Back to Journals » OncoTargets and Therapy » Volume 13
Human Endogenous Retroviruses in Clear Cell Renal Cell Carcinoma: Biological Functions and Clinical Values
Authors Cao W , Kang R, Xiang Y, Hong J
Received 23 April 2020
Accepted for publication 13 July 2020
Published 7 August 2020 Volume 2020:13 Pages 7877—7885
DOI https://doi.org/10.2147/OTT.S259534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Wenjun Cao,1 Ran Kang,2 Yining Xiang,1 Jidong Hong1
1Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2Department of Urology, The First Affiliated Hospital of University of South China, Hengyang, Hunan, People’s Republic of China
Correspondence: Jidong Hong Department of Oncology
Xiangya Hospital, Central South University, 87 Xiangya Road, Changsha, Hunan Province 410008, People’s Republic of China
Email [email protected]
Abstract: Human endogenous retroviruses (HERVs) form an important part of the human genome, commonly losing their coding ability and exhibiting only rare expression in healthy tissues to promote the stability of the genome. However, overexpression of HERVs has been observed in various malignant tumors, including clear cell renal cell carcinoma (ccRCC), and may be closely correlated with tumorigenesis and progression. HERVs may activate the interferon (IFN) signaling pathway by a viral mimicry process to enhance antitumor immune responses. There is increasing interest in the diagnostic and prognostic value of HERVs in cancers, and they may be candidate targets for tumor immunotherapy. The review will introduce the biological functions of HERVs in ccRCC and their clinical value, especially in regard to immunotherapy.
Keywords: clear cell renal cell carcinoma, human endogenous retroviruses, immunotherapy, long terminal repeat, biomarker
Introduction
Millions of years ago, retroviruses invaded and integrated into the cells of human hosts, and the resulting genomic components are known as human endogenous retroviruses (HERVs), which are vertically inherited by offspring and persist throughout human evolution. HERVs form a vital component of the human genome, accounting for ~8%. To date, more than 50 HERV groups have been found and divided into classes I–III. For example, HERV-E and HERV-H belong to class I, whereas HERV-K belongs to class II.1 Full-length HERVs comprise gag, pol, env and two gene regulatory sequences on both sides of the 5ʹ and 3ʹ ends, namely, a long terminal repeat (LTR) (Figure 1).2 HERVs often lost coding ability but exist permanently in the genome due to deletion, mutation and hypermethylation.3 There are only a few genes that encode functional proteins such as HERV-K and HERV-W.3,4 HERV-W encodes Syncytin proteins, which are essential for placental syncytiotrophoblast cell development.4 Inactivation of HERVs can also be attributed to solo-LTRs, which are generated by non-allelic homologous recombination between the 5ʹ and 3ʹ LTRs.5,6 LTRs make great contributions to the complexity of the genome in providing promoters, enhancers, repressors, poly A signals and alternative splicing sites for human genes. The majority of HERVs reside in human genomic DNA as solo-LTRs, some of which retain primary transcriptional regulatory motifs and hence a gene regulatory capacity.6,7
A substantial body of research has indicated that dysregulated expression of HERVs is strongly correlated with the occurrence and development of clear cell renal cell carcinoma (ccRCC).8 Similar findings have been revealed for melanoma,9 breast cancer10 and bladder transitional cell carcinoma.11 ccRCC is one of the most prevalent types of renal cancer, accounting for ~85% of cases.12–14 ccRCC is highly resistant to radiotherapy and chemotherapy; first-line treatment relies on surgery, but the clinical efficacy is not satisfactory. Due to the lack of specific clinical manifestations in the early stage, approximately 30% of ccRCC patients are diagnosed at the metastatic stage, missing the opportunity for surgical intervention.15 ccRCC patients with American Joint Committee on Cancer (AJCC; 6th ed) stage I disease have a relatively good prognosis, but the five-year survival rate of AJCC stage IV ccRCC patients is only 18%.16 Recent breakthroughs have been achieved in ccRCC using targeted therapy and immunotherapy, but a considerable proportion of patients are resistant to targeted therapy and insensitive to immunotherapy, which is linked to a poor prognosis.17,18 Accumulating evidence suggests that HERVs are of great significance for the occurrence and development of ccRCC and have evolved to become promising diagnostic and therapeutic targets.8,19 Based on the available evidence, the review will elucidate the biological functions of HERVs in ccRCC and their potential involvement as targets for diagnosis and treatment and make recommendations for future investigations.
