Back to Journals » Drug Design, Development and Therapy » Volume 14
Hyaluronic Acid Capped, Irinotecan and Gene Co-Loaded Lipid-Polymer Hybrid Nanocarrier-Based Combination Therapy Platform for Colorectal Cancer
Authors Wang Z, Zang A, Wei Y, An L, Hong D, Shi Y, Zhang J, Su S, Fang G
Received 8 September 2019
Accepted for publication 5 February 2020
Published 12 March 2020 Volume 2020:14 Pages 1095—1105
DOI https://doi.org/10.2147/DDDT.S230306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Zhiyu Wang, Aimin Zang, Yaning Wei, Lin An, Dan Hong, Yan Shi, Jingnan Zhang, Shenyong Su, Guotao Fang
Hebei Key Laboratory of Cancer Radiotherapy and Chemotherapy, Department of Medical Oncology, Affiliated Hospital of Hebei University, Baoding 071000, People’s Republic of China
Correspondence: Guotao Fang
Department of Medical Oncology, Affiliated Hospital of Hebei University, No. 648 Dongfeng East Road, Lianchi District, Baoding 071000, Hebei Province, People’s Republic of China
Tel +86-312-5983042
Email [email protected]
Background: The current approach for treating colorectal cancer favors the use of drug and gene combination therapy, and targeted nano-systems are gaining considerable attention for minimizing toxicity and improving the efficacy of anticancer treatment. The aim of this study was to develop ligand-modified, irinotecan and gene co-loaded lipid-polymer hybrid nanocarriers for targeted colorectal cancer combination therapy.
Methods: Hyaluronic acid modified, irinotecan and gene co-loaded LPNs (HA-I/D-LPNs) were prepared using a solvent-evaporation method. Their average size, zeta potential, drug and gene loading capacity were characterized. The in vitro and in vivo gene transfection and anti-tumor ability of this nano-system were evaluated on colorectal cancer cells and mice bearing colorectal cancer model.
Results: HA-I/D-LPNs had a size of 182.3 ± 5.1, over 80% drug encapsulation efficiency and over 90% of gene loading capacity. The peak plasma concentration (Cmax) and half-life (T1/2) achieved from HA-I/D-LPNs were 41.31 ± 1.58 μg/mL and 12.56 ± 0.67 h. HA-I/D-LPNs achieved the highest tumor growth inhibition efficacy and the most prominent transfection efficiency in vivo.
Conclusion: HA-I/D-LPNs exhibited the most remarkable tumor inhibition efficacy and best gene transfection efficiency in the tumor, which could prove the effects of the drug and gene combination therapy.
Keywords: colorectal cancer, combination therapy, lipid-polymer hybrid nanoparticles, hyaluronic acid, irinotecan
Introduction
Colorectal cancer (CRC) is the world’s fourth most deadly cancer which causes the death of 700,000 people every year.1–3 The current approaches to treating CRC favor the use of combination cytotoxic therapy. First-line treatments include the doublet cytotoxic combinations of fluorouracil, leucovorin, irinotecan, leucovorin, and oxaliplatin.4,5 Irinotecan, an extract from the Chinese tree Camptotheca acuminate, was first approved in the United States in 1996 for the treatment of metastatic CRC refractory to 5-fluorouracil (5-FU).6 Clinical trials have shown that irinotecan has a survival advantage in patients with metastatic colorectal cancer, making irinotecan one of the most important drugs in the management of metastatic CRC.7 Unfortunately, chemotherapy using irinotecan may be limited by multidrug resistance, inability to have a selective distribution, or other adverse effects.8
 |
Table 1 Characterization of LPNs (Mean ± SD, n=6) |
 |
Table 2 Plasma Pharmacokinetic Parameters (Mean ± SD, n=8) |
