Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Hyaluronan Dermal Fillers: Efforts Towards a Wider Biophysical Characterization and the Correlation of the Biophysical Parameters to the Clinical Outcome
Authors La Gatta A, Schiraldi C , Zaccaria G, Cassuto D
Received 20 June 2019
Accepted for publication 20 November 2019
Published 28 January 2020 Volume 2020:13 Pages 87—97
DOI https://doi.org/10.2147/CCID.S220227
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Annalisa La Gatta,1 Chiara Schiraldi,1 Giovanna Zaccaria,2 Daniel Cassuto2
1Department Experimental Medicine, Section of Biotechnology, Medical Histology and Molecular Biology, School of Medicine, University of Campania “L. Vanvitelli”, Naples 80138, Italy; 2Private Practice, Milan, Italy
Correspondence: Chiara Schiraldi via De Crecchio 7, Naples 80138, Italy
Tel +39 081 5667546
Fax +39 081 5665897
Email [email protected]
Daniel Cassuto Dr. Daniel Cassuto, Piazza V Giornate 1, Milan 20129, Italy
Tel +39 02 36723960
Email [email protected]
Introduction: Hyaluronic Acid (HA) fillers are among the most used products in cosmetic medicine. Companies offer different formulations to allow full facial treatment and/or remodeling. Gels are being studied to establish the biophysical properties behind the specific clinical use and a correlation between the gel biophysical properties and their clinical performance. Clinicians’ awareness is growing about the potential benefit deriving from such biophysical characterization.
Aim: The Aliaxin® line of HA dermal fillers is the object of this study. The study aimed to widen the biophysical characterization of these gels by investigating a variety of properties to better support their optimal use. Further, we aimed to provide some clinical findings to gain a deeper insight into the correlation between filler features and clinical outcome.
Methods: The four gels of the line were investigated, for the first time, for their cohesivity and stability to Reactive Oxygen Species (ROS). Additional secondary rheological parameters; evidence of relative water-uptake ability; and some clinical findings on product safety, palpability and duration of the aesthetic effect are provided.
Results and conclusion: The gels proved highly cohesive and sensitive to ROS action with stability declining with the decrease in the overall gel elasticity. The G* and complex viscosity values at clinically relevant frequencies and gel water-uptake ability are consistent with the relative clinical indication related to gel projection and hydration capacity. Clinical outcomes showed the safety of the products and a perception of palpability well correlating with the cohesive/viscosity properties of the gels. A similar duration of the aesthetic effect (up to 1 year) was observed despite the diverse in vitro gel stability. The results broaden our knowledge of these gels and may contribute to optimize their clinical use towards the improvement of patient safety and satisfaction. Initial clinical observation indicated that gel biophysical properties allow for a reliable prediction of gel palpability, while in vitro data on gel stability cannot be related to the duration of the observed skin improvement. The latter finding further corroborates the idea of a skin restoration process activated by the gels besides the physical volumetric action.
Keywords: dermal filler, degradation, cohesivity, palpability
Introduction
In the field of aesthetic medicine, hyaluronic (HA)-based dermal fillers are among the most used products for the non-invasive treatment of skin defects.1–16 Characterization studies of the commercial gels are intensifying since strongly aiding clinicians in the optimal use of available products also contributing to the establishment of the correlation between the biophysical parameters of a gel and the clinical outcome.
The biophysical properties of fillers (rheological parameters, hydration capacity, resistance to degradation, etc.) have been gaining growing importance and exposure in the literature, but the clinical implications of each parameter remain ill-defined. The capacity of a filler to integrate into the surrounding tissues is crucial for a natural effect. In this respect, viscosity and deformability of a filler are lately investigated as they may determine tissue integration properties. Specifically, the higher the deformability (low G′, G* values) and the lower the viscosity, the better the gel spreads within the tissue. Other studies have focused on “cohesivity”.8–10 This is intended as the capacity of a filler to maintain its integrity without dissociating. This property is thought to correlate with a more uniform distribution in tissues, probably reducing the risk of lumps' formation and allowing a very superficial use without Tyndall effect. The association of low G′ and viscosity with good spreading ability and high cohesivity could therefore be particularly attractive.