Biological Functions of HERVs in ccRCC
HERVs in the Occurrence and Progression of ccRCC: Mainly Related to LTRs
One mechanism of tumorigenesis is that the reactivation of LTRs drives ectopic expression.20 Research on the renal cell carcinoma (RCC) genome shows that reactivation of the hypoxia-inducible factor (HIF)-dependent dormant promoters embedded in LTRs mainly upregulates the expression of the stem cell transcription factor Pou5f1 in ccRCC patients, which promotes tumorigenesis by affecting transcriptional dysregulation.21 It is worth noting that self-renewing RCC stem cells may be a small group of tumor cells in patients that can produce the Pou5f1 protein.21 Similarly, abnormal activation of LTR promoters in Hodgkin lymphoma (HL) patients can drive the expression of the proto-oncogene colony-stimulating factor-1 receptor (CSF1R), while healthy controls do not exhibit LTR-driven CSF1R transcripts.22 Reactivation of LTRs is also controlled by the level of DNA methylation.23 Recently, pioneering research found that the HERV-E 5ʹ LTR, located on chromosome 6q, is hypomethylated in ccRCC and that the combination of HIF and a HIF response element (HRE) of the 5ʹ LTR can lead to overexpression of HERV-E transcripts and tumor antigen CT-RCC-1 in tumor tissues but not in normal tissues.8,24 Sporadic ccRCC has somatic mutations in the VHL gene, resulting in reduced degradation of HIF.25,26 The regulation of the VHL gene is particularly important for HERV-E activation. The VHL gene was introduced into a VHL-deficient ccRCC cell line, resulting in the inhibition of HERV-E expression.24 The B7/CD28 family members are important modulators of T-cell responses in the tumor immunity process, such as programmed cell death-1 (PD-1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA4). HERV-H LTR-associating protein 2 (HHLA2), a novel member of the B7 family, is related to the inhibition of the T-cell receptor. A recent study indicated that HHLA2 shows increased expression in ccRCC tissues compared to normal tissues and that HHLA2 may promote the progression of ccRCC by inactivating efficient antitumor T-cells and increasing tumor angiogenesis.19
HERVs affect tumorigenesis and prognosis in a variety of different ways. Some studies considered Np9, encoded by HERV-K type 1, as a novel viral oncogene that can activate several signaling pathways that confer proliferative and survival advantages to stem/progenitor leukemia cells, such as the β-catenin, AKT, Notch1 and ERK pathways.27,28 Studies on breast cancer, melanoma and germ cell tumors have shown that HERVs are oncogenic and act by activating the ERK1/2 cell signaling pathway.9,10 Abnormal expression of HERV elements has been detected in prostate cancer,29 breast cancer,30 lung cancer31 and colorectal cancer32 and is closely related to the occurrence and development of these tumors, but the underlying molecular mechanisms require further elucidation.
Immunomodulatory Effects of HERVs
For a long time, the immunomodulatory effects of HERVs have attracted much attention. Healthy people have low or no expression of HERVs and can generally tolerate the basal expression of HERVs to avoid autoimmune reactions. However, the expression of HERVs often increases in autoimmune diseases and tumors. Derived from retroviruses, HERVs and their products have high immunogenicity that may activate the antitumor immune response.33–35 The interferon (IFN) response is inducible by the double-stranded RNA (dsRNA) and single-stranded RNA (ssRNA) arising from HERVs in a process called “viral mimicry”.36,37 The dsRNA and ssRNA can activate its vital sensors including toll-like receptors (TLRs), RLRs retinoic acid-inducible gene I (RIG-I), and melanoma differentiated associated gene 5 (MDA 5), resulting in the expression of type I IFN by activating NF-κB, which can induce IFN-regulatory factor-3 and 7 (IRF-3 and IRF-7).36–39 IFNs can increase the expression of MHC type I on tumor cells, which may promote T-cells to recognize tumor cells (Figure 2).36,37 Anti-HERV-K gag and env antibodies have been detected in melanoma patients, which proves that HERV-K proteins can activate the humoral immune response, and the HERV-K antigen is the target of CTLs and is presented by HLA-A2 of HLA class I on melanoma cells.40,41 Tumor antigen CT-RCC-1 acts as the target antigen of cytotoxic T lymphocytes (CTLs) to increase immune infiltration in ccRCC patients.8 Smith et al42 discussed the mechanisms underlying the immune regulation of HERVs in ccRCC: (i) HERV 4700, located on chromosome 6q, is also known as CT-RCC HERV-E, which can trigger antitumor immunological effects as a direct target of the immune system or a new biomarker of anti-programmed death receptor 1 (aPD1) responsiveness, and (ii) the authors divided HERVs into 2 groups, group 2 HERVs may increase the immunosuppression of tumor microenvironment by downregulating the RIG-I-like pathway through an unknown mechanism. There are significant differences between the biological effects of different types of HERVs. HERV-H is important for tumor immune evasion and metastasis.43 HHLA2 may inactivate efficient antitumor T-cells and promote ccRCC tumor cell escape from immune attack.19 In short, the majority of HERVs are beneficial for activating the antitumor effect, but the tumor microenvironment may weaken this effect.
