study has provided strong evidence to support the preventive role of anti-inflammatory agents on carcinogenesis in vivo, and this study represents the first in vivo evaluation of hydrocortisone treatment in HCC progression showing some beneficial effect.
In 2006, Zhang et al47 reported that dexamethasone inhibited apoptosis of HCC cells and the release of inflammatory cytokines by fibroblasts in vitro. In our study, hydrocortisone treatment was found to reduce the early paraneoplastic inflammation and microvessel density and to attenuate HCC progression at Week 12 before advanced solid tumors developed without showing obvious adverse effects (Figures 1–5). Nevertheless, at Week 16 post-HCC induction, both hydrocortisone and aspirin showed no obviously protective effect on the HCC pathological grade and morphological results in rats, which suggests that anti-inflammatory treatment plays a preventive role rather than an anti-tumorigenesis role (Figure 1). While the poor blood supply and more dependence on the environment in early HCC colonization could explain the result partially,48 this phenomenon still needs to be investigated further in future studies.
The correlation between paraneoplastic inflammation and HCC was proved again in our study. Increased levels of TNF-α and p38 phosphorylation indicate the increased inflammatory level in the paraneoplastic microenvironment, which is consistent with a previous report.49 The paraneoplastic inflammation was inhibited significantly by hydrocortisone treatment during the early progression of HCC before Week 12 (Figures 3 and 4). Unfortunately, the paraneoplastic inflammation surrounding advanced HCC was found to be resistant to hydrocortisone treatment and remained unresolved when the animals received continuous DEN. In clinical practice, glucocorticoid-resistant inflammation still puzzles the physicians.50 However, results from the current study suggest that hydrocortisone at this dosage inhibits HCC induction by reducing paraneoplastic inflammation but it does not have an anti-cancer activity after advanced solid tumors have formed.
The chronic inflammation in paraneoplastic tissue has been considered an important contributing factor in HCC progression. However, the mechanisms and relevant signaling molecules of the macrophage modulation are still largely unknown. Classic macrophages are considered to be essential in chronic hepatic inflammation and therefore contribute to hepatic carcinogenesis.51 The modulatory role that paraneoplastic macrophages, especially M2 polarized macrophages, play in chronic inflammation has been demonstrated in recent investigations.52,53 In a rodent model, Ambade et al52 demonstrated that macrophages were predominant in livers of alcohol-DEN -treated animals, and that both total macrophages and M2 polarized macrophages were present at higher densities in paraneoplastic tissues when compared to healthy liver. Moreover, M2 polarized macrophages promote liver angiogenesis and accelerate hepatic carcinogenesis.54,55 Consistent with these studies, our results suggest that hydrocortisone treatment inhibits paraneoplastic macrophage polarization and reduces release of associated cytokines from M1 (TNF-α) (Figure 4B) and M2 (IL-10) (Figure 4F) polarized macrophages and TNF-α stimulated fibroblasts (HGF and TGF-β1) (Figure 5), as well as downregulates inflammation-regulatory transcription factor NF-κB (Figure 4E) and MAPK p38 activation (Figure 4D). Previous studies have suggested that the overexpressed inflammatory cytokine (TNF-α56), anti-inflammatory cytokine (IL-1052), growth factors HGF57–59 and TGF-β1,60,61 and NF-κB62–64 in the hepatic microenvironment could form a vicious cascade to promote HCC progression.
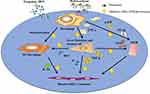
Angiogenesis in paraneoplastic tissue plays important roles in the vicious cascade to promote HCC progression. An increased microvessel density (MVD) in paraneoplastic tissue suggests initial transformation in carcinogenesis.21 MVD and the major angiogenic cytokine, VEGF, have been shown to be obviously regulated by TGF-β and NF-κB.20,63 The current study showed that hydrocortisone treatment suppresses MVD (Figure 2A and B) and consistently the expression of VEGF (Figure 2C). These suggest that when hydrocortisone is administrated, the cascade may have been controlled and the early HCC colonization may have been inhibited. Although the regulation of macrophages and macrophage polarization by anti-inflammatory agents still need to be investigated further in hepatic carcinogenesis in future studies, a potential mechanism for hydrocortisone treatment-induced attenuated HCC progression can be proposed from data of the current study: hydrocortisone attenuates HCC progression by suppressing macrophage-mediated chronic inflammation and angiogenesis in paraneoplastic tissue (Figure 6).