Besides the rheological behavior and the hydration capacity, another crucial aspect for filler performance is its permanence in vivo mainly depending on its sensitivity to degradation due to hyaluronidases and ROS action. To predict relative in vivo permanence of HA gels, many in vitro studies have been performed on HA filler stability to enzymatic action while stability to ROS has been poorly investigated up to now.6,10–13
The current trend in HA-filler use aims to a “natural-looking” full face remodeling thanks to the availability of diverse formulations designed for specific needs, including Aliaxin gels.
These specific gels have already been investigated in terms of flow behavior, insoluble vs. soluble HA amount, and resistance to enzymatic degradation.13 A 3D full-thickness skin model was also used to evaluate the effect on skin remodeling.13 Here we aimed at widening the knowledge of these gels by investigating their cohesivity and their sensitivity to ROS and by providing additional rheological parameters (G*, tan delta, etc.). In addition, we here provide clinical data derived from a retrospective analysis on the use of gels in subjects with the aim of moving a step towards the correlation between the gel biophysical properties and the specific clinical effect.
Materials and Methods
Materials
The following HA fillers were evaluated: Aliaxin® (A) line, AEV, AGP, AFL and ASR distributed by IBSA Farmaceutici Italia S.R.L. (Lodi, Italy). They are all BDDE-crosslinked HA hydrogels and the different products of the line are claimed to contain diverse combinations of HA molecular weights: from 500 to 2000kDa.13 Specifically, 1000 and 2000 kDa for AEV and AGP, 500 and 1000 kDa for AFL, and a combination of three molecular weights (500, 1000, and 2000 kDa) for ASR. All fillers were provided by IBSA for this in vitro arm of the study.
Dulbecco’s Phosphate Buffered Saline (PBS) without calcium and magnesium was purchased from Lonza Sales Ltd (Switzerland). Hydrogen peroxide, 30% w/w in water was purchased from Sigma Aldrich (Italy), cat. N.H1009. Copper (II) sulfate (≥99%) was a Fluka product, cat. N. 61230 (Sigma Aldrich, Germany).
For the clinical part, patients were treated in a standard private practice setup. Fillers were regularly purchased by the office and patients were charged normally for the injections in order to obtain realistic satisfaction feedback. All patients had previously received other HA injections.
Methods
Hydration Capacity
An equal amount of each filler (160 mg) was stained with toluidine blue (Tb). Specifically, 5 μL of Tb (0.1% w/w in PBS pH7.4) were added to each gel. After mixing, samples were centrifuged (10 min at 10,000×g) in order to remove air and obtain a homogeneously stained gel. PBS was added to 1 mL final volume to each gel. Samples were allowed to reach the equilibrium swelling and then, centrifuged (13,000×g for 5 min). The tubes containing the samples were inverted to better observe the phase separation between the hydrated gel and the liquid not incorporated in the gel. Images of the tubes were captured. Staining was performed to better visualize the samples that were, otherwise, transparent. A tube containing the stained sample before the addition of PBS was also included.
Rheological Studies
Measurements were carried out using a Physica MCR301 oscillatory rheometer (Anton Paar, Germany) equipped with a parallel plate geometry, 25 mm plate diameter, 1.0 mm gap and a Peltier temperature control, as reported elsewhere for the characterization of other marketed or newly developed HA-based dermal fillers.13 Measurements were performed at 37°C.
For the determination of tanδ and complex modulus (G*) values, a strain range of 0.01–100% was applied in no time setting mode while keeping the oscillation frequency at 1.59 Hz. Tanδ values (Gʺ/G′) and G* were derived in the linear viscoelastic range. A shear stress vs strain curve was derived.
For the determination of the complex viscosity values at diverse frequencies, a constant strain, within the linear viscoelastic range, was applied and the oscillation frequency varied from 0.159 to 10 Hz. Complex viscosity values at 0.159, 0.7 and 2 Hz frequency were registered.