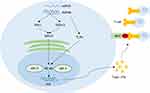 |
Figure 2 HERVs can up-regulate tumor immune signal and trigger subsequent antitumor immune by a viral mimicry process. The double-stranded RNA (dsRNA) and single-stranded RNA (ssRNA) arising from HERVs can active toll-like receptors (TLRs), RLRs retinoic acid-inducible gene I (RIG-I), and melanoma differentiated associated gene 5 (MDA 5), resulting in the activation of NF-κB. Consequently, NF-κB induces the production of type I interferon (IFN) by activating IFN-regulatory factor-3 and 7 (IRF-3 and IRF-7). IFNs can increase the expression of MHC type I on tumor cells to promote T-cells to recognize tumor cells. This process is called viral mimicry.Notes: Data from these studies.36–39 |
Potential Clinical Values of HERVs in ccRCC
Developing novel anticancer therapeutic targets depends upon identifying target antigens that exhibit differential expression between normal and tumor tissues. Overexpression of HERVs has been observed in various malignant tumors, and many studies show that HERVs have potential clinical values in the early diagnosis, prognostic prediction and immunotherapy of tumors. The lack of specific biomarkers and effective therapeutic targets are serious obstacles to improving the prognosis of ccRCC patients. The overexpression of HERVs and CTL responses against HERVs in ccRCC patients highlight HERVs as biomarkers for early diagnosis, targets for immunotherapy and predictors of prognosis.
HERVs Acting as Candidate Targets for Immunotherapy
HERVs Acting as Tumor Vaccine Targets
Effective tumor-associated antigens (TAAs) that can be recognized by T-cells have attracted great attention due to their ability to minimize the risk of autoimmunity. However, the lack of identified TAAs is still a major obstacle in developing tumor vaccines. To date, therapeutic vaccines for RCC have not achieved significant clinical efficacy;44 thus, it is necessary to develop novel specific TAAs as vaccine targets. HERVs are usually not expressed in noncancerous populations, and tumor vaccines based on HERVs may induce long-lasting CTL responses for the clearance of HERV-overexpressing cancer cells.
Previous studies have indicated the differential expression of HERVs between normal and tumor tissues and CTL responses against HERVs that highlight HERVs as targets for preventive and therapeutic tumor vaccines in ccRCC.8 Renal cancer vaccination directed against HERV-K gag and env protein has achieved preliminary success in animal models.45,46 Intravenous injection of cells expressing the HERV-K gag protein into a syngeneic murine renal cancer model can cause formation of pulmonary metastases. Vaccination with the recombinant vaccinia virus expressing the HERV-K gag protein can reduce the growth of pulmonary metastases.45 A previous study by the same author showed that prophylactic vaccination against the HERV-K env protein reduces tumor formation and metastasis of RCC, but vaccination safety and effectiveness in humans have not yet been evaluated.46 HERV-E env and LTRs are source of antigens recognized by T-cells in ccRCC patients; thus, the HERV-E env and LTRs products may act as vaccine targets.8,24,35 Moreover, studies have suggested that HERV-H Xp22.3 should be included in clinical vaccination programs for colorectal tumors, but further work is still required to estimate safety.47 To improve the safety of vaccines, future studies should develop tumor vaccines against single HERV site targets.