Previously, aspirin was reported to prevent HCC progression, thus, aspirin was used as a positive anti-inflammatory control in the current study.29 We showed that both aspirin and hydrocortisone had an inhibitory effect on MVD (Figure 2) and HCC pathological deterioration (Table 1 and Figure 1). However, aspirin showed no obvious inhibitory effects on the total number of macrophages (Figure 3A) and M2 polarized macrophages (Figure 3B). Moreover, aspirin treatment showed no obvious inhibitory effects on expression of the tested cytokines (Figure 4B–F). These findings suggest that aspirin did not influence M2 polarization and macrophage-related inflammation. Although hydrocortisone had an effect similar to that of aspirin on HCC progression, hydrocortisone exhibited a greater anti-inflammatory effect in the paraneoplastic tissue. On the other hand, our study suggested that aspirin influences the angiogenesis by a pathway other than the TGF-β and VEGF pathways, and the result is consistent with the report from Etulain et al15. Thus, our results suggest that hydrocortisone and aspirin have different mechanisms in attenuating carcinogenesis.
Clinically, the use of GCs alone for hepatitis without assistance of liver protection and anti-viral medication are relatively dangerous due to significant side effects.65 According to The Merck Index, glucuronolactone is used as a detoxicant,66 which is the most commonly used hepatoprotective medication in some countries such as China.67 Therefore, the glucuronolactone was used for this manner in the current study. We showed that, while the single use of glucuronolactone showed no effect on controlling HCC progression, the combination uses of hydrocortisone with glucuronolactone showed protective effect in the early stage of HCC.
Although our results suggest that hydrocortisone attenuates HCC progression through inhibiting paraneoplastic inflammation and angiogenesis, the precise pathways for this effect in HCC remain largely unclear. However, our study provides evidence to indicate the regulatory role of paraneoplastic macrophages and angiogenesis and their associated cytokines/growth factors in the anti-inflammatory effects of hydrocortisone and its inhibitory effect on HCC progression. Thus, the impact of hydrocortisone on macrophage activation and polarization and angiogenesis will be investigated further in our future work.
Conclusion
While previously inflammation has been implicated in hepatocellular carcinogenesis, and treatment with long-acting glucocorticoid dexamethasone was shown to prevent liver carcinogenesis in mice, the current study has demonstrated that treatment with hydrocortisone (a short-acting glucocorticoid clinically known to have fewer adverse effects compared to the long-acting glucocorticoids) can suppress paraneoplastic inflammation and angiogenesis and attenuate HCC progression at the early stage in rats. The present study has demonstrated that, in a rat model of DEN-induced HCC, hydrocortisone treatment can inhibit the chronic paraneoplastic inflammation induced by macrophages and angiogenesis and thus attenuate the early-stage HCC progression. This study has provided evidence for the strong correlation between paraneoplastic inflammation/angiogenesis and HCC progression. This study shows benefits and supports potential clinical use of hydrocortisone in attenuating early HCC progression.
Abbreviations
HCC, hepatocellular carcinoma; TNF, tumor necrosis factor; p-p38, phosphorylated p38; NF, nuclear factor; IL, interleukin; HGF, hepatocyte growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor; GCs, glucocorticoids.
Ethics Approval And Consent To Participate
All animal procedures were carried out according to the Guidelines for the Welfare and Use of Animals in Cancer Research and were approved by the Animal Care and Use Committee of Southern Medical University [approval No. 2015-026].
Availability Of Data And Material
All data generated or analyzed during this study are included in this published article.
Acknowledgements
This work was supported by the Medical Scientific Research Foundation of Guangdong Province (B2013261) and The Basic Research Start-up Foundation of Southern Medical University (QD201403). LW is supported by Australian National Health and Medical Research Council (NHMRC) Fellowship (1158402), and CJX was supported by Australian NHMRC Senior Research Fellowship (1042105). The funding bodies had no roles in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
2. Tanaka H, Tanaka M, Chen W, et al. Proposal for a cooperative study on population-based cancer survival in selected registries in East Asia. Asian Pac J Cancer Prev. 2009;10(6):1191–1198.
3. de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62(4):1190–1200.