The application of nano-systems offers new prospects for the effective therapy of CRC.9 Targeted nano-systems could utilize the surface-modified ligands, which could interact with the expressed molecules on the surface of tumor cells, enabling the effective delivery of antitumor agents.10 One of the targeted ligands applied is hyaluronic acid (HA), a natural anionic polysaccharide. The high binding affinity of HA to the CD44 receptors overexpressed on the tumor cells surfaces made it a promising moiety for anticancer drug delivery.11 It was reported that the prominent expression of CD44 has been considered as a marker of highly tumorigenic CRC cells and a component of the colorectal cancer stem cell gene signature that predicts disease recurrence in CRC patients. Therefore, CD44 is a potential therapeutic target for the treatment of CRC.12
Co-delivery of plasmid DNA and anti-cancer drugs using a single nano-system has emerged as a strategy to combine the advantages of gene therapy and chemotherapy.13 Currently used nano-systems for the co-delivery of genes and anticancer drugs include liposomes,14 lipid nanoparticles,15 polymeric nanoparticles,16 inorganic nanoparticles,17 and so on. Polymeric nanoparticles provide significant stability, high drug loading ability, controlled drug release, and excellent biocompatibility, thus was widely applied as gene and drug delivery system.18–20 In our previous study, colorectal cancer was treated with 5-Fluorouracil (5-FU) and enhanced GFP (EGFP) co-delivered polymeric nanoparticles and achieved effective combination results.21 Lipid nanoparticles/liposomes are biocompatible and can be used for the specific delivery of gene/drug to tumor tissues and also render them long circulatory lifetime.22 In the research carried out by Han et al, plasmid DNA and doxorubicin were co-delivered by solid lipid nanoparticles for lung cancer therapy.15
Lipid-polymer hybrid nanoparticles (LPNs) are core-shell nanoparticle structures comprising polymer cores and lipid shells, which combined the advantages of both polymeric nanoparticles and lipid nanoparticles/liposomes, particularly in terms of their physical stability and biocompatibility.23 In the present study, we would like to construct an HA modified, irinotecan and gene co-loaded LPNs for targeted colorectal cancer combination therapy. The in vitro and in vivo gene transfection and anti-tumor ability of this nano-system were evaluated.
Materials and Methods
Materials
pEGFP-N1 was obtained from Solarbio Life Sciences (Beijing, China). Egg yolk lecithin (EYL, PC-98T) was purchased from Kewpie Corporation (Tokyo, Japan). Poly (D,L-lactic-co-glycolic) (PLGA, 50:50, MW 20,000) was purchased from Shandong Institute of Medical Instrument (Shandong, China). HA-PEG-DSPE was provided by Xi’an Ruixi Biological Technology Co., Ltd. (Xi’an, China). Dimethyl sulfoxide (DMSO) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). SW480 cells (ATCC® CCL-228™, human Dukes’ type B, colorectal adenocarcinoma), and Human Umbilical Vein Endothelial Cells (HUVEC) were purchased from American Type Culture Collection (ATCC, Manassas, VA). BALB/c nude mice (6–8 weeks old) were purchased from Beijing Vital River Experimental Animal Technical Co., Ltd (Beijing, China). All the animal experiments complied with the ARRIVE guidelines and should be carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments and were approved by the Medical Ethics Committee of Hebei University.
Preparation of Hyaluronic Acid Modified, Irinotecan and Gene Co-Loaded LPNs
Hyaluronic acid modified, irinotecan and gene co-loaded LPNs (HA-I/D-LPNs, Figure 1A) were prepared using a solvent-evaporation method.23 Irinotecan (50 mg) and PLGA (200 mg) was dissolved in acetone (10 mL) (organic phase). Then, the pEGFP (100 mg), HA-PEG-DSPE (100 mg), and EYL (200 mg) were dispersed in water (90 mL) (aqueous phase). The organic phase was added dropwise into the constantly stirring aqueous phase to form an oil-in-water (o/w) emulsion. Then, the organic solvent was removed by stirring for another 4 h. Non-ligand modified, irinotecan and gene co-loaded LPNs (I/D-LPNs) were prepared using PEG-DSPE instead of HA-PEG-DSPE. Blank LPNs (LPNs) were prepared without adding irinotecan and pEGFP. Free irinotecan and gene solution (Free I/D) were prepared by mixing irinotecan (100 mg) with pEGFP (100 mg).