HERVs as Specific Targets for Adoptive Cell Therapy (ACT) and Monoclonal Antibody (mAb) Therapy
ACT and mAb therapy are effective cancer immunotherapy modalities. The common goals of these therapies are to reduce toxicity and increase efficacy by improving the specificity of T-cell responses. Isolated from ccRCC patients with tumor regression after hematopoietic stem cell transplantation (HSCT), CTLs can specifically recognize HERV-E env and LTRs expression products, which are promising targets for ACT and mAb therapy in ccRCC.24
The safety and efficacy of these therapies have been confirmed in other tumor (Table 1). HERV-K env-specific chimeric antigen receptor T (CAR-T) cells can reduce primary tumor and metastatic burden of murine xenograft model by recognizing and killing HERV-K env-overexpressing melanoma cells without attacking normal cells.48 Zhou et al49 observed a notable reduction or no metastasis to multiple organs in a murine breast cancer model after HERV-K env-specific CAR-T cell treatment, and they confirmed that HERV-K env products act as cancer-promoting proteins to promote oncogenesis related to the activation of Ras–ERK and MDM2 signaling. Wang-Johanning’s team verified that the anti-HERV-K env protein-specific mAb can suppress human breast cancer cell proliferation and limit tumor growth in breast tumor-bearing mice.34 As a result, they proposed HERV-specific mAbs as effective antitumor treatments earlier. In a phase IIb clinical trial involving 270 multiple sclerosis (MS) patients, GNbAC1, a humanized mAb targeting the HERV-W env protein, reduced brain atrophy, and showed a good safety with no hypersensitivity response or infusion reactions and uninduced immunogenicity.50,51
 |
Table 1 Efficacy of HERV-Specific CAR-T Cell or mAb in Different Diseases |
Epigenetic Therapy and Immune Checkpoint Inhibitor (ICI) Combinatory Treatment
Epigenetic therapy is an emerging strategy in anticancer therapy. DNA methyltransferase inhibitors (DNMTis), including azacitidine, decitabine and guadecitabine, are widely effective agents of epigenetic therapy that have exhibited remarkable clinical efficacy in hematological malignancies.52 DNMTis can reactivate HERVs to express dsRNA which can activate IFN signaling pathway by a viral mimicry process to up-regulate tumor immune signal and trigger subsequent antitumor immune responses (Figure 2).37,53,54 Roulois et al55 proved that the lasting anti-tumor effect of low-dose 5-aza-2-deoxycytidine (5-AZA-CdR) on colorectal cancer-initiating cells (CICs) induced by the production of dsRNA activates the viral mimicry process. The anti-tumor effect of 5-AZA-CdR can be simulated by transfecting dsRNA into CICs.55 PD ligand 1 (PD-L1)-PD-1 or CD80/86-CTLA-4 interactions can induce the immunosuppressive activities of immune checkpoints which can be disrupted by ICIs to promote T-cell activation.56 The viral mimic effect can also upregulate PD-L1 and CLAT4 to improve the sensitivity of ICIs.37,53 When used alone, both decitabine and ICI showed poor efficacy in a murine ovarian cancer model, but anti-CTLA-4 therapy showed enhanced efficacy when combined with decitabine.57 ICIs rarely induce a complete response (CR) in relapsed or refractory classical HL, but, in a clinical trial, all 5 patients treated with 5-azacitidine followed by ICI achieved a CR, while only 2 of the other 4 patients who received the ICI alone achieved a CR.58
Based on the above evidences that DNMTis can activate IFN signal pathway and enhance the efficacy of ICIs, accumulating clinical trials are addressing the safety and efficacy of DNMTis and ICI combinatory treatment (Table 2). The poor prognosis of ccRCC patients is closely related to resistance to targeted therapy and insensitivity to immunotherapy, but HERVs have raised hopes for novel therapies. At present, the combination of durvalumab and guadecitabine is being evaluated in a multicenter phase Ib/II clinical trial for advanced renal cancer (ClinicalTrials.gov identifier: NCT03308396). The final results are expected to demonstrate the effectiveness and safety of combinatory treatment in ccRCC patients. In addition, high HERV expression is related to a poor prognosis in ccRCC patients who may receive greater benefit from combinatory treatment.42
 |
Table 2 Clinical Trials Combining Epigenetic and Immune Therapy |
Roles of HERVs in ccRCC Early Diagnosis and Prognosis Prediction
Due to the lack of specific clinical manifestations in the early stage, the discovery of novel specific biomarkers, ideally measurable by noninvasive methods, is urgently required for early detection of ccRCC and for improving prognosis. Some findings have reported that HERV-K is overexpressed in early-stage breast tumor tissues but hardly expressed in healthy control samples raising the idea that HERV-K may be biomarker for the early detection of breast cancer.59 While prostate-specific antigen (PSA) is used as a biomarker for the early detection of prostate cancer, the overall sensitivity of the serum PSA level decreases with aging. In a study involving 294 prostate cancer patients and 135 healthy males, the HERV-K gag mRNA abundance in the blood was significantly higher in the patients with prostate cancer than in the healthy controls, and further results suggested HERV-K as potential biomarkers for the early detection of prostate cancer; the joint detection of HERV-K and PSA may improve diagnostic efficiency in prostate cancer.60 Moreover, HERV-K env can serve as a potential diagnostic biomarker of ovarian cancer.61 Given the above, HERVs are potential tumor-specific biomarkers and more studies are needed to determine the diagnostic values of these biomarkers in ccRCC, especially in early diagnosis.
