Back to Journals » Drug Design, Development and Therapy » Volume 9
Human colon carcinogenesis is associated with increased interleukin-17-driven inflammatory responses
Authors Xie Z, Qu Y, Leng Y, Sun W, Ma S, Wei J, Hu J, Zhang X
Received 17 December 2014
Accepted for publication 2 February 2015
Published 18 March 2015 Volume 2015:9 Pages 1679—1689
DOI https://doi.org/10.2147/DDDT.S79431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Zhaohui Xie,1 Yine Qu,2 Yanli Leng,2 Wenxiu Sun,2 Siqi Ma,2 Jingbo Wei,2 Jiangong Hu,3 Xiaolan Zhang1
1Department of Gastroenterology, Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 2Department of Histology and Embryology, Hebei United University School of Basic Medicine, Tangshan, Hebei, People’s Republic of China; 3Department of Pathology, the Second Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China
Abstract: Inflammation is known to contribute to carcinogenesis in human colorectal cancer. Proinflammatory cytokine interleukin-17 (IL-17 or IL-17A) has been shown to play a critical role in colon carcinogenesis in mouse models. However, few studies have investigated IL-17A in human colon tissues. In the present study, we assessed IL-17-driven inflammatory responses in 17 cases of human colon adenocarcinomas, 16 cases of human normal colon tissues adjacent to the resected colon adenocarcinomas, ten cases of human ulcerative colitis tissues from biopsies, and eight cases of human colon polyps diagnosed as benign adenomas. We found that human colon adenocarcinomas contained the highest levels of IL-17A cytokine, which was significantly higher than the IL-17A levels in the adenomas, ulcerative colitis, and normal colon tissues (P<0.01). The levels of IL-17 receptor A (IL-17RA) were also the highest in human colon adenocarcinomas, followed by adenomas and ulcerative colitis. The increased levels of IL-17A and IL-17RA were accompanied with increased IL-17-driven inflammatory responses, including activation of extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK) pathways, increase in expression of matrix metalloproteinase (MMP)9, MMP7, MMP2, B-cell lymphoma (Bcl-2), and cyclin D1, decrease in Bcl-2-associated X protein (BAX) expression, and increase in vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR) expression that were associated with increased angiogenesis. These findings suggest that IL-17 and its signaling pathways appear as promising new targets in the design and development of drugs for cancer prevention and treatment, particularly in colorectal cancer.
Keywords: colitis, colorectal cancer, inflammation, IL-17
Introduction
Interleukin-17 (IL-17 or IL-17A) is a proinflammatory cytokine.1 It binds to a heterodimer of IL-17 receptor A (IL-17RA) and IL-17 receptor C (IL-17RC) complex, and subsequently activates nuclear factor-κB (NF-κB) activator 1 (Act1) through a similar expression to fibroblast growth factor genes, IL-17 receptors, and the Toll–IL-1R (SEFIR) domain.2–6 Act1 is an E3 ubiquitin ligase, which activates tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) through lysine-63-linked ubiquitination.7 The polyubiquitinated TRAF6 activates transforming growth factor-β-activated kinase 1 (TAK1) and then IκB kinase (IKK) complex, which subsequently activates the NF-κB pathway to initiate transcription of a variety of chemokines, cytokines, and growth factor, such as C-X-C motif ligand 1 (CXCL1), IL-6, and vascular endothelial growth factor (VEGF).8–10 Some previous studies have demonstrated that IL-17 can stabilize downstream CXCL1 messenger (m)RNA through an inducible kinase IKKi-dependent Act1–TRAF2–TRAF5 complex, which binds to splicing factor 2 (SF2) (also named alternative splicing factor [ASF]) and prevents SF2/ASF-mediated mRNA degradation.11,12 IL-17 also activates mitogen-activated protein kinases (MAPK), including the extracellular signal-regulated kinase (ERK)1/2, p38, and c-Jun N-terminal kinase (JNK1/2/3) pathways. ERK1/2 and p38 may act through increasing target mRNA stability.13,14 The JNK pathway acts through activator protein-1 (AP-1) transcription factor.15,16