Characterization of the LPNs
Particle Size, Polydispersity, and Zeta Potential
LPNs were diluted by ultrapure water. Surface morphology of HA-I/D-LPNs was recorded by a transmission electron microscopy (JEM-1010, JEOL, Japan). The particle size, polydispersity, and zeta potential of the LPNs were determined by dynamic light scattering (DLS) using a NanoZS Zetasizer (Malvern Instruments Ltd., Malvern, UK).24
TEM pictures are Morphologies and size of NI/HA-DDP-PNPs and NI/HA-DDP-LPNs were recorded by a transmission electron microscopy.
Drug Entrapment Efficiency and Gene Loading Capacity
The irinotecan entrapment efficiency (EE) was determined by high-performance liquid chromatography method.25 Irinotecan was separated by a C18 column (200×4.6 mm, 5 μm). The mobile phase consisted of acetonitrile and water (40/60, v/v) with a flow rate of 1.0 mL/min. The fluorescence detection was set at 370 nm. Gene loading capacity (GL) of LPNs was determined by the PicoGreen-fluorometry method.26 pEGFP was isolated from LPNs by centrifugation (15,000 rpm, 30 min). The concentration of pEGFP was assessed by a fluorescence spectrophotometer at 480 nm.
Serum Stability
LPNs were added into the phosphate-buffered saline (PBS, pH 7.4) containing 10% FBS (v/v) at 37°C under gentle stirring to exam the stability in serum.27 LPNs formulas were incubated with 10% FBS (v/v) solution for 1, 2, 4, 8, 24, 48, or 72 h. At predetermined time points, 1 mL of each sample was taken out and the particle size and polydispersity were measured.
In vitro Drug and Gene Release
In vitro release of irinotecan and pEGFP from LPNs were performed in PBS (pH 7.4) by suspending samples in different Eppendorf® tubes and vortexed.28 The tubes were placed in a shaking water bath (100 rpm, 37°C). At predetermined time points, the LPNs suspensions were centrifuged (15,000 rpm, 30 min) and the amount of irinotecan and pEGFP released was analyzed by methods assay mentioned in “Drug entrapment efficiency and gene loading capacity” section.
Cell Culture
All cell lines were cultured in DMEM containing 5% fetal bovine serum and incubated at 37°C with 5% CO2 and 90–100% relative humidity. When the cells had reached 80–90% fusion, they were sub-cultured.
Cellular Uptake
Cellular uptake characteristics of LPNs were analyzed by flow cytometry.29 HA-I/D-LPNs and I/D-LPNs (200 mg/mL) were added at concentrations of into SW480 cells equilibrated with Hank’s buffered salt solution (HBSS) at 37°C. After incubation for 24 h, the cells were washed once with 1 mL of PBS; detached with trypsin/EDTA, centrifuged at 1500 rpm, 4°C for 5 min; resuspended in 300 μL of PBS and directly introduced to a flow cytometer.
In vitro Cytotoxicity
In vitro cytotoxicity of LPNs was estimated by MTT assay.29 Briefly, SW480 cells and HUVEC were seeded in 96-well plates (2, 000 cells/well) and incubated for 24 h. Drug and gene loaded LPNs and Free I/D at various concentrations were added and incubated for 72 h. MTT (5 mg/mL) was then added and incubated for another 4 h. The formazan crystals were dissolved in DMSO (100 μL) and the absorbance of the formed dye was measured at 570 nm using a microplate reader. Cell viability (%) was calculated according to the equation: (Absorbance of test cells)/(Absorbance of control)×100.
In vivo Pharmacokinetics and Anticancer Activity
Colorectal cancer-bearing mice were produced by injecting SW480 cells (106 in 100 µL 0.9% saline) to the dorsal side of the mice. When tumors reached 4–5 mm in diameter, mice were randomly divided into three groups. HA-I/D-LPNs (10 mg irinotecan per kg mice), I/D-LPNs (10 mg irinotecan per kg mice), and Free I/D (10 mg irinotecan per kg mice) were administered to the mice via the tail vein.30 Blood samples which were taken from the retro-orbital plexus at predetermined time points, centrifuged (4,000 rpm, 15 min) and plasma was collected and stored at −20°C until further analyzed by methods assay mentioned in “Drug entrapment efficiency and gene loading capacity” section.