The most common system for evaluating tumor progression and prognosis is the TNM staging system; however, it is insufficient for malignant tumors. Recently, there is gaining interest for prognostic values of HERVs in cancers (Table 3). High endogenous retrovirus (ERV) 1 expression is associated with poor patient survival in RCC.62 As mentioned before, LTRs play a key role in upregulating Pou5f1 expression, which is related to the progression of RCC and poor overall survival.21 Overexpressed HERV-K env is related to metastasis and a poor prognosis for breast cancer patients; thus, HERV-K env can be used as a new prognostic predictor for breast cancer patients.10,63 A study found that melanoma patients with anti-HERV-K antibodies had a significantly reduced overall survival rate.41 They reported that serological HERV-K responses might be an independent prognostic biomarker for a decreased survival rate in melanoma patients at AJCC stage I–III.41 Moreover, ERV3-2 overexpression in responders compared with nonresponders in a research involving 24 patients with metastatic ccRCC receiving PD-1/PD-L1 blockade suggested a possible role for HERVs as predictors of the antitumor immune response in renal cancers.64 The abnormal expression of HHLA2 has prognostic significance in gastric cancer and bladder urothelial carcinoma.65,66 The increased expression of HHLA2 in ccRCC tissues results in a shorter overall survival indicating that HHLA2 may be a potential prognostic marker of ccRCC.19
 |
Table 3 The Roles of HERVs in the Prognosis Prediction of Cancer |
Conclusion
With the discovery of abnormal expression of HERVs and anti-HERV antibodies in various tumors, the correlation between HERVs and cancers that has been suggested does not reveal a cause–effect relationship but provides evidence for HERVs as potential targets for cancer diagnosis and therapy. Great effort is needed to unravel possible contact and discover the molecular mechanisms involving HERVs in cancers.
HERV elements and expression products are intricately connected to cancers. The efficient reactivation of LTRs poses a significant threat to genome stability and serves vital biological roles in the occurrence and progression of ccRCC.8,19,21 HERVs may activate the IFN signaling pathway through a viral mimicry process to enhance antitumor immune responses (Figure 2).36–39 Epigenetic therapy may increase the sensitivity of cancer patients to ICIs through this process.37,53 Therefore, DNMTis and ICI combinatory treatment brings hope to cancer patients, especially for patients with poor response to ICIs. HERV-K-directed vaccination has achieved preliminary success in animal models.45 HERV-specific ACT and mAb therapy have shown efficiency in vitro experiments for melanoma and breast cancer and might represent promising novel therapeutic approaches in ccRCC, but clinical trials are required in the future for the assessment of the safety and efficacy of new therapies. Tumor immunotherapy targeting HERVs may be a key to developing breakthroughs in ccRCC therapy, especially in advanced-stage tumors.34,48,49 In addition, the great majority of the studies performed to date, both for disease etiopathogenesis and diagnosis, measured the expression of an entire HERV group. To link HERVs and cancer or biomarker usage to specific locus, future research should focus on the identification of single HERV sites.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang X, Huang J, Zhu F. Human endogenous retroviral envelope protein syncytin-1 and inflammatory abnormalities in neuropsychological diseases. Front Psychiatry. 2018;9:422. doi:10.3389/fpsyt.2018.00422
2. Garazha A, Ivanova A, Suntsova M, et al. New bioinformatic tool for quick identification of functionally relevant endogenous retroviral inserts in human genome. Cell Cycle. 2015;14(9):1476–1484. doi:10.1080/15384101.2015.1022696
3. Vargiu L, Rodriguez-Tome P, Sperber GO, et al. Classification and characterization of human endogenous retroviruses; mosaic forms are common. Retrovirology. 2016;13(1):7. doi:10.1186/s12977-015-0232-y
4. Denner J. Expression and function of endogenous retroviruses in the placenta. APMIS. 2016;124(1–2):31–43. doi:10.1111/apm.12474