Gel Degradation Due to ROS Action
The stability of the gels to ROS action was studied using the H2O2/Cu2+ system for generating radicals, as recently reported.10 Briefly, aqueous solutions of H2O2 and CuSO4 were added to 1 mL of each gel to have H2O2 375mM and CuSO4 3.75mM (1.3 mL final volume). The suspensions were mixed and evaluated by rheological measurements. Specifically, the storage modulus was measured as a function of the time while maintaining constant frequency and strain. The acquisition of the G′ values started 4 mins after the addition of the ROS generating system. G′ values were registered, under the same conditions, for control samples, obtained by adding water, in place of the H2O2/Cu2+ system, to the gels.
Gel degradation was monitored by measuring the G′ decrease (% in respect to the control) as a function of the incubation time (up to 10ʹ) with the ROS generating system.
Cohesivity
Cohesivity was evaluated following a recent protocol reported by Sundaram and collaborators with slight modifications.8–10 Specifically, 10 μL of Tb (0.1% w/w in PBS, pH7.4) were added to 1 g of each gel.10 Staining was performed as indicated above. Samples were carefully drawn into 1 mL syringes and extruded under reported conditions.8,9 Immediately after extrusion, the magnetic stirring started and movies were recorded; in addition, images were obtained at specific time points (15, 70 and 90 s).
Cohesivity was evaluated independently by four raters that assigned, for each sample at each time point, a value of cohesivity (from 1 to 5) referring to the Gavard–Sundaram Cohesivity Scale.9 Results were reported as the mean score ± SD.
We retrieved the data from 56 patients (5 men and 51 women), age ranging from 21 to 79 years (mean age 53) treated in a real-life setting. All patients had previously received different types of HA fillers (not restricted to Aliaxin products) injections. However, no data on the results from these previous treatments were available. Patients could express a relative degree of satisfaction.
Patients were treated according to their clinical needs and assessment, not by a standard protocol. Unlike most studies performed under strictly standardized protocols, we wanted to receive data from a real-life setting with the idea that these data could be more useful for daily practice than those obtained under standard study conditions. The treated areas, accordingly to the patient’s needs, were upper orbital and temporal, pre-auricular region, infraorbital, cheek, chin and mandibular border. An average of two areas per patient were injected, with a mean injection volume of 2 mL per area, i.e. 1 mL per side.
The four types of HA gel were injected by the last author, according to the quality and amount of soft-tissue coverage and the needed support.
The skin was prepared with alcohol prior to injection. The gel was injected through a 22G/50mm or 23G/40mm blunt-tipped cannula (SoftFil®, 55 Bd Pereire, Paris, France) in the immediate subdermal plan. One cubic centimetre of HA was injected per side following a fan-shaped pattern, except for the mandibular line that was injected linearly. Gentle and slow advancement of the blunt tipped cannula was used during all injections attempting to deflect any eventually encountered blood vessel or nerve. Extremely slow retrograde extrusion of the gel in the thin tract left by the receding cannula avoided the use of any anesthesia. Patients were evaluated by Clinical Digital Photography before, immediately after, and up to 1 year after last treatment by an independent evaluator. Patients were required to express their degree of satisfaction by the following scale: 1 – not satisfied, 2 – partially satisfied, 3 – fully satisfied. Similar degrees of satisfaction were also required by the independent evaluator. Injection sites were examined for evidence of any tissue reaction such as erythema, edema, hematoma, nodules' formation, inflammation.
All the patients signed an Informed Consent allowing the use of photographic record for scientific publications, in which they declared to be aware of the products' indications and treatment. According to Italian Law (GU 214/2002 & DL 211/2003), considering the fact that all the data presented in the manuscript derived from a retrospective descriptive analysis, there was no need to have a specific approval from Ethical Committee.
Results
Hydration Capacity in Physiological Condition: In vitro Assessment
The results of the swelling experiments are shown in Figure 1. A photo showing 160 mg (0.16 mL) of AEV gel after staining with Tb and centrifugation is shown on the left side; it is representative of all the Aliaxin gels. As expected, no phase separation is observed which indicates that the gels, in their commercial formulations, do not exploit their total hydration capacity; therefore, if allowed to equilibrate in the aqueous medium, they expand by adsorbing water.