For the anticancer activity evaluation, colorectal cancer-bearing mice were randomly divided into five groups. HA-I/D-LPNs (5 mg irinotecan, 10 mg pEGFP per kg mice), I/D-LPNs (5 mg irinotecan, 10 mg pEGFP per kg mice), LPNs, Free I/D (10 mg irinotecan, 10 mg pEGFP per kg mice), and 0.9% saline were administered to the mice via the tail vein every 3 days. The tumors were measured every 3 days with calipers during the period of study and were calculated according to the equation: (longest diameter × shortest diameter2)/2. Mice were weighed at the time of treatment and the body weight of mice was monitored as an index of systemic toxicity.
In vivo Transfection Efficiency
Colorectal cancer-bearing mice were administered with the same five samples as mentioned in the anticancer activity evaluation section. The mice were sacrificed at 24 or 72 h after administration and the tumor tissue samples were taken out, homogenized, washed three times with PBS.31 The cells were finally obtained after centrifugation (4°C, 1000 rpm, 5 min) and were seeded into 24-well plates in 1 mL of DMEM with 10% FBS. The fluorescent cells were observed using an inversion fluorescence microscope. Then, the cells were quantified using flow cytometry.
Statistical Analysis
Experiments were performed at least three times and expressed as mean ± standard deviation (mean ± SD). The statistical analysis was performed using a post hoc test following ANOVA. * P < 0.05 was considered as statistical significance and ** P < 0.01 as extreme statistical significance.
Results and Discussion
Characterization of LPNs
HA modified nanoparticles have been widely used for the delivery of drugs and genes for tumor targeting.32–34 They were expected to accumulate in cancer tissues as a combined function of the magnetic targeting-enhanced EPR effect and HA-mediated active targeting after intravenous injection.35 Particle sizes smaller than 200 nm are conducive to drug accumulation at the tumor site based on the EPR effect, thereby reducing the drug dose and minimizing toxicity.36 The size of HA-I/D-LPNs was 182.3 ± 5.1 (Table 1), which was smaller than 200 nm and larger than that of I/D-LPNs and LPNs. Zeta potential of nanoparticles higher than 20 mV was reported to make the nanoparticles repel each other, thereby avoiding particle aggregation and keeping the long-term stability of nanoparticles.37 The zeta potentials of LPNs were between −21.3 ± 2.2 and −39.1 ± 2.6 mV, which was high enough to keep a stable system. Good drug encapsulation efficacy and gene loading capacity are required for the construction of a successful nanoparticle system.38 Over 90% of GL and EE above 80% proved good loading ability of LPNs. HA-I/D-LPNs were spherical particles (Figure 1B). Serum stability was tested to simulate the in vivo hemocompatibility of LPNs.39 The adsorption of proteins on the nanoparticles could cause aggregation, thus leading to increase in particle size. The size and polydispersity showed no obvious change during the tested time, indicating the stability of the LPNs in serum (Figure 2).