5. Gemmell P, Hein J, Katzourakis A, Matsen IV FA. Phylogenetic analysis reveals that ervs “die young” but HERV-H is unusually conserved. PLoS Comput Biol. 2016;12(6):e1004964. doi:10.1371/journal.pcbi.1004964
6. Hughes JF, Coffin JM. Human endogenous retrovirus K solo-LTR formation and insertional polymorphisms: implications for human and viral evolution. Proc Natl Acad Sci U S A. 2004;101(6):1668–1672. doi:10.1073/pnas.0307885100
7. Belshaw R, Watson J, Katzourakis A, et al. Rate of recombinational deletion among human endogenous retroviruses. J Virol. 2007;81(17):9437–9442. doi:10.1128/JVI.02216-06
8. Cherkasova E, Scrivani C, Doh S, et al. Detection of an immunogenic HERV-E envelope with selective expression in clear cell kidney cancer. Cancer Res. 2016;76(8):2177–2185. doi:10.1158/0008-5472.CAN-15-3139
9. Lemaitre C, Tsang J, Bireau C, Heidmann T, Dewannieux M. A human endogenous retrovirus-derived gene that can contribute to oncogenesis by activating the ERK pathway and inducing migration and invasion. PLoS Pathog. 2017;13(6):e1006451. doi:10.1371/journal.ppat.1006451
10. Zhou F, Li M, Wei Y, et al. Activation of HERV-K Env protein is essential for tumorigenesis and metastasis of breast cancer cells. Oncotarget. 2016;7(51):84093–84117. doi:10.18632/oncotarget.11455
11. Yu H, Liu T, Zhao Z, et al. Mutations in 3ʹ-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene. 2014;33(30):3947–3958. doi:10.1038/onc.2013.366
12. Inamura K. Renal cell tumors: understanding their molecular pathological epidemiology and the 2016 WHO classification. Int J Mol Sci. 2017;18(10):2195. doi:10.3390/ijms18102195
13. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part a: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi:10.1016/j.eururo.2016.02.029
14. Lipworth L, Morgans AK, Edwards TL, et al. Renal cell cancer histological subtype distribution differs by race and sex. BJU Int. 2016;117(2):260–265. doi:10.1111/bju.12950
15. Dabestani S, Thorstenson A, Lindblad P, Harmenberg U, Ljungberg B, Lundstam S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol. 2016;34(8):1081–1086. doi:10.1007/s00345-016-1773-y
16. Feng X, Zhang L, Tu W, Cang S. Frequency, incidence and survival outcomes of clear cell renal cell carcinoma in the United States from 1973 to 2014: a SEER-based analysis. Medicine (Baltimore). 2019;98(31):e16684. doi:10.1097/MD.0000000000016684
17. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi:10.1056/NEJMoa1510665
18. Lalani AA, McGregor BA, Albiges L, et al. Systemic treatment of metastatic clear cell renal cell carcinoma in 2018: current paradigms, use of immunotherapy, and future directions. Eur Urol. 2019;75(1):100–110. doi:10.1016/j.eururo.2018.10.010
19. Chen D, Chen W, Xu Y, et al. Upregulated immune checkpoint HHLA2 in clear cell renal cell carcinoma: a novel prognostic biomarker and potential therapeutic target. J Med Genet. 2019;56(1):43–49. doi:10.1136/jmedgenet-2018-105454
20. Wiesner T, Lee W, Obenauf AC, et al. Alternative transcription initiation leads to expression of a novel ALK isoform in cancer. Nature. 2015;526(7573):453–457. doi:10.1038/nature15258
21. Siebenthall KT, Miller CP, Vierstra JD, et al. Integrated epigenomic profiling reveals endogenous retrovirus reactivation in renal cell carcinoma. EBioMedicine. 2019;41:427–442. doi:10.1016/j.ebiom.2019.01.063
22. Lamprecht B, Walter K, Kreher S, et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med. 2010;16(5):571–579. doi:10.1038/nm.2129
23. Wu Z, Mei X, Zhao D, et al. DNA methylation modulates HERV-E expression in CD4+ T cells from systemic lupus erythematosus patients. J Dermatol Sci. 2015;77(2):110–116. doi:10.1016/j.jdermsci.2014.12.004
24. Takahashi Y, Harashima N, Kajigaya S, et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Invest. 2008;118(3):1099–1109. doi:10.1172/JCI34409C1
25. Frew IJ, Moch H. A clearer view of the molecular complexity of clear cell renal cell carcinoma. Annu Rev Pathol. 2015;10(1):263–289. doi:10.1146/annurev-pathol-012414-040306
26. Gattolliat CH, Couve S, Meurice G, et al. Integrative analysis of dysregulated microRNAs and mRNAs in multiple recurrent synchronized renal tumors from patients with von hippel-lindau disease. Int J Oncol. 2018;53(4):1455–1468. doi:10.3892/ijo.2018.4490
