Back to Journals » Infection and Drug Resistance » Volume 13
Human Umbilical Cord Mesenchymal Stem Cells for Adjuvant Treatment of a Critically Ill COVID-19 Patient: A Case Report
Authors Zhu Y, Zhu R, Liu K, Li X, Chen D, Bai D, Luo J, Liu Y, Zhang Y, Li L, Hu J, Xu D, Liu Y , Zhao RC
Received 17 July 2020
Accepted for publication 3 September 2020
Published 28 September 2020 Volume 2020:13 Pages 3295—3300
DOI https://doi.org/10.2147/IDR.S272645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yue Zhu,1,* Rongjia Zhu,2,* Kun Liu,3,* Xin Li,1 Dezhong Chen,4 Dunyao Bai,5 Jieli Luo,5 Yixun Liu,6 Yan Zhang,7 Li Li,8 Junfang Hu,9 Dayong Xu,10 Yan Liu,10 Robert Chunhua Zhao2,11
1Stem Cell Lab, Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei 430081, People’s Republic of China; 2Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing 100005, People’s Republic of China; 3Otorhinolaryngology, Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei 430081, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei 430081, People’s Republic of China; 5Molecular Laboratory, Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei 430081, People’s Republic of China; 6Gonadal Biology Research Group, Institute of Zoology, Chinese Academy of Sciences, Beijing, 100101, People’s Republic of China; 7National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, People’s Republic of China; 8The Ministry of Science and Education, Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei 430081, People’s Republic of China; 9Department of Pharmacy, Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei 430081, People’s Republic of China; 10Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei 430081, People’s Republic of China; 11School of Life Sciences, Shanghai University, Shanghai 200444, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Robert Chunhua Zhao
Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing 100005, People’s Republic of China
Email [email protected]
Yan Liu
Stem Cell Lab, Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei 430081, People’s Republic of China
Email [email protected]
Background: COVID-19 (coronavirus disease 2019) has become a global public health emergency since patients were first detected in Wuhan, China, in December 2019. Currently, there are no satisfying antiviral medications and vaccines available.
Case Presentation: We reported the treatment process and clinical outcome of a 48-year-old man critically ill COVID-19 patient who received transfusion of allogenic human umbilical cord mesenchymal stem cells (UC-MSCs).
Conclusions: We proposed that UC-MSC transfusion might be a new option for critically ill COVID-19. Although only one case we were shown, more similar clinical cases are inquired for further evidence providing the potential effectiveness of UC-MSC treatment.
Keywords: COVID-19, UC-MSCs, critically ill, cell transplantation, case report
Background
The COVID-19 has become a global public health emergency since patients were first detected in Wuhan, China, in December 2019, which spread quickly to 211 countries worldwide and presented a serious threat to public health.1 It is mainly characterized by fever, dry cough, shortness of breath and dyspnea.2 Currently, there are no satisfying antiviral medications and vaccines available. Hence, there is an unmet need of a safe and effective treatment for COVID-19 patients, especially the critically ill cases.
Recently, some clinical researches on the COVID-19 suggested that various inflammatory cells’ infiltration and inflammatory cytokines’ secretion were found in patients’ lungs, alveolar epithelial cells and capillary endothelial cells were damaged, causing acute lung injury.1,3 So it is suggested that inhibiting inflammatory response maybe the key way to cure the COVID-19. The first step of the SARS-CoV-2 pathogenesis is that the virus specifically recognizes the angiotensin I converting enzyme 2 receptor (ACE 2) by its spike Protein.3,4 This receptor is abundant in lung and small intestinal tissues, but is also highly expressed in vascular endothelial cells and smooth muscle cells in almost all organs, including the nervous system and skeletal muscle.5 Therefore, when the initial symptom is discomfort of other systems in the early stage, it is often easy to be misdiagnosed and delay treatment.