Human colorectal cancer remains the third most common malignancy and third leading cause of cancer-related deaths in the United States.17 The American Cancer Society estimates that there were approximately 136,830 new cases and 50,310 deaths due to colorectal cancer in American men and women in 2014.17 In People’s Republic of China, colorectal cancer incidence and mortality ranked the third and sixth, respectively, in most areas from 1993 to 1997.18 In recent years, the colorectal cancer incidence in the People’s Republic of China has increased.19 There are several factors that have been associated with the increased risk of developing colorectal cancer, such as old age, polyps in the colorectum, ulcerative colitis and Crohn’s disease, a family history of colorectal cancer, African American or Ashkenazi ethnicity, type 2 diabetes, and certain family syndromes, such as familial adenomatous polyposis (FAP) or hereditary nonpolyposis colon cancer (HNPCC), also called Lynch syndrome.20 Some lifestyle-related factors have also been linked to a higher risk of colorectal cancer, such as diet with red meats (beef, lamb, or liver) and processed meats (like hot dogs and bologna), cooking meats at very high heat (frying, broiling, or grilling), lack of exercise, overweight and obesity, smoking, and heavy alcohol use.20–22 Inflammatory bowel diseases (including ulcerative colitis and Crohn’s disease) are well-known for their risk to develop colorectal cancer.23 Of particular interest, IL-17 expression has been associated with both ulcerative colitis and Crohn’s disease.24 Furthermore, neutralization of IL-17 in the multiple intestinal neoplasia (Min) mouse model inhibited intestinal cancer formation,25 which was confirmed independently by studies using IL-17A knockout and IL-17F knockout mice.26,27 While IL-17A’s role in promoting colon carcinogenesis has been confirmed by many independent research groups,25,26 IL-17F has been found to have a protective role in colon cancer development in other study.28 Guo et al reported that IL-17 inhibited TNF-α-induced expression of IL-12P35 and CXCL11 in human colon cancer cell line HT-29.29 However, Wang et al found that IL-17 enhanced TNFα-induced expression of CXCL1, CXCL2, CXCL5, IL-8, and CCL20 in HT29 cells.30 Hölttä et al found that mRNA expression of IL-17, IL-22, and IL-6 was increased in the human colon samples from Crohn’s disease and ulcerative colitis.31 Wedebye Schmidt et al showed that neutralization of both IL-17A and IL-17F ameliorated intestinal inflammation in a mouse model of colitis.32 Hyun et al demonstrated that IL-17A knockout mice developed fewer and smaller colon tumors compared with wild-type mice, in a mouse model of colitis-associated cancer.33 Recently, Qi et al reported that IL-17A expression was increased in a mouse model of colitis-associated cancer and that anti-IL-17A antibody treatment could suppress cancer development in the mice.34 Finally, De Simone et al reviewed the published experimental data on the role of T helper (Th)17 cytokines in colorectal tumorigenesis.35 However, given the consistent findings regarding IL-17A’s tumor-promoting role in the preclinical animal models, very few studies have been performed to validate these findings in human colorectal cancer.36 Therefore, we conducted the present study, the purpose of which was to assess IL-17A and IL-17RA levels and to assess the biological changes that IL-17 signaling may have caused in human colon adenocarcinoma, in comparison with human normal colon, ulcerative colitis, and adenoma of the colon.