27. Bhardwaj N, Montesion M, Roy F, Coffin JM. Differential expression of HERV-K (HML-2) proviruses in cells and virions of the teratocarcinoma cell line tera-1. Viruses. 2015;7(3):939–968. doi:10.3390/v7030939
28. Chen T, Meng Z, Gan Y, et al. The viral oncogene Np9 acts as a critical molecular switch for co-activating beta-catenin, ERK, Akt and Notch1 and promoting the growth of human leukemia stem/progenitor cells. Leukemia. 2013;27(7):1469–1478. doi:10.1038/leu.2013.8
29. Wang-Johanning F, Frost AR, Jian B, et al. Detecting the expression of human endogenous retrovirus E envelope transcripts in human prostate adenocarcinoma. Cancer. 2003;98(1):187–197. doi:10.1002/cncr.11451
30. Frank O, Verbeke C, Schwarz N, et al. Variable transcriptional activity of endogenous retroviruses in human breast cancer. J Virol. 2008;82(4):1808–1818. doi:10.1128/JVI.02115-07
31. Kahyo T, Tao H, Shinmura K, et al. Identification and association study with lung cancer for novel insertion polymorphisms of human endogenous retrovirus. Carcinogenesis. 2013;34(11):2531–2538. doi:10.1093/carcin/bgt253
32. Perot P, Mullins CS, Naville M, et al. Expression of young HERV-H loci in the course of colorectal carcinoma and correlation with molecular subtypes. Oncotarget. 2015;6(37):40095–40111. doi:10.18632/oncotarget.5539
33. Madeira A, Burgelin I, Perron H, Curtin F, Lang AB, Faucard R. MSRV envelope protein is a potent, endogenous and pathogenic agonist of human toll-like receptor 4: relevance of GNbAC1 in multiple sclerosis treatment. J Neuroimmunol. 2016;291:29–38. doi:10.1016/j.jneuroim.2015.12.006
34. Wang-Johanning F, Rycaj K, Plummer JB, et al. Immunotherapeutic potential of anti-human endogenous retrovirus-K envelope protein antibodies in targeting breast tumors. J Natl Cancer Inst. 2012;104(3):189–210. doi:10.1093/jnci/djr540
35. Grandi N, Tramontano E. HERV envelope proteins: physiological role and pathogenic potential in cancer and autoimmunity. Front Microbiol. 2018;9:462. doi:10.3389/fmicb.2018.00462
36. Gonzalez-Cao M, Iduma P, Karachaliou N, Santarpia M, Blanco J, Rosell R. Human endogenous retroviruses and cancer. Cancer Biol Med. 2016;13(4):483–488. doi:10.20892/j.issn.2095-3941.2016.0080
37. Chiappinelli KB, Strissel PL, Desrichard A, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2015;162(5):974–986. doi:10.1016/j.cell.2015.07.011
38. Alcazer V, Bonaventura P, Depil S. Human endogenous retroviruses (HERVs): shaping the innate immune response in cancers. Cancers (Basel). 2020;12(3):610. doi:10.3390/cancers12030610
39. Grandi N, Tramontano E. Human endogenous retroviruses are ancient acquired elements still shaping innate immune responses. Front Immunol. 2018;9:2039. doi:10.3389/fimmu.2018.02039
40. Schiavetti F, Thonnard J, Colau D, Boon T, Coulie PG. A human endogenous retroviral sequence encoding an antigen recognized on melanoma by cytolytic T lymphocytes. Cancer Res. 2002;62(19):5510–5516.
41. Hahn S, Ugurel S, Hanschmann KM, et al. Serological response to human endogenous retrovirus K in melanoma patients correlates with survival probability. AIDS Res Hum Retroviruses. 2008;24(5):717–723. doi:10.1089/aid.2007.0286
42. Smith CC, Beckermann KE, Bortone DS, et al. Endogenous retroviral signatures predict immunotherapy response in clear cell renal cell carcinoma. J Clin Invest. 2018;128(11):4804–4820. doi:10.1172/JCI121476
43. Kudo-Saito C, Yura M, Yamamoto R, Kawakami Y. Induction of immunoregulatory CD271+ cells by metastatic tumor cells that express human endogenous retrovirus H. Cancer Res. 2014;74(5):1361–1370. doi:10.1158/0008-5472.CAN-13-1349
44. Rini BI, Stenzl A, Zdrojowy R, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, Phase 3 trial. Lancet Oncol. 2016;17(11):1599–1611. doi:10.1016/S1470-2045(16)30408-9
45. Kraus B, Fischer K, Sliva K, Schnierle BS. Vaccination directed against the human endogenous retrovirus-K (HERV-K) gag protein slows HERV-K gag expressing cell growth in a murine model system. Virol J. 2014;11(1):58. doi:10.1186/1743-422X-11-58
46. Kraus B, Fischer K, Buchner SM, et al. Vaccination directed against the human endogenous retrovirus-K envelope protein inhibits tumor growth in a murine model system. PLoS One. 2013;8(8):e72756. doi:10.1371/journal.pone.0072756