Mesenchymal stem cells (MSCs) are widely used in basic research and clinical application. MSCs, owing to their powerful immunomodulatory ability, may have beneficial effects on preventing or attenuating the cytokine storm. They proved to migrate to damaged tissues, exert anti-inflammatory and immunoregulatory functions, promote the regeneration of damaged tissues and inhibit tissue fibrosis.6–8 MSCs could secrete many types of cytokines by paracrine secretion or make direct interactions with immune cells, leading to immunomodulation.9,10 They significantly reduced acute lung injury in mice caused by H9N2 and H5N1 viruses by reducing the levels of proinflammatory cytokines and the recruitment of inflammatory cells into the lungs.10 MSCs also can secretion MSC-secretome, made of free-soluble protein and EVs, emerges as a promising cell-free therapeutic tool for the treatment of acute and chronic lung diseases, as it displays anti-inflammatory, immunomodulatory, regenerative, pro-angiogenic and anti-protease properties.11–13 MSC-secretome formulated as a freeze-dried powder and administered by intravenous injection (or inhalation), may represent a well-suited approach for the treatment of patients with COVID-19 pneumonia, especially for the ones in critically severe condition.12 Compared with MSCs from other sources, UC-MSCs have been widely applied to various diseases due to their convenient collection, no ethical controversy, low immunogenicity, and rapid proliferation rate.14,15
Here, we introduce a COVID-19 case of a critically ill male patient in China. Then we explore the safety and effectiveness of UC-MSC treatment and provide a new potential means for COVID-19.
Case Presentation
A 48-year-old male patient with COVID-19 without previous medical history. The patient developed a cough with a small amount of white sticky sputum on January 21, 2020. On January 31, he underwent Chest computerized tomography (CT) images in Wuhan Yangtze River Shipping General Hospital, and showed ground glass opacity in both lungs, which indicated that he had a bilateral pulmonary infectious disease. On February 1, he developed fever, with the highest body temperature of 39.2°C, so he received treatment in Tongji Hospital Sino-French New City Campus for 2 days, this patient was not confirmed as COVID-19, he was treated with routine bacterial pneumonia. On February 3, the nucleic acid test was confirmed he was diagnosed as COVID-19, and the patient was admitted to our hospital on February 4. On admission, his temperature was 38.4°C while other vital signs were normal. Meanwhile, on February 4, he showed higher than abnormal levels of neutrophil (NEU) ratio (90.7%), aspartate aminotransferase (AST) (89.9 U/L), alanine transaminase (ALT) (100 U/L), blood urea nitrogen (Bun) (8.41 mmol/L), indicators of inflammation C-reactive protein (CRP) (150 mg/L) and procalcitonin (PCT) (0.766 ng/mL). At the same time, the patient had low blood oxygen saturation (SpO2: 90%), so the nasal catheter was given oxygen with a flow rate of 3 L/min.
On February 6, the patient’s SpO2 fluctuated at 75–80%, and the asthmatic chest tightness was obvious in the quiet state. Then he was given noninvasive ventilator (FiO2: 100%) to assist ventilation, and the SpO2 rose to 91%.
On February 7, NEU and NEU rate increased to 6.32×109/L and 94.2%, respectively. The concentrations of Bun (10.47 mmol/L) and D-dimer (15.46 μg/mL) were higher than 3 days ago.
On February 7, the patient was confirmed as critically ill COVID-19, presenting with acute respiratory distress, multiple organ injury (hepatic respiratory system), immunosuppression and other symptoms. These resulted suggested that the current therapy was not effective, considering the severe organ damage caused by the inflammatory response and side effects, the adoptive transfer therapy of UC-MSCs was proposed under the recommendation and guidance of the expert group.
Cell transplantation: The clinical grade UC-MSCs were supplied for free by Shanghai University, Qingdao Co-orient Watson Biotechnology group co. LTD and the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. The cell product has been certified by the National Institutes for Food and Drug Control of China (authorization number: 2004L04792, 2006L01037, CXSB1900004). Before the intravenous drip, UC-MSCs were suspended in 100 mL of normal saline, and the dosage was calculated by 1 × 106 cells per kilogram of weight. The injection was performed about one hour with a speed of ~25 drops per minute. Antibiotics were discontinued on the day of UC-MSC input, and the rest of the treatment remained unchanged.
The patient was assessed by the investigators through the 14-day observation after UC-MSC treatment. The clinical laboratory and radiological outcomes were recorded and certified by a trained group of doctors.