Materials and methods
Human tissue specimens
Human tissue specimens were obtained from the Third Hospital of Chengde City, Hebei Province, People’s Republic of China, from January to December 2013. This study was approved by the Institutional Review Board of the Third Hospital of Chengde City. All samples were anonymously coded in accordance with local ethical guidelines, and written informed consents were obtained from the patients. The procedures to obtain human colon tissues were in accordance with the Ethical Principles for Medical Research Involving Human Subjects, as formulated in the World Medical Association Declaration of Helsinki (revised in 2008). During surgical resection, we collected tumor tissues from 17 cases of colorectal adenocarcinomas, and the normal colon tissues approximately 5 cm away from the macroscopic margin of the resected tumors. We collected tissues from eight patients whose colon polyps were endoscopically removed and diagnosed as colon adenomas. We also collected tissues from ten patients who were endoscopically biopsied and diagnosed with ulcerative colitis. Approximately half of each specimen was used for pathologic examination, and the other half was used for protein extraction. The patient ages were between 18 and 86 years old, with a median age of 60 years old. There were 24 male and eleven female patients. None of the patients had received any radiotherapy or chemotherapy prior to biopsy or surgery.
Reagents
Mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Anbo Biotech Co., Ltd (San Francisco, CA, USA). Rabbit antibodies against cyclin D1, ERK1/2, phospho-ERK (P-ERK)1/2, JNK, phospho-JNK (P-JNK), IL-17, IL-17RA, matrix metalloproteinase (MMP)2, MMP9 B-cell lymphoma (Bcl-2), and Bcl-2-associated X protein (BAX) were obtained from Epitomics (Burlingame, CA, USA). Rabbit anti-MMP7 antibodies were purchased from Eterlife, Ltd (Birmingham, UK). Mouse anti-β-actin antibodies were ordered from Affinity Biosensors (Changzhou, People’s Republic of China). Radioimmunoprecipitation assay (RIPA) buffer was purchased from Bestbio (Shanghai, People’s Republic of China). An IL-17 Enzyme-linked Immunosorbent Assay (ELISA) kit was obtained from MultiSciences Biotech Co., Ltd (Hangzhou, People’s Republic of China). MMP9, VEGF, and cluster of differentiation 34 antigen (CD34) immunohistochemical (IHC) staining kits were purchased from 4A Biotech Co., Ltd (Beijing, People’s Republic of China).
ELISA assay
The IL-17A protein levels in human colon tissues were measured using an ELISA kit, according to the manufacturer’s instructions. Each sample was analyzed in duplicate wells in 96-well plates. The mean of the duplicate readings was used to calculate IL-17A cytokine concentrations (unit: pg/mL) using a linear regression curve derived from a series of IL-17A protein standards provided in the kit. Then, IL-17A cytokine concentration was normalized by the protein concentration of each sample (unit: g/mL), thus the tissue IL-17A cytokine levels were presented as IL-17A cytokine amount per gram of tissue protein (unit: pg/mL).
Western blot analysis
Proteins were extracted from the tissues using RIPA lysis buffer and an electric homogenizer on ice. Equal amounts of proteins were fractionated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. The membranes were blocked with 5% nonfat dry milk in TBST buffer (25 mM tris(hydroxymethyl)aminomethane [Tris]-HCl, 125 mM NaCl, and 0.1% Tween 20) for 2 hours and probed with the indicated primary antibodies overnight and then, with horseradish peroxidase-conjugated secondary antibodies. The horseradish peroxidase substrate reaction was performed using a chemiluminescence detection system with a charge-coupled device camera. For loading control, the membranes were stripped and probed for either β-actin or GAPDH. Three samples from each group were randomly selected for western blot analysis. Quantification of the western blot signals was performed using an ImageJ system (image analysis software developed by the US National Institutes of Health, Bethesda, MD, USA). The integrated density values of IL-17A and IL-17RA were normalized by those of β-actin. The integrated density values of MMP9, MMP7, MMP2, Bcl-2, BAX, cyclin D1, vascular endothelial growth factor (VEGF), and VEGF receptor (VEGFR) were normalized by those of GAPDH. The integrated density values of P-ERK1/2 and P-JNK were normalized by those of ERK1/2 and JNK, respectively. The ratios represent the relative level of the proteins. The data represent the mean and standard error of the mean (SEM) of three samples from each group (n=3).