In vitro Drug and Gene Release
PEG is the most important prolonged circulation modification moiety, which provides a very attractive combination of properties such as excellent solubility in aqueous solutions, high flexibility of its polymer chain, very low toxicity, immunogenicity, and PEG can be used as a linker for covalent attachment of active targeting moieties.40 In this study, HA was conjugated to the end of the PEG chain and used for the modification of LPNs. Figure 3 revealed that HA modified LPNs showed slower drug/gene release than that of unmodified LPNs. We also found that the DNA release was faster than drug release in the same kind of LPNs, this may be explained by the loading of gene was at the outer layer of the LPNs.21
In vitro Cellular Uptake and Cytotoxicity
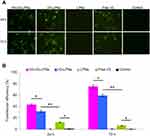
Figure 4A illustrated that the cellular uptake of HA-I/D-LPNs was higher than I/D-LPNs (P < 0.05), which may be the evidence that HA-I/D-LPNs have CD44-targeting effect.41 In vitro cytotoxicity results showed that HA-I/D-LPNs exhibited remarkable better cell inhibition efficiency than I/D-LPNs on SW480 cells (Figure 4B, P < 0.05). However, on HUVEC, cytotoxicity of HA-I/D-LPNs and I/D-LPNs exhibited no significant difference (Figure 4C). This may be due to the HA in the surface of LPNs that could bind to CD44 receptors overexpressed on the tumor cell surface thus bring about higher cytotoxicity.42 Higher cell inhibition ability observed by I/D-LPNs than free I/D (P < 0.05) indicated that the LPNs might have the enhanced ability to adhere to the cell membrane due to the similar nature of the lipids and the cell membrane.43 Blank LPNs did not show any cytotoxicity compared with control, indicating the safety and biocompatibility of the materials used for the preparation of the LPNs.44 The biocompatibility of LPNs is consistent with previous findings on LPNs and proved that this nano-system can be safely used as a drug/gene delivery vehicle.45
In vivo Pharmacokinetics and Anticancer Activity
The plasma drug concentration versus time profiles and the pharmacokinetic parameters were summarized (Figure 5A, Table 2). The peak plasma concentration (Cmax) achieved from HA-I/D-LPNs (41.31 ± 1.58 μg/mL) was significantly higher than that from free I/D (33.72 ± 1.85 μg/mL, P < 0.05). The half-life (T1/2) of irinotecan in HA-I/D-LPNs, I/D-LPNs, and free I/D was 12.56 ± 0.67, 8.78 ± 0.49, and 6.35 ± 0.32 h. Area Under Curve (AUC) of HA-I/D-LPNs was 1.8-fold greater than that of I/D-LPNs and 7.9-fold longer than free I/D. It was reported that nanoparticles can be excreted by the kidney or stealthy enough to evade the macrophage phagocytic system (MPS), formerly the reticuloendothelial system (RES).46 It was found that the structure of ligand-modified nanoparticles was propitious to reduce the capture of MPS, and benefit to selectively accumulate at the tumor site after intravenous injection via active tumor targeting cooperated with the enhanced permeability and retention (EPR) effect.47 The in vivo antitumor effects of LPNs were assessed using colorectal cancer-bearing mice. It was observed that the tumor volumes of HA-I/D-LPNs group were smaller than those of I/D-LPNs group (Figure 5B, P < 0.01). This may be explained by the HA CD44-receptor-mediated tumor targeting, indicating that HA-I/D-LPNs can inhibit the tumor growth most significantly, which is consistent with the results of the cytotoxicity assay. No antitumor effect was observed in the control group and blank LPNs group, while free I/D showed effectively inhibit tumor growth than the control (P < 0.01). I/D-LPNs illustrated better antitumor ability than that of free I/D (P < 0.01) even at a lower dose (10 mg/kg irinotecan for free I/D and 5 mg/kg irinotecan for I/D-LPNs), indicating the remarkable efficiency of the LPNs. The mice treated with HA-I/D-LPNs and I/D-LPNs depicted no obvious body weight changes from the day of the administration of different formulations to the end of the experiment (Figure 5C). However, free I/D caused reduction of body weight along with time. This weight loss induced by free I/D was higher than that of LPNs groups.
In vivo Transfection Efficiency
HA-I/D-LPNs showed the most prominent fluorescence during the in vivo transfection experiment (Figure 6A). For gene loaded LPNs groups, better transfection efficiencies were achieved at 72 h than 24 h (Figure 6B). This could be explained by the sustained release of the LPNs.48 On the contrary, free I/D showed weaker fluorescence at 72 h than 24 h. At 72 h post administration, HA-I/D-LPNs showed 75.3 ± 3.3% fluorescence positivity, which is higher than I/D-LPNs (59.4 ± 2.2%, P < 0.05) and free I/D (6.5 ± 0.6%, P < 0.01). Co-delivery of drug and DNA into the same tumor site is a key for achieving synergistic effect in the combined drug and gene therapy of cancer.16 In the present study, HA-I/D-LPNs exhibited the most remarkable tumor inhibition efficacy and best gene transfection efficiency in the tumor, which could prove the effects of the drug and gene combination therapy.