Photos showing the same amount of each formulation after being equilibrated in PBS (1 mL final volume) are reported. Images clearly show the differences in water uptake. Among the Aliaxin formulations, AEV and AGP showed phase separation thus indicating that the gels are at the maximum of their water retention capability. AEV has the lowest water uptake (hydrating capacity), AGP proved higher absorption/expansion in PBS: less water volume remained outside the gel; ASR and AFL still did not show phase separation under the experimental conditions which indicates that 160 mg of these formulations expand to more than 1 mL final volume.
Rheological Investigation
The results of the rheological investigation are reported in Figure 2. Specifically, Figure 2A and 2B shows the complex modulus and the tanδ values, calculated within the linear viscoelastic range, for each filler. G* values span in the range 40–160 Pa. AEV proved the less deformable (highest G*value) followed by AGP and then AFL and ASR.
The Aliaxin gels show tanδ values covering the range 0.15–0.51 with AEV and ASR showing the lowest and the highest value, respectively.
Figure 2C shows the shear stress vs strain curves for the fillers, which allow us to better appreciate differences in deformability. Actually, under constant stress (see the horizontal line in the graph), the lowest deformation is registered for AEV, while the highest for AFL and ASR. Specifically, at 0.1 Pa stress, ASR and AFL exhibit a deformation about 4fold higher compared to AEV; for AGP the strain found was about twofold than AEV.
Figure 2D reports the complex viscosity values at different frequencies. In particular, the examined frequencies cover the whole range (0.1–2 Hz) recognized as physiologically relevant.17 The rank in viscosity reflects the one in rigidity (G′) and viscoelasticity (G*): AEV is the most rigid, viscoelastic and viscous, followed by AGP and then by AFL and ASR, the less crosslinked among the Aliaxin gels.
Gel Degradation Due to ROS Action
The results of the degradation studies in the presence of ROS are reported in Figure 3. Specifically, the G′ values during incubation of each gel with the ROS generating system and the ones for the related control are reported in Figure 3A. All the gels showed a rigidity decreasing during incubation with the H2O2/Cu2+ system thus indicating degradation due to ROS action. A diverse rate of G′ reduction, indicating diverse sensitivity, is evident by comparing the profiles obtained for the four gels. A quantitative analysis of the extent of degradation is reported in Figure 3B. Specifically, the G′ values registered in the presence of ROS, normalized to the G′ values for the control (G′t=0) are reported for each gel. At 4 mins of incubation, AEV preserved about 20% of the initial rigidity while, for ASR, only 5% of residual stiffness was observed. AEV and ASR proved the most stable and the most sensitive gel, respectively, up to the longest time tested. At 5 and 8 mins of incubation, AGP behave similarly to AEV while AFL showed intermediate stability. At the longest time tested, all the gels retained less than 2% of rigidity with ASR showing the lowest residual G′ value and the other gels showing comparable degradation.
Cohesivity
Data on fillers' cohesivity are reported in Figure 4. In particular, representative images captured at 15, 70 and 90ʺ for the diverse gels are reported in Figure 4A. The values for cohesivity, as attributed to the fillers by the raters (the Sundaram–Gavard scale), are reported in Figure 4B. As shown in Figure 4A, the gels preserved their “integrity” all over the experiment. An optimal retention of the gel structure was observed: no fragmentation or dispersion of the gels was recorded. Based on the behavior observed, all the Aliaxin gels were rated as “full cohesive” (score for cohesivity: “5”) throughout the experimentation.
Clinical Experience
All injected fillers showed excellent extrudability, which allowed for very low extrusion pressure. For this reason, no anesthesia was necessary. The blunt tipped cannula, the slow movements and the relatively slow injection also ensured safety by avoiding intravascular placement of HA. Examination of injection sites evidenced no serious tissue reaction such as erythema, edema, hematoma, small lumps, inflammation.