IHC staining
Formalin-fixed and paraffin-embedded samples were cut into 4 μm serial sections that were processed for hematoxylin and eosin (H&E) and IHC staining according to the manufacturer’s instructions. IHC staining was performed using antibodies against MMP9, VEGF, and CD34 (to identify microvessels). The negative control used nonimmune serum instead of the primary antibodies. The positive control used a sample that was known to express the target protein. Representative photomicrographs were presented. Six samples from each group were randomly selected for image analysis. Ten high-power (×400) fields of each sample were randomly and automatically selected using the ImageJ system. The intensity of staining was normalized by the area of the selected region. The data represent the mean and SEM of six samples of each group (n=6). Six samples from each group were randomly selected for CD34 staining and counting of microvessel density. Five high-power (×200) fields were randomly selected from each sample to count the number of microvessels. The average number from the five high-power fields represents each sample. The data represent the mean and SEM of six samples in each group (n=6).
Statistical analysis
The results were presented as means ± SEM. Statistical significance was determined by one-way analysis of variance (ANOVA) and Tukey’s tests, using GraphPad Prism software version 6.1 (GraphPad Software, La Jolla, CA, USA). P-value <0.05 was considered as statistically significant.
Results
Human colon cancer tissues contain the highest levels of IL-17A
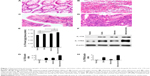
We measured the levels of IL-17A cytokine in 17 cases of human colon adenocarcinomas, 16 cases of human normal colon tissues adjacent to the resected colon adenocarcinomas, ten cases of human ulcerative colitis tissues from biopsies, and eight cases of human colon polyps diagnosed as benign adenomas. The diagnosis of each case was reconfirmed by a pathologist (Figure 1A–D). Using an ELISA kit, we found that human colon adenocarcinomas contained the highest levels of IL-17A cytokine, which was significantly higher than the IL-17A levels in adenomas, ulcerative colitis, and normal colon tissues (P<0.01) (Figure 1E). Both adenomas and ulcerative colitis tissues contained higher levels of IL-17A cytokine than did normal colon tissues (P<0.01) (Figure 1E), but there was no statistically significant difference between adenomas and ulcerative colitis tissues. To further confirm the ELISA results, we randomly selected three samples from each group and performed western blot analyses of IL-17A and IL-17RA levels. We found that, consistent with the ELISA results, colon adenocarcinomas had the highest levels of IL-17A, which was followed by adenomas and ulcerative colitis tissues, whereas IL-17A levels were barely detectable in normal colon tissues when using western blot analysis (Figure 1F–H). To our surprise, we also found that the IL-17RA levels were the highest in colon adenocarcinomas, followed by ulcerative colitis and then adenomas, while the IL-17RA levels were very low in normal colon tissues (Figure 1F–H).
The MAPK ERK1/2 and JNK pathways are activated in neoplastic colon lesions
In our search to find which IL-17 signaling pathways were activated, we performed western blot analyses on three samples randomly selected from each group. We found that P-ERK1/2 levels were the highest in colon adenocarcinomas, followed by adenomas and then ulcerative colitis, with a low basal level found in normal colon tissues (Figure 2A and B). A similar trend was observed in P-JNK levels, except that the signals were almost identical between adenomas and adenocarcinomas (Figure 2A and C).