4. Kassebaum NJ, Lopez AD, Murray CJ, Lozano R. A comparison of maternal mortality estimates from GBD 2013 and WHO. Lancet. 2014;384(9961):2209–2210.
5. Knolle P, Schlaak J, Uhrig A, Kempf P. Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22(2):226–229.
6. Lin Y, Yang X, Liu W, et al. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene. 2017;36(25):3599–3608.
7. Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol. 2015;62(6):1420–1429.
8. Shlomai A, de Jong YP, Rice CM. Virus associated malignancies: the role of viral hepatitis in hepatocellular carcinoma. Semin Cancer Biol. 2014;26:78–88.
9. Cubero FJ. Shutting off inflammation: A novel switch on hepatic stellate cells. Hepatology. 2016;63(4):1086–1089.
10. Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46(2):590–597.
11. Liu XL, Li FQ, Liu LX, Li B, Zhou ZP. TNF-alpha, HGF and macrophage in peritumoural liver tissue relate to major risk factors of HCC recurrence. Hepatogastroenterology. 2013;60(125):1121–1126.
12. Ohtsuki T, Kimura K, Tokunaga Y, et al. M2 macrophages play critical roles in progression of inflammatory liver disease in hepatitis C virus transgenic mice. J Virol. 2015;90(1):300–307.
13. Pandey P, Rahman M, Bhatt PC, et al. Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin. Nanomedicine (Lond). 2018;13(8):849–870.
14. Mendes F, Domingues C, Rodrigues-Santos P, et al. The role of immune system exhaustion on cancer cell escape and anti-tumor immune induction after irradiation. Biochim Biophys Acta. 2016;1865(2):168–175.
15. Etulain J, Fondevila C, Negrotto S, Schattner M. Platelet-mediated angiogenesis is independent of VEGF and fully inhibited by aspirin. Br J Pharmacol. 2013;170(2):255–265.
16. Murri AM, Hilmy M, Bell J, et al. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br J Cancer. 2008;99(7):1013–1019.
17. Claus M, Dychus N, Ebel M, et al. Measuring the immune system: a comprehensive approach for the analysis of immune functions in humans. Arch Toxicol. 2016;90(10):2481–2495.
18. Afzal M, Kazmi I, Khan R, et al. Thiamine potentiates chemoprotective effects of ibuprofen in DEN induced hepatic cancer via alteration of oxidative stress and inflammatory mechanism. Arch Biochem Biophys. 2017;623–624:58–63.
19. Verma A, Singh D, Anwar F, Bhatt PC, Al-Abbasi F, Kumar V. Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kB pathway. Inflammopharmacology. 2018;26(1):133–146.
20. Krishnan S, Szabo E, Burghardt I, Frei K, Tabatabai G, Weller M. Modulation of cerebral endothelial cell function by TGF-beta in glioblastoma: VEGF-dependent angiogenesis versus endothelial mesenchymal transition. Oncotarget. 2015;6(26):22480–22495.
21. D’Alessio A, Proietti G, Lama G, et al. Analysis of angiogenesis related factors in glioblastoma, peritumoral tissue and their derived cancer stem cells. Oncotarget. 2016;7(48):78541–78556.
22. Peng SH, Deng H, Yang JF, et al. Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol. 2005;11(41):6521–6524.
23. Morise Z, Sugioka A, Fujita J, Hoshimoto S, Kato T, Ikeda MS. 1 plus cisplatin combination therapy for the patients with primary liver carcinomas. Hepatogastroenterology. 2007;54(80):2315–2318.
24. Zou H, Zhu XX, Zhang GB, Ma Y, Wu Y, Huang DS. Silibinin: an old drug for hematological disorders. Oncotarget. 2017;8:89307.
25. Kumar V, Bhatt PC, Rahman M, et al. Fabrication, optimization, and characterization of umbelliferone beta-D-galactopyranoside-loaded PLGA nanoparticles in treatment of hepatocellular carcinoma: in vitro and in vivo studies. Int J Nanomedicine. 2017;12:6747–6758.
26. Rahman M, Al-Ghamdi SA, Alharbi KS, et al. Ganoderic acid loaded nano-lipidic carriers improvise treatment of hepatocellular carcinoma. Drug Deliv. 2019;26(1):782–793.
27. Kawano Y, Miyanishi K, Takahashi S, et al. Hepatitis C virus reactivation due to antiemetic steroid therapy during treatment of hepatocellular carcinoma. J Infect Chemother. 2017;23(5):323–325.