47. Mullins CS, Linnebacher M. Endogenous retrovirus sequences as a novel class of tumor-specific antigens: an example of HERV-H env encoding strong CTL epitopes. Cancer Immunol Immunother. 2012;61(7):1093–1100. doi:10.1007/s00262-011-1183-3
48. Krishnamurthy J, Rabinovich BA, Mi T, et al. Genetic engineering of T cells to target HERV-K, an ancient retrovirus on melanoma. Clin Cancer Res. 2015;21(14):3241–3251. doi:10.1158/1078-0432.CCR-14-3197
49. Zhou F, Krishnamurthy J, Wei Y, et al. Chimeric antigen receptor T cells targeting HERV-K inhibit breast cancer and its metastasis through downregulation of ras. Oncoimmunology. 2015;4(11):e1047582. doi:10.1080/2162402X.2015.1047582
50. Diebold M, Derfuss T. The monoclonal antibody GNbAC1: targeting human endogenous retroviruses in multiple sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419833574. doi:10.1177/1756286419833574
51. Derfuss T, Curtin F, Guebelin C, et al. A phase IIa randomized clinical study testing GNbAC1, a humanized monoclonal antibody against the envelope protein of multiple sclerosis associated endogenous retrovirus in multiple sclerosis patients - a twelve month follow-up. J Neuroimmunol. 2015;285:68–70. doi:10.1016/j.jneuroim.2015.05.019
52. Tsai HC, Li H, Van Neste L, et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012;21(3):430–446. doi:10.1016/j.ccr.2011.12.029
53. Chiappinelli KB, Zahnow CA, Ahuja N, Baylin SB. Combining Epigenetic and Immunotherapy to Combat Cancer. Cancer Res. 2016;76(7):1683–1689. doi:10.1158/0008-5472.CAN-15-2125
54. Zhang M, Liang JQ, Zheng S. Expressional activation and functional roles of human endogenous retroviruses in cancers. Rev Med Virol. 2019;29(2):e2025. doi:10.1002/rmv.2025
55. Roulois D, Loo Yau H, Singhania R, et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell. 2015;162(5):961–973. doi:10.1016/j.cell.2015.07.056
56. Wrangle J, Wang W, Koch A, et al. Alterations of immune response of non-small cell lung cancer with azacytidine. Oncotarget. 2013;4(11):2067–2079. doi:10.18632/oncotarget.1542
57. Wang L, Amoozgar Z, Huang J, et al. Decitabine enhances lymphocyte migration and function and synergizes with CTLA-4 blockade in a murine ovarian cancer model. Cancer Immunol Res. 2015;3(9):1030–1041. doi:10.1158/2326-6066.CIR-15-0073
58. Falchi L, Sawas A, Deng C, et al. High rate of complete responses to immune checkpoint inhibitors in patients with relapsed or refractory Hodgkin lymphoma previously exposed to epigenetic therapy. J Hematol Oncol. 2016;9(1):132. doi:10.1186/s13045-016-0363-1
59. Wang-Johanning F, Li M, Esteva FJ, et al. Human endogenous retrovirus type K antibodies and mRNA as serum biomarkers of early-stage breast cancer. Int J Cancer. 2014;134(3):587–595. doi:10.1002/ijc.28389
60. Wallace TA, Downey RF, Seufert CJ, et al. Elevated HERV-K mRNA expression in PBMC is associated with a prostate cancer diagnosis particularly in older men and smokers. Carcinogenesis. 2014;35(9):2074–2083. doi:10.1093/carcin/bgu114
61. Rycaj K, Plummer JB, Yin B, et al. Cytotoxicity of human endogenous retrovirus K-specific T cells toward autologous ovarian cancer cells. Clin Cancer Res. 2015;21(2):471–483. doi:10.1158/1078-0432.CCR-14-0388
62. Zapatka M, Borozan I, Brewer DS, et al. The landscape of viral associations in human cancers. Nat Genet. 2020;52(3):320–330. doi:10.1038/s41588-019-0558-9
63. Zhao J, Rycaj K, Geng S, et al. Expression of human endogenous retrovirus type K envelope protein is a novel candidate prognostic marker for human breast cancer. Genes Cancer. 2011;2(9):914–922. doi:10.1177/1947601911431841
64. Panda A, de Cubas AA, Stein M, et al. Endogenous retrovirus expression is associated with response to immune checkpoint blockade in clear cell renal cell carcinoma. JCI Insight. 2018;3(16). doi:10.1172/jci.insight.121522.
65. Wei L, Tang L, Chang H, Huo S, Li Y. HHLA2 overexpression is a novel biomarker of malignant status and poor prognosis in gastric cancer. Hum Cell. 2020;33(1):116–122.
66. Lin G, Ye H, Wang J, Chen S, Chen X, Zhang C. Immune checkpoint human endogenous retrovirus-H long terminal repeat-associating protein 2 is upregulated and independently predicts unfavorable prognosis in bladder urothelial carcinoma. Nephron. 2019;141(4):256–264. doi:10.1159/000495887
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