Several IL-17 downstream genes are differentially expressed in neoplastic colon lesions
We found that MMP9 levels were the highest in colon adenocarcinomas, followed by ulcerative colitis and adenomas, while the MMP9 levels were barely detectable in normal colon tissues (Figure 3A and B). MMP7 and MMP2 levels were only dramatically increased in adenomas and adenocarcinomas, with slightly higher levels of MMP2 in adenocarcinomas than in adenomas (Figure 3A, C, and D). In addition, we found that Bcl-2 and cyclin D1 levels were dramatically increased in adenocarcinomas, followed by adenomas, whereas neither was detectable in ulcerative colitis or normal colon tissues (Figure 3A, E, and G). In contrast, BAX levels were dramatically reduced, to almost undetectable levels, in adenocarcinomas compared with the other three groups (Figure 3A and F). Using IHC staining, we found that MMP9 protein was not detectable in normal colon epithelium (Figure 4A), but it was seen in a few inflammatory cells in the stroma (Figure 4A). In ulcerative colitis tissues, more inflammatory cells were stained positive for MMP9 (Figure 4B). In contrast, the neoplastic epithelium in adenomas was stained moderately positive for MMP9 (Figure 4C), while many stromal inflammatory cells were stained positive for MMP9 (Figure 4C). Further, the malignant epithelium in adenocarcinomas was stained strongly positive for MMP9 (Figure 4D), with many stromal inflammatory cells being stained positive for MMP9 (Figure 4D). MMP9 staining intensity was the highest in adenocarcinomas, followed by adenomas and colitis, with the lowest in normal colon tissues (Figure 4E).
VEGF levels are increased in neoplastic colon lesions accompanied with increased angiogenesis
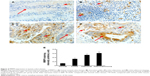
We found that VEGF levels were the highest in adenocarcinomas, followed by adenomas, whereas VEGF levels were not detectable in ulcerative colitis and normal colon tissues (Figure 5A and K). VEGFR levels were also the highest in adenocarcinomas, followed by ulcerative colitis and adenomas, with a low basal level in normal colon tissues (Figure 5A and L). Compared with a negative staining section (Figure 5B), VEGF protein was not detectable in normal colon tissues under IHC staining (Figure 5C). In contrast, VEGF protein was readily detectable in ulcerative colitis (Figure 5D), and strong staining of VEGF was seen in adenomas (Figure 5E) and adenocarcinomas (Figure 5F). VEGF protein was mostly detected in the epithelium (Figure 5D–F), but a few stromal cells were also stained positive. VEGF staining intensity was the highest in adenocarcinomas, followed by adenomas and colitis, with the lowest in normal colon tissues (Figure 5M). As VEGF is known for its role in angiogenesis, we assessed microvessels in the colon tissues, using CD34 staining. We found that microvessels were sparsely distributed in the stroma of normal colon tissues (Figure 5G), with somewhat increase in numbers in ulcerative colitis (Figure 5H). In contrast, we observed dramatically increased numbers of microvessels in the stroma of adenomas (Figure 5I) and adenocarcinomas (Figure 5J). The microvessel density was the highest in adenocarcinomas, followed by adenomas and colitis, with the lowest found in normal colon tissues (Figure 5N).
Discussion
In the present study, we found that IL-17A cytokine and IL-17RA levels were increased in human colon adenocarcinomas, adenomas, and ulcerative colitis, compared with the normal colon tissues. Particularly, both IL-17A and IL-17RA levels were the highest in human colon adenocarcinomas. Of note, the differences of IL-17A levels between the groups appeared more dramatic in the western blot analysis (Figure 1F) than in ELISA assays (Figure 1E), which could be due to the higher sensitivity of the western blot analysis. Previously it has been reported that IL-17A mRNA and IL-17-positive immune cell numbers are increased in human colon cancer tissues.36 Consequently, we found that both ERK1/2 and JNK pathways were highly activated in adenomas and adenocarcinomas, which may be due to the increased levels of IL-17A and IL-17RA because IL-17 has been shown to activate these MAPK pathways.37–41 IL-17A has been shown to activate ERK1/2 signaling in human colon cancer cell line HT-29.29 Activation of these pathways may upregulate the IL-17 downstream genes. Indeed, we found that expression of MMP9, MMP7, and MMP2 was increased in colon adenomas and adenocarcinomas. MMP9 and MMP2 are known IL-17 downstream genes that may promote tumor invasion and metastasis.42 They have also been found to play a role in lung cancer development.43 MMP7 has been shown to be a key factor in development of invasive prostate cancer in a mouse model.44 Our findings suggest that IL-17 may act through upregulation of MMP9, MMP7, and MMP2 to promote development of colon adenomas and adenocarcinomas.