Conclusion
In summary, an HA modified, irinotecan and gene co-loaded LPNs were prepared for targeted colorectal cancer combination therapy. HA-I/D-LPNs achieved the highest tumor growth inhibition efficacy and the most prominent transfection efficiency in vivo, which could prove the drug and gene combination therapy effects of the system.
Disclosure
The authors report no conflict of interest in this paper.
References
1. Brody H. Colorectal cancer. Nature. 2015;521(7551):S1. doi:10.1038/521S1a
2. Gupta S, Provenzale D, Regenbogen SE, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 3.2017. J Natl Compr Canc Netw. 2017;15(12):1465–1475. doi:10.6004/jnccn.2017.0176
3. Williams CD, Grady WM, Zullig LL. Use of NCCN guidelines, other guidelines, and biomarkers for colorectal cancer screening. J Natl Compr Canc Netw. 2016;14(11):1479–1485. doi:10.6004/jnccn.2016.0154
4. Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33(16):1809–1824. doi:10.1200/JCO.2014.59.7633
5. Pedrosa L, Esposito F, Thomson TM, Maurel J. The tumor microenvironment in colorectal cancer therapy. Cancers (Basel). 2019;11:8. doi:10.3390/cancers11081172
6. Fujita K, Kubota Y, Ishida H, Sasaki Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J Gastroenterol. 2015;21(43):12234–12248. doi:10.3748/wjg.v21.i43.12234
7. Liu D, Li J, Gao J, Li Y, Yang R, Shen L. Examination of multiple UGT1A and DPYD polymorphisms has limited ability to predict the toxicity and efficacy of metastatic colorectal cancer treated with irinotecan-based chemotherapy: a retrospective analysis. BMC Cancer. 2017;17(1):437. doi:10.1186/s12885-017-3406-2
8. Bartoş A, Bartoş D, Szabo B, et al. Recent achievements in colorectal cancer diagnostic and therapy by the use of nanoparticles. Drug Metab Rev. 2016;48(1):27–46. doi:10.3109/03602532.2015.1130052
9. Dong Z, Cui MY, Peng Z, et al. Nanoparticles for colorectal cancer targeted drug delivery and MR imaging: current situation and perspectives. Curr Cancer Drug Targets. 2016;16(6):536–550. doi:10.2174/1568009616666151130214442
10. Cisterna BA, Kamaly N, Choi WI, Tavakkoli A, Farokhzad OC, Vilos C. Targeted nanoparticles for colorectal cancer. Nanomedicine (Lond). 2016;11(18):2443–2456. doi:10.2217/nnm-2016-0194
11. Song Y, Cai H, Yin T, et al. Paclitaxel-loaded redox-sensitive nanoparticles based on hyaluronic acid-vitamin E succinate conjugates for improved lung cancer treatment. Int J Nanomedicine. 2018;13:1585–1600. doi:10.2147/IJN
12. Jian YS, Chen CW, Lin CA, et al. Hyaluronic acid-nimesulide conjugates as anticancer drugs against CD44-overexpressing HT-29 colorectal cancer in vitro and in vivo. Int J Nanomedicine. 2017;12:2315–2333. doi:10.2147/IJN.S120847
13. Teo PY, Cheng W, Hedrick JL, Yang YY. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv Drug Deliv Rev. 2016;98:41–63. doi:10.1016/j.addr.2015.10.014
14. Peng Z, Wang C, Fang E, Lu X, Wang G, Tong Q. Co-delivery of doxorubicin and SATB1 shRNA by thermosensitive magnetic cationic liposomes for gastric cancer therapy. PLoS One. 2014;9(3):e92924. doi:10.1371/journal.pone.0092924
15. Han Y, Zhang P, Chen Y, Sun J, Kong F. Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. Int J Mol Med. 2014;34(1):191–196. doi:10.3892/ijmm.2014.1770
16. Liu C, Liu F, Feng L, Li M, Zhang J, Zhang N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials. 2013;34(10):2547–2564. doi:10.1016/j.biomaterials.2012.12.038