Objective Evaluation of the Aesthetic Effect
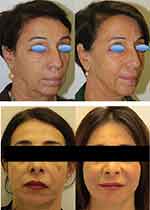
Figure 5 shows, as an example, images of the treated areas before and 1 year after filler’s application in two subjects. This first subject received AGP (2 mL in the upper orbital margin and temporal), AEV (2 mL in the preauricular and mandibular area) and AFL (4 mL in the midface, maxillary and perioral areas) treatments at 3-months distance. The second subject received ASR (3 mL in the forehead and midface areas) AFL (2 mL in the midface area) AGP (2 mL in the areas of temples and brows) AEV (2 mL in the preauricular area) treatments at 3-months distance. The pictures have been obtained before treatment and 12 months after the last treatment. A part from this example, all patients showed subjective and objective (aesthetic) improvement after gels injection. The (aesthetic) improvement was still evident in patients’ images 1 year after the last injection without exception both to a Board Certified independent evaluator and ourselves.
Patient Satisfaction
Immediate satisfaction was excellent, except in cases where periocular edema was observed (7/38 cases). This was due to immediate complete correction of the defect in severely depleted cases that, in retrospect, would have deserved a more gradual correction.
All patients reported satisfaction at 3, 6 and 12 months and the majority reported full satisfaction. The degree of satisfaction at the different intervals is reported in Table 1. The most remarkable feedback was the early lack of implant palpability together with a good longevity of the aesthetic improvement. There was also an overall improvement in perceived tissue quality by all patients, compared to the adjacent untreated areas. A similar degree of satisfaction was reported by an independent evaluator (Table 1).
 |
Table 1 Degree of Satisfaction Determined 3, 6 and 12 Months After Injections in the 56 Patients |
Side Effects
Only 20% of the treated patients presented a slight erythema in the injected area that disappeared within 30 mins and was probably due to the vasodilating effect of the alcohol used to disinfect the skin. Most of these patients had relatively fair skin types (I–III) with mild to moderate “couperose” due to sunlight exposure. Local edema was observed in seven periocular cases; however, it subsided spontaneously within 72 hrs. Overcorrection that required hyaluronidase occurred in one case. No case of inflammation occurred.
Observations
The injected products were no longer palpable at 1 week after injection both subjectively (by patients) and objectively (by the injector). We observed that 9 of 10 patients could not palpate the material a few minutes after the injection in the case of Aliaxin FL.
In the temples, very small irregularities were visible immediately after injection due to superficial venous engorgement. They resolved spontaneously in a few days without any treatment, probably thanks to good tissue integration.
Discussion
The four formulations of the HA dermal fillers are consistent with the current clinical approach of a whole face restoration achieved by using diverse products. A previous characterization of these gels highlighted differences in their biophysical properties that represent a scientific rationale to the specific clinical indications.13 A further characterization was here performed to increase our knowledge of these gels and to better support their use.
In the attempt to provide a complete panel of the flow behavior and of the hydration capacity of the gels, key properties for clinical outcome, secondary rheological parameters and evidence of the diverse water-uptake ability of the formulations were presented (Figures 1 and 2).6,13–22
The water absorption capacity of a filler is related to its in vivo hydro-action. The results of the hydration test (Figure 1) are highly indicative of the huge hydration capacity of the Aliaxin gels. Further, the evident relative extent in water up-take is consistent with the ranking in hydro-action indicated by the distributor and with the quantitative results reported elsewhere.13 Fillers' behavior, in physiological buffer, suggests an in vivo expansion of the gels after injection. However, it is worth underlining that these data suggest a ranking in filler water up-take and, therefore, hydration capacity; however, the expansion registered in vitro does not correspond to the one expected in vivo, the latter depending not only on the gel swelling ability but also on the surrounding tissues consistency (e.g., depending on the tensile force of the muscle and derma in the face) and on gel resistance to deformation.