28. Wang J, Wang R, Wang H, et al. Glucocorticoids suppress antimicrobial autophagy and nitric oxide production and facilitate mycobacterial survival in macrophages. Sci Rep. 2017;7(1):982.
29. Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22(48):10512–10522.
30. Dunford EC, Riddell MC. The metabolic implications of glucocorticoids in a high-fat diet setting and the counter-effects of exercise. Metabolites. 2016;6(4):44.
31. Maceiras AR, Almeida SCP, Mariotti-Ferrandiz E, et al. T follicular helper and T follicular regulatory cells have different TCR specificity. Nat Commun. 2017;8:15067.
32. Samuels AL, Heng JY, Beesley AH, Kees UR. Bioenergetic modulation overcomes glucocorticoid resistance in T-lineage acute lymphoblastic leukaemia. Br J Haematol. 2014;165(1):57–66.
33. Ma R, Zhang W, Tang K, et al. Switch of glycolysis to gluconeogenesis by dexamethasone for treatment of hepatocarcinoma. Nat Commun. 2013;4:2508.
34. Ren H, Liang D, Jiang X, et al. Variance of spinal osteoporosis induced by dexamethasone and methylprednisolone and its associated mechanism. Steroids. 2015;102:65–75.
35. Lee PC, Yeh CM, Hu YW, et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann Surg Oncol. 2016;23(Suppl 5):874–883.
36. Li JH, Wang Y, Xie XY, et al. Aspirin in combination with TACE in treatment of unresectable HCC: a matched-pairs analysis. Am J Cancer Res. 2016;6(9):2109–2116.
37. Jin X, Zhao T, Shi D, Ye MB, Yi Q. Protective role of fucoxanthin in diethylnitrosamine-induced hepatocarcinogenesis in experimental adult rats. Drug Dev Res. 2019;80(2):209–217.
38. Fathy AH, Bashandy MA, Bashandy SAE, Mansour AM, Elsadek B. Sequential analysis and staging of a diethylnitrosamine-induced hepatocellular carcinoma in male Wistar albino rat model. Can J Physiol Pharmacol. 2017;95(12):1462–1472.
39. Zhang CL, Zeng T, Zhao XL, Yu LH, Zhu ZP, Xie KQ. Protective effects of garlic oil on hepatocarcinoma induced by N-nitrosodiethylamine in rats. Int J Biol Sci. 2012;8(3):363–374.
40. Wang WQ, Liu L, Xu HX, et al. The combination of HTATIP2 expression and microvessel density predicts converse survival of hepatocellular carcinoma with or without sorafenib. Oncotarget. 2014;5(11):3895–3906.
41. Nosratabadi R, Alavian SM, Zare-Bidaki M, Shahrokhi VM, Arababadi MK. Innate immunity related pathogen recognition receptors and chronic hepatitis B infection. Mol Immunol. 2017;90:64–73.
42. Lee M, Chung GE, Lee JH, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66:1556–1569.
43. He XL, Xing Y, Gu XZ, et al. The synthesis and antitumor activity of lithocholic acid and its derivatives. Steroids. 2017;125:54–60.
44. Silva LN, Alves SL, Venugopal J, et al. Differences of lipid membrane modulation and oxidative stress by digoxin and 21-benzylidene digoxin. Exp Cell Res. 2017;359:291–298.
45. Malyarenko OS, Dyshlovoy SA, Kicha AA, et al. The inhibitory activity of luzonicosides from the starfish echinaster luzonicus against human melanoma cells. Mar Drugs. 2017;15(7):227.
46. McNamara KM, Kannai A, Sasano H. Possible roles for glucocorticoid signalling in breast cancer. Mol Cell Endocrinol. 2017;466:38–50.
47. Zhang C, Kolb A, Mattern J, et al. Dexamethasone desensitizes hepatocellular and colorectal tumours toward cytotoxic therapy. Cancer Lett. 2006;242(1):104–111.
48. Kudo M, Tochio H. Intranodular blood supply correlates well with biological malignancy grade determined by tumor growth rate in pathologically proven hepatocellular carcinoma. Oncology. 2008;75(Suppl 1):55–64.