17. Zhao D, Zhuo RX, Cheng SX. Modification of calcium carbonate based gene and drug delivery systems by a cell-penetrating peptide. Mol Biosyst. 2012;8(12):3288–3294. doi:10.1039/c2mb25233c
18. Zhao K, Li D, Shi C, et al. Biodegradable polymeric nanoparticles as the delivery carrier for drug. Curr Drug Deliv. 2016;13(4):494–499. doi:10.2174/156720181304160521004609
19. Banerjee K, Gautam SK, Kshirsagar P, et al. Amphiphilic polyanhydride-based recombinant MUC4β-nanovaccine activates dendritic cells. Genes Cancer. 2019;10(3–4):52–62. doi:10.18632/genesandcancer.189
20. Hu J, Sheng Y, Shi J, Yu B, Yu Z, Liao G. Long circulating polymeric nanoparticles for gene/drug delivery. Curr Drug Metab. 2018;19(9):723–738. doi:10.2174/1389200219666171207120643
21. Wang Z, Wei Y, Fang G, et al. Colorectal cancer combination therapy using drug and gene co-delivered, targeted poly(ethylene glycol)-ε-poly(caprolactone) nanocarriers. Drug Des Devel Ther. 2018;12:3171–3180. doi:10.2147/DDDT.S175614
22. Gandhi NS, Tekade RK, Chougule MB. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release. 2014;28(194):238–256. doi:10.1016/j.jconrel.2014.09.001
23. Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85(3):427–443. doi:10.1016/j.ejpb.2013.07.002
24. Zhang L, Zhu D, Dong X, et al. Folate-modified lipid-polymer hybrid nanoparticles for targeted paclitaxel delivery. Int J Nanomedicine. 2015;10:2101–2114. doi:10.2147/IJN.S77667
25. Zhang L, Cui H. HAase-sensitive dual-targeting irinotecan liposomes enhance the therapeutic efficacy of lung cancer in animals. Nanotheranostics. 2018;2(3):280–294. doi:10.7150/ntno.25555
26. Yu W, Liu C, Liu Y, Zhang N, Xu W. Mannan-modified solid lipid nanoparticles for targeted gene delivery to alveolar macrophages. Pharm Res. 2010;27(8):1584–1596. doi:10.1007/s11095-010-0149-z
27. Li S, Wang L, Li N, Liu Y, Su H. Combination lung cancer chemotherapy: design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed Pharmacother. 2017;95:548–555. doi:10.1016/j.biopha.2017.08.090
28. Yu W, Liu C, Ye J, Zou W, Zhang N, Xu W. Novel cationic SLN containing a synthesized single-tailed lipid as a modifier for gene delivery. Nanotechnology. 2009;20(21):215102. doi:10.1088/0957-4484/20/21/215102
29. Gao Z, Li Z, Yan J, Wang P. Irinotecan and 5-fluorouracil-co-loaded, hyaluronic acid-modified layer-by-layer nanoparticles for targeted gastric carcinoma therapy. Drug Des Devel Ther. 2017;11:2595–2604. doi:10.2147/DDDT
30. Wang L, Liu Z, Liu D, Liu C, Juan Z, Zhang N. Docetaxel-loaded-lipid-based-nanosuspensions (DTX-LNS): preparation, pharmacokinetics, tissue distribution and antitumor activity. Int J Pharm. 2011;413(1–2):194–201. doi:10.1016/j.ijpharm.2011.04.023
31. Li P, Liu D, Miao L, et al. A pH-sensitive multifunctional gene carrier assembled via layer-by-layer technique for efficient gene delivery. Int J Nanomedicine. 2012;7:925–939. doi:10.2147/IJN.S26955
32. Liu L, Cao F, Liu X, et al. Hyaluronic acid-modified cationic lipid-PLGA hybrid nanoparticles as a nanovaccine induce robust humoral and cellular immune responses. ACS Appl Mater Interfaces. 2016;8(19):11969–11979. doi:10.1021/acsami.6b01135
33. Pramanik N, Ranganathan S, Rao S, et al. A composite of hyaluronic acid-modified graphene oxide and iron oxide nanoparticles for targeted drug delivery and magnetothermal therapy. ACS Omega. 2019;4(5):9284–9293. doi:10.1021/acsomega.9b00870