Tan delta (Gʺ/G′) is a measure of the ratio between the viscous and elastic component of the gel. It indicates the presence and the extent of elasticity in a gel: the lower is the tan delta, the higher is the elasticity (predominance of G′ over Gʺ).17,21 Data reported in Figure 2B indicate, for the Aliaxin formulations, the presence of elasticity with tan delta values in the range typical for crosslinked HA dermal fillers (e.g. 0.05–0.80) and consistently decreasing (elasticity increases) with the increase of the crosslinking degree (ASR>AFL>AGP>AEV; p<0.05).21 It is worth to note that two fillers can have very different G′ but similar tanδ values, the latter being a derived value (ratio between moduli). Thus, it does not discriminate between high and low G′ gels but simply indicates the elasticity extent of each sample. Therefore, this parameter should be cautiously used to compare dermal fillers' flow properties.22
The complex modulus is a measure of the overall viscoelasticity of the gels considering both the elastic and viscous components (G′ and Gʺ). It is also referred to as gel hardness and measures the resistance to deformation/the resilience to shape modification. The higher is the complex modulus, the lower is the deformability. All the gels evaluated here have G* values slightly exceeding G′ values, as expected for viscoelastic materials (purely elastic materials have G*=G′ while purely viscous materials have G*=Gʺ).17,21 Data indicate that G* does not give additional valuable information on fillers flow behavior compared to G′, in agreement with the findings reported by other authors.22
The stress vs strain curves (Figure 2C) can be considered highly indicative of the relative deformation/flow properties of the gels evidencing the deformation occurring in each filler at a certain applied stress. The rank in deformability, derived from the curves, is consistent with the storage and complex modulus values for these fillers. It is indicative of the site/depth most suitable for filler placement. Highly deformable gels (AFL and ASR) are better suited for more superficial placement, while the gels with the highest capacity to maintain their shape under the stress (AEV and then AGP) are better indicated for deeper placement where palpability is not a concern. These results are consistent with what reported previously and with the clinical indication for these gels.13 As expected, the rank in viscosity (Figure 2D) reflects the one in rigidity (G′) and viscoelasticity (G*).
Cohesivity is a parameter that is gaining more and more significance. Specifically, it is thought that the tissue integration pattern of a filler depends on both cohesivity and viscosity. A low viscosity and highly cohesive gel could fill the area of interest by uniformly spreading into the interstitial spaces of the tissue thus finally resulting in a more “natural” aesthetic effect, at least for superficial injection layers. The cohesivity and viscosity data allow us to predict, for the Aliaxin formulations, an integration with the surrounding tissue improving with the decrease in crosslinking (ASR>AFL>AGP>AEV). Even we do not have a formal comparison with other commercial fillers, the viscosity values and the utmost cohesivity of the gel formulations tested as compared to the values reported for other fillers intended for the same clinical use suggest a similar or improved tissue integration for the Aliaxin fillers.10 Taken together, the hydration, rheological and cohesivity data allow us to predict a singular aspect of the tested gels. A high hydration capacity is generally desired for a dermal filler; however, a wide expansion after injection is often perceived as an issue related to edema or overcorrection. This should not be the case for the Aliaxin formulations due to their flow properties (higher deformability, lower viscosity in respect to other commercial gels) which, together with high cohesivity, allow for an efficient hydro-action whilst well spreading in the surrounding tissue. A strong tissue expansion and better tissue integration and spreading are expected when moving from AEV to ASR.8,9 Finally, it is worth underlining that a better spreading filler is less capable of forming nodular deposits that represent the most filler complications.23,24
The duration of the aesthetic effect is certainly related to the gel permanence in vivo that, in turn, rationally depends on filler sensitivity to hyaluronidases and ROS.25 It is worth to notice that, despite no differences in degradation rate had previously been found in case of gel exposure to Bovine Testicular Hyaluronidase (BTH), the sensitivity to ROS action decreased with the increase of gel crosslinking degree (Figure 3). Taken together, data on gel resistance to BTH and ROS action suggest for AEV the longest permanence in vivo, while the shortest residence time would be expected for ASR. The results also prompt further investigation to better unravel the parameters (e.g. crosslinking degree, molecular weight of the polymer undergoing crosslinking, etc.) that more strongly affect final gel stability under these two main hydrolysis conditions.