First, we summarized the symptoms and treatment of the critically ill COVID-19 patient. In Figure 1, the patient was admitted with fever, cough, sputum, dyspnea, poor appetite, poor mental state and fatigue. After the UC-MSC transfusion, no obvious side effects were observed, indicating it was well tolerated. On February 9, the patient’s SpO2 returned to the normal level (95%). On February 13, the patient tried to take off the ventilator. After the weaning, the patient’s condition was stable and the SpO2 remained stable at the normal level. At the same time, the cough symptoms of the patient were relieved after treatment, and there was no sputum when coughing.
 |
Figure 1 The major symptoms and treatment of this critically ill COVID-19 patient. Light color indicates mild symptoms, while dark color indicates severe symptoms. |
The clinical laboratory features of the patient are shown in Table 1. The number of NEU returned to normal on 20 February (5.88 × 109/L). The number of lymphocyte was 0.23 × 109/L on February 7, gradually recovered after intravenous injection of UC-MSCs, and returned to normal on February 20 (1.32 × 109/L). Before February 9, AST and ALT levels were higher, and on February 17, both decreased to normal (AST: 18.3 U/L, ALT: 46.4 U/L). BUN also decreased gradually from 10.47 mmol/L on February 7, and returned to the normal of 6.01 mmol/L on February 12, which decreased by half. CRP gradually decreased from 150 mg/L before treatment, and returned to the normal level of 1.28 mg/L on February 20. PCT decreased from 0.766 ng/mL to 0.024 ng/mL, returned to normal level. The patient’s throat swab tested positive for the novel coronavirus nucleic acid on 9 February and negative on 20 February.
 |
Table 1 The Clinical Laboratory Characteristics of This Critically Ill COVID-19 Patient |
The CT and X-ray are shown in Figure 2. On February 4, CT showed multiple patchy ground glass density shadows, blurred boundary, and severe pulmonary fibrosis in double lungs. CT showed that the ground-glass opacity and pneumonia infiltration had obviously reduced on the 7th day after UC-MSC transplantation. The SpO2 increased to the normal range after UC-MSC transplantation 2 days, and the non-invasive ventilator was successfully removed after 6 days.
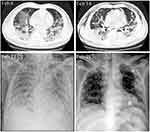 |
Figure 2 CT and X-ray images of this critically ill COVID-19 patient. |
From Table 2, the percentages of various types of lymphocytes in the patient were within the normal range, but the absolute number of lymphocytes had been at a low level. After the UC-MSC reinfusion treatment, the absolute number of lymphocytes increased to a certain extent, from 201/μL to 330/μL on the second day after treatment, and to 651/μL one week later. On February 7, the total absolute number of T lymphocytes was 152/μL, rose to 251/μL on February 9, and 547/μL on February 15. The absolute number of B lymphocytes increased from 21.8/μL to 46.8/μL, while the absolute number of NK cells increased from 17.9/μL to 29.3/μL. After the UC-MSC adjuvant treatment, the absolute number of lymphocytes of the patient was significantly increased. Thus, we speculated that UC-MSCs might adjust the body’s immune function, to improve inflammatory reaction, improve lung function and multiple organ functions to improve the outcome of this critically ill patient.
 |
Table 2 Lymphocyte Subpopulation Analysis of This Critically Ill COVID-19 Patient |
Conclusions
The patient with COVID-19 was a 48-year-old man without previous medical history. This hospitalization was due to the infection of SARS-CoV-2. This was a typical case of COVID-19. If the low SpO2 could not be corrected in time, the patient might die at any time. From Table 2, the percentages of various types of lymphocytes in the patient were within the normal range, but the absolute number of lymphocytes had been at a low level. After the UC-MSC adjuvant treatment, the absolute number of lymphocytes of the patient was significantly increased. Thus, we speculated that UC-MSCs might adjust the body’s immune function, to improve inflammatory reaction, improve lung function and multiple organ functions to improve the outcome of this critically ill patient.
We proposed that the adoptive transfer therapy of UC-MSCs might be an ideal choice to be used. Although only one case was shown here, it would also be very important to inspire more similar clinical practice to treat such critically ill COVID-19 patients.