34. Lee JE, Yin Y, Lim SY, et al. Enhanced transfection of human mesenchymal stem cells using a hyaluronic acid/calcium phosphate hybrid gene delivery system. Polymers (Basel). 2019;11:5. doi:10.3390/polym11050798
35. Fang Z, Li X, Xu Z, et al. Hyaluronic acid-modified mesoporous silica-coated superparamagnetic Fe(3)O(4) nanoparticles for targeted drug delivery. Int J Nanomedicine. 2019;14:5785–5797. doi:10.2147/IJN.S213974
36. Zhang R, Ru Y, Gao Y, Li J, Mao S. Layer-by-layer nanoparticles co-loading gemcitabine and platinum (IV) prodrugs for synergistic combination therapy of lung cancer. Drug Des Devel Ther. 2017;11:2631–2642. doi:10.2147/DDDT
37. Zheng G, Zheng M, Yang B, Fu H, Li Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed Pharmacother. 2019;116:109006. doi:10.1016/j.biopha.2019.109006
38. Cui T, Zhang S, Sun H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment. Oncol Rep. 2017;37(2):1253–1260. doi:10.3892/or.2017.5345
39. Ma P, Li T, Xing H, et al. Local anesthetic effects of bupivacaine loaded lipid-polymer hybrid nanoparticles: in vitro and in vivo evaluation. Biomed Pharmacother. 2017;89:689–695. doi:10.1016/j.biopha.2017.01.175
40. Xu G, Chen Y, Shan R, Wu X, Chen L. Transferrin and tocopheryl-polyethylene glycol-succinate dual ligands decorated, cisplatin loaded nano-sized system for the treatment of lung cancer. Biomed Pharmacother. 2018;99:354–362. doi:10.1016/j.biopha.2018.01.062
41. Sun Y, Li X, Zhang L, et al. Cell permeable NBD peptide-modified liposomes by hyaluronic acid coating for the synergistic targeted therapy of metastatic inflammatory breast cancer. Mol Pharm. 2019;16(3):1140–1155. doi:10.1021/acs.molpharmaceut.8b01123
42. Tran TH, Choi JY, Ramasamy T, et al. Hyaluronic acid-coated solid lipid nanoparticles for targeted delivery of vorinostat to CD44 overexpressing cancer cells. Carbohydr Polym. 2014;114:407–415. doi:10.1016/j.carbpol.2014.08.026
43. Wang H, Sun G, Zhang Z, Ou Y. Transcription activator, hyaluronic acid and tocopheryl succinate multi-functionalized novel lipid carriers encapsulating etoposide for lymphoma therapy. Biomed Pharmacother. 2017;91:241–250. doi:10.1016/j.biopha.2017.04.104
44. Hu CM, Kaushal S, Tran cao HS, et al. Half-antibody functionalized lipid−polymer hybrid nanoparticles for targeted drug delivery to carcinoembryonic antigen presenting pancreatic cancer cells. Mol Pharm. 2010;7(3):914–920. doi:10.1021/mp900316a
45. Chan JM, Zhang L, Yuet KP, et al. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30:1627–1634. doi:10.1016/j.biomaterials.2008.12.013
46. Kobayashi H, Turkbey B, Watanabe R, Choyke PL. Cancer drug delivery: considerations in the rational design of nanosized bioconjugates. Bioconjug Chem. 2014;25(12):2093–2100. doi:10.1021/bc500481x
47. Deng T, Peng Y, Zhang R, et al. Water-solubilizing hydrophobic ZnAgInSe/ZnS QDs with tumor-targeted cRGD-sulfobetaine-PIMA-histamine ligands via a self-assembly strategy for bioimaging. ACS Appl Mater Interfaces. 2017;9(13):11405–11414. doi:10.1021/acsami.6b16639
48. Date T, Nimbalkar V, Kamat J, Mittal A, Mahato RI, Chitkara D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J Control Release. 2018;271:60–73. doi:10.1016/j.jconrel.2017.12.016
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.