IL-17 may indirectly affect apoptosis and cellular proliferation. It has been shown that IL-17 can upregulate IL-6 expression.8,45 In turn, IL-6 can upregulate Bcl-2 and Bcl-xL, both of which are antiapoptotic proteins.46 We found that Bcl-2 levels were increased in colon adenomas and adenocarcinomas, particularly in the later, which may be associated with the increased IL-17 signaling in these tissues. On the other hand, the proapoptotic BAX protein levels were dramatically decreased in colon adenocarcinomas. The decrease of the BAX/Bcl-2 ratio may favor the survival of colon adenocarcinoma cells as it has been shown previously that the BAX/Bcl-2 ratio determines survival or apoptosis of the cells.47 We found that cyclin D1 levels were increased in colon adenomas and adenocarcinomas, which is associated with increased cellular proliferation often seen in adenomas and adenocarcinomas. However, we do not know whether cyclin D1 is directly or indirectly regulated by IL-17A. It has been reported that in a mouse model of colitis-associated colon cancer, cyclin D1 expression is reduced in IL-17A-deficient mice, and this is associated with reduced proliferation scores and tumorigenesis compared with those in IL-17A wild-type mice.33 In another mouse model of skin cancer, cyclin D1 expression is also reduced in IL-17A-deficient mice, which is associated with reduced Ki67-positive cell number and tumorigenesis compared with IL-17A wild-type mice.48
We found that VEGF and VEGFR levels were increased in colon adenomas and adenocarcinomas, particularly in adenocarcinomas. IL-17 has been demonstrated to be able to increase VEGF expression.49,50 Therefore, we speculate that the increased levels of VEGF and VEGFR are associated with the increased levels of IL-17 and IL-17RA in adenomas and adenocarcinomas. It has been reported that IL-17 can enhance VEGF’s angiogenic action.51 Thus, it is not surprising that we found the numbers of microvessels were dramatically increased in adenomas and adenocarcinomas. We believe that this finding is consistent with the high levels of IL-17A/IL-17RA and VEGF/VEGFR in adenomas and adenocarcinomas, according to the previous report.51 It has been demonstrated that blockade of IL-17 signaling by IL-17RC knockout leads to decreased VEGF expression and, consequently, reduced angiogenesis, in a mouse model of prostate cancer.52 It has also been well-known that angiogenesis is able to promote tumor growth.53
In summary, this study provides data from human colon tissues to demonstrate that IL-17A and IL-17RA levels are increased in colon adenomas and adenocarcinomas, which are associated with increased IL-17-driven inflammatory responses, including activation of ERK1/2 and JNK pathways, increase in expression of MMP9, MMP7, MMP2, Bcl-2, and cyclin D1, decrease in BAX expression, and increase in VEGF and VEGFR expression. These responses are involved in many hallmarks of cancer, such as inflammation, invasion and metastasis, resistance to cell death, sustained proliferative signaling, and angiogenesis.54 Therefore, IL-17 and its signaling pathways appear as promising new targets in the design and development of drugs for cancer prevention and treatment, particularly in colorectal cancer. This conclusion is consistent with the previous reports. Our findings provide evidence from human specimens to support this conclusion. However, our findings need to be interpreted together with the results from the studies performed using the cell lines and animal models, in order to reach any definite conclusion. Preclinical animal studies have shown that anti-IL-17 antibodies are effective in suppressing IL-17-mediated colon and skin carcinogenesis, and SR1001 (an inhibitor of Th17 cell differentiation) has