Abbreviations
COVID-19, coronavirus disease 2019; UC-MSCs, human umbilical cord mesenchymal stem cells; MSCs, mesenchymal stem cells; ACE2, angiotensin I converting enzyme 2 receptor; NEU, neutrophil; AST, aspartate aminotransferase; ALT, alanine transaminase; Bun, blood urea nitrogen; CRP, C-reactive protein; PCT, procalcitonin; SpO2, blood oxygen saturation; CT, chest computerized tomography; LYM, lymphocyte; WBC, white blood cell; CREA, blood creatinine.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Ethics Approval and Consent to Participate
The study was conducted in Puren Hospital Affiliated to Wuhan University of Science and Technology, and approved by the ethics committee of the hospital (Number: 2020-001), and issued in ClinicalTrials.gov (NCT04339660), and informed consent was confirmed by the participant.
Acknowledgments
The clinical grade UC-MSCs were supplied for free by Shanghai University, Qingdao Co-orient Watson Biotechnology group co. LTD and the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. The cell product has been certified by the National Institutes for Food and Drug Control of China (authorization number: 2004L04792, 2006L01037, CXSB1900004).
Author Contributions
YZ, RJZ and KL: Transport MSCs, screening cases, execution, sample collection, data statistics, writing and revising manuscript.
XL: Transport MSCs, screening cases, follow up patients and writing manuscript.
DYB and JLL: Lymphocyte subgroup analysis, related testing and writing manuscript.
DZC, LL and JFH: Informed consent and enrolled patients, acquisition of data and writing manuscript.
YXL, YanZ and DYX: Technical guidance, analysis and interpretation, revising manuscript.
YL and Robert CHZ: Study design, project manage, overall planning, analysis, interpretation and revising manuscript.
All authors read and approved the final manuscript. All authors had agreed on the journal to which the article will be submitted. All authors agreed to take responsibility and be accountable for the contents of the article.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFA0101000, 2016YFA0101003, 2018YFA0109800, 2020YFC0844000), CAMS Innovation Fund for Medical Sciences (2017-I2M-3-007) and the 111 Project (B18007). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Munster VJ, Koopmans M, van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692–694. doi:10.1056/NEJMp2000929
2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi:10.1016/S0140-6736(20)30183-5
3. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi:10.1016/S0140-6736(20)30251-8
4. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi:10.1007/s11427-020-1637-5
5. Zhang Q, Lu S, Li T, et al. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. CR. 2019;38:173. doi:10.1186/s13046-019-1156-5
6. Wilson JG, K D L, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a Phase 1 clinical trial. Lancet Respiratory Med. 2015;3:24–32. doi:10.1016/S2213-2600(14)70291-7
7. Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi:10.1016/S1474-4422(11)70305-2
8. Hashmi S, Ahmed M, Murad MH, et al. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol. 2016;3:e4552. doi:10.1016/S2352-3026(15)00224-0
9. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi:10.1016/j.stem.2013.09.006
10. Darwish I, Mubareka S, Liles WC. Immunomodulatory therapy for severe influenza. Expert Rev Anti Infect Ther. 2011;9:807–822. doi:10.1586/eri.11.56
11. Muraca M, Pessina A, Pozzobon M, et al. Mesenchymal stromal cells and their secreted extracellular vesicles as therapeutic tools for COVID-19 pneumonia? Published online ahead of print, 2020 Jul 3. J Control Release. 2020;325:135–140. doi:10.1016/j.jconrel.2020.06.036
12. Bari E, Ferrarotti I, Saracino L, Perteghella S, Torre ML, Corsico AG. Mesenchymal stromal cell secretome for severe COVID-19 infections: premises for the therapeutic use. Cells. 2020;9(4):924. doi:10.3390/cells9040924
13. Bari E, Ferrarotti I, Torre ML, Corsico AG, Perteghella S. Mesenchymal stem/stromal cell secretome for lung regeneration: the long way through “pharmaceuticalization” for the best formulation. J Control Release. 2019;309:11–24. doi:10.1016/j.jconrel.2019.07.022
14. Esposito M, Lucariello A, Costanzo C, et al. Differentiation of human umbilical cord-derived mesenchymal stem cells, WJ-MSCs, into chondrogenic cells in the presence of pulsed electromagnetic fields. In Vivo (Brooklyn). 2013;27:495–500.
15. Shu Y, Yang C, Ji X, et al. Reversibly immortalized human umbilical cord-derived mesenchymal stem cells (UC-MSCs) are responsive to BMP9-induced osteogenic and adipogenic differentiation. J Cell Biochem. 2018;119:8872–8886. doi:10.1002/jcb.27140
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