Clinical observations indicated a comparable duration of the aesthetic effect regardless of the specific delivered gel. This would suggest an improvement of skin quality not only related to in vivo permanence of the gels. However, this would be in line with recent findings indicating that injections of hyaluronic acid can improve soft-tissue quality by positively influencing the behavior of fibroblasts towards the stimulation of ECM production, not only by providing better temporary hydration and volume.3,13,26–31
Since the beginning of their use, patients have perceived the presence and duration of a filler by its palpability, though this is hardly a desirable feature. Even this clinical data were not designed to address specifically the presence and the duration of the filler, all patients in our series have reported that their implant became impalpable either immediately or a few days after injection, this correlates well with tissue-like behavior. The relative time interval for gel palpability is consistent with the gel relative “level” of tissue integration, based on their cohesive/viscosity properties thus suggesting the rheological/cohesive properties of a gel as a tool to predict this aspect of filler performance.
Conclusion
New data on cohesivity and stability to ROS action and additional rheological parameters were provided for the Aliaxin fillers. Such data can contribute to clinician awareness of filler properties thus further supporting their optimal clinical use. Based on the acquired biophysical data, differences in gel tissue integration and longevity can be predicted. Clinical observations showed the safety of the products and a consistent correlation of gel palpability with the relative viscosity/cohesive properties. Even if the observed duration of the aesthetic effect did not parallel the in vitro gel degradation data, as discussed, it corroborates the current idea of a biological effect of the gels on skin restoration besides the physical volumetric action.
Acknowledgments
The authors are grateful to Giovanna Damia for help in writing the manuscript. IBSA Farmaceutici Italia Srl sponsored the medical writing of the manuscript.
Disclosure
The material for in vitro experiments has been provided by IBSA Farmaceutici Italia Srl. The authors report no other conflicts of interest in this work.
References
1. Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009;25(2):86–94. doi:10.1055/s-0029-1220647
2. Callan P, Goodman GJ, Carlisle I, et al. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: a 24-month study. Clin Cosmet Investig Dermatol. 2013;6:81–89. doi:10.2147/CCID.S40581
3. Fagien S, Cassuto D. Reconstituted injectable hyaluronic acid: expanded applications in facial aesthetics and additional thoughts on the mechanism of action in cosmetic medicine. Plast Reconstr Surg. 2012;130(1):208–217. doi:10.1097/PRS.0b013e318254b3f6
4. Gaffney J, Matou-Nasri S, Grau-Olivares M, Slevin M. Therapeutic applications of hyaluronan. Mol Biosyst. 2010;6(3):437–443. doi:10.1039/B910552M
5. Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39(11):1602–1612. doi:10.1111/dsu.12343
6. La Gatta A, Schiraldi C, Papa A, De Rosa M. Comparative analysis of commercial dermal fillers based on crosslinked hyaluronan: physical characterization and in vitro enzymatic degradation. Polym Degrad Stab. 2011;96(4):630–636. doi:10.1016/j.polymdegradstab.2010.12.025
7. Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol Ther. 2006;19(3):141–150. doi:10.1111/dth.2006.19.issue-3
8. Sundaram H, Fagien S. Cohesive polydensified matrix hyaluronic acid for fine lines. Plast Reconstr Surg. 2015;136(5 Suppl):149S–163S. doi:10.1097/PRS.0000000000001835
9. Sundaram H, Rohrich RJ, Liew S, et al. Cohesivity of hyaluronic acid fillers: development and clinical implications of a novel assay, pilot validation with a five-point grading scale, and evaluation of six U.S. Food and Drug Administration-approved fillers. Plast Reconstr Surg. 2015;136(4):678–686. doi:10.1097/PRS.0000000000001638
10. La Gatta A, Salzillo R, Catalano C, et al. Hyaluronan-based hydrogels as dermal fillers: the biophysical properties that translate into a “volumetric” effect. PLoS One. 2019;14(6):e0218287. doi:10.1371/journal.pone.0218287
11. Jones D, Tezel A, Borrell M. In vitro resistance to degradation of hyaluronic acid dermal fillers by ovine testicular hyaluronidase. Dermatol Surg. 2010;36:804–809. doi:10.1111/j.1524-4725.2010.01550.x
12. Park S, Park KY, Yeo IK, et al. Investigation of the degradation-retarding effect caused by the low swelling capacity of a novel hyaluronic acid filler developed by solid-phase crosslinking technology. Ann Dermatol. 2014;26(3):357–362. doi:10.5021/ad.2014.26.3.357
13. La Gatta A, De Rosa M, Frezza MA, Catalano C, Meloni M, Schiraldi C. Biophysical and biological characterization of a new line of hyaluronan-based dermal fillers: a scientific rationale to specific clinical indications. Mater Sci Eng C Mater Biol Appl. 2016;68:565–572. doi:10.1016/j.msec.2016.06.008
14. Falcone SJ, Doerfler AM, Berg RA. Novel synthetic dermal fillers based on sodium carboxymethylcellulose: comparison with crosslinked hyaluronic acid-based dermal fillers. Dermatol Surg. 2007;33(Suppl 2):S136–S143. doi:10.1111/j.1524-4725.2007.33353.x
15. Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(Suppl 1):302–312. doi:10.1111/j.1524-4725.2008.01046.x
16. Stocks D, Sundaram H, Michaels J, Durrani MJ, Wortzman MS, Nelson DB. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011;10(9):974–980.
17. Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4 Suppl 2):5S–21S. doi:10.1097/PRS.0b013e31829d1d40
18. La Gatta A, Ricci G, Stellavato A, et al. Hyaluronan hydrogels with a low degree of modification as scaffolds for cartilage engineering. Int J Biol Macromol. 2017;103:978–989. doi:10.1016/j.ijbiomac.2017.05.091
19. La Gatta A, Papa A, Schiraldi C, De Rosa M. Hyaluronan dermal fillers via crosslinking with 1,4-butandiol diglycidyl ether: exploitation of heterogeneous reaction conditions. J Biomed Mater Res B Appl Biomater. 2016;104(1):9–18. doi:10.1002/jbm.b.33329
20. Salzillo S, Schiraldi C, Corsuto L, et al. Optimization of hyaluronan-based eye drop formulations. Carbohydr Polym. 2016;153:275–283. doi:10.1016/j.carbpol.2016.07.106
21. Pierre S, Liew S, Bernardin A. Basics of dermal filler rheology. Dermatol Surg. 2015;41(Suppl 1):S120–S126. doi:10.1097/DSS.0000000000000334
22. Sundaram H. Going with the flow: an overview of soft tissue filler rheology and its potential clinical applications (2 of 3). Pract Dermatol. 2011;6:23–28.
23. Cassuto D, Pignatti M, Pacchioni L, Boscaini G, Spaggiari A, De Santis G. Management of complications caused by permanent fillers in the face: a treatment algorithm. Plast Reconstr Surg. 2016;138(2):215e–227e. doi:10.1097/PRS.0000000000002350
24. Weinberg MJ, Solish N. Complications of hyaluronic acid fillers. Facial Plast Surg. 2009;25(5):324–328. doi:10.1055/s-0029-1243081
25. Fakhari A, Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013;9(7):7081–7092. doi:10.1016/j.actbio.2013.03.005
26. Landau M, Fagien S. Science of hyaluronic acid beyond filling: fibroblasts and their response to the extracellular matrix. Plast Reconstr Surg. 2015;136(5 Suppl):188S–95S. doi:10.1097/PRS.0000000000001823
27. Mochizuki M, Aoi N, Gonda K, Hirabayashi S, Komuro Y. Evaluation of the in vivo kinetics and biostimulatory effects of subcutaneously injected hyaluronic acid filler. Plast Reconstr Surg. 2018;142:112–121. doi:10.1097/PRS.0000000000004496
28. Paliwal S, Fagien S, Sun X, et al. Skin extracellular matrix stimulation following injection of a hyaluronic acid–based dermal filler in a rat model. Plast Reconstr Surg. 2014;134:1224–1233. doi:10.1097/PRS.0000000000000753
29. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143(2):155–163. doi:10.1001/archderm.143.2.155
30. Quan T, Wang F, Shao Y, et al. Enhancing structural support of the dermal microenvironment activates fibroblasts, endothelial cells, and keratinocytes in aged human skin in vivo. J Invest Dermatol. 2013;133(3):658–667. doi:10.1038/jid.2012.364
31. Bukhari SNA, Roswandi NL, Waqas M, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol. 2018;120(Pt B):1682–1695. doi:10.1016/j.ijbiomac.2018.09.188
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.