49. Zhou FH, Foster BK, Zhou XF, Cowin AJ, Xian CJ. TNF-alpha mediates p38 MAP kinase activation and negatively regulates bone formation at the injured growth plate in rats. J Bone Miner Res. 2006;21(7):1075–1088.
50. Keenan CR, Radojicic D, Li M, Radwan A, Stewart AG. Heterogeneity in mechanisms influencing glucocorticoid sensitivity: the need for a systems biology approach to treatment of glucocorticoid-resistant inflammation. Pharmacol Ther. 2015;150:81–93.
51. Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66(6):1300–1312.
52. Ambade A, Satishchandran A, Saha B, et al. Hepatocellular carcinoma is accelerated by NASH involving M2 macrophage polarization mediated by hif-1alphainduced IL-10. Oncoimmunology. 2016;5(10):e1221557.
53. Luo HL, Chen J, Luo T, et al. Downregulation of macrophage-derived T-UCR uc.306 associates with poor prognosis in hepatocellular carcinoma. Cell Physiol Biochem. 2017;42(4):1526–1539.
54. Capece D, Fischietti M, Verzella D, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204.
55. Kono H, Fujii H, Furuya S, et al. Macrophage colony-stimulating factor expressed in non-cancer tissues provides predictive powers for recurrence in hepatocellular carcinoma. World J Gastroenterol. 2016;22(39):8779–8789.
56. Nong Y, Wu D, Lin Y, Zhang Y, Bai L, Tang H. Tenascin-C expression is associated with poor prognosis in hepatocellular carcinoma (HCC) patients and the inflammatory cytokine TNF-alpha-induced TNC expression promotes migration in HCC cells. Am J Cancer Res. 2015;5(2):782–791.
57. Jiang J, Ye F, Yang X, et al. Peri-tumor associated fibroblasts promote intrahepatic metastasis of hepatocellular carcinoma by recruiting cancer stem cells. Cancer Lett. 2017;404:19–28.
58. Ilangumaran S, Villalobos-Hernandez A, Bobbala D, Ramanathan S. The hepatocyte growth factor (HGF)-MET receptor tyrosine kinase signaling pathway: diverse roles in modulating immune cell functions. Cytokine. 2016;82:125–139.
59. Pedraza-Brindis EJ, Sanchez-Reyes K, Hernandez-Flores G, et al. Culture supernatants of cervical cancer cells induce an M2 phenotypic profile in THP-1 macrophages. Cell Immunol. 2016;310:42–52.
60. Chen J, Liu WB, Jia WD, et al. Embryonic morphogen nodal is associated with progression and poor prognosis of hepatocellular carcinoma. PLoS One. 2014;9(1):e85840.
61. Li DP, Fan J, Wu YJ, Xie YF, Zha JM, Zhou XM. MiR-155 up-regulated by TGF-beta promotes epithelial-mesenchymal transition, invasion and metastasis of human hepatocellular carcinoma cells in vitro. Am J Transl Res. 2017;9(6):2956–2965.
62. Kang HJ, Chung DH, Sung CO, et al. SHP2 is induced by the HBx-NF-kappaB pathway and contributes to fibrosis during human early hepatocellular carcinoma development. Oncotarget. 2017;8(16):27263–27276.
63. Nam SY, Ko YS, Jung J, et al. A hypoxia-dependent upregulation of hypoxia-inducible factor-1 by nuclear factor-kappaB promotes gastric tumour growth and angiogenesis. Br J Cancer. 2011;104(1):166–174.
64. Kumar V, Bhatt PC, Rahman M, Al-Abbasi FA, Anwar F, Verma A. Umbelliferon-alpha-d-glucopyranosyl-(2(I)–>1(II))-alpha-Dglucopyranoside ameliorates Diethylnitrosamine induced precancerous lesion development in liver via regulation of inflammation, hyperproliferation and antioxidant at pre-clinical stage. Biomed Pharmacother. 2017;94:834–842.
65. Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365(19):1781–1789.
66. Petit A, Karila L, Lejoyeux M. [Abuse of energy drinks: does it pose a risk?]. Presse Med. 2015;44(3):261–270.
67. Zhang Q, Zhong FY, Wu M, Zhang XP. Efficacy of Jian’ganle () versus Hugan Pian (), glucuronolactone and reduced glutathione in prevention of antituberculosis drug-induced liver injury. J Huazhong Univ Sci Technolog Med Sci. 2014;34(3):450–455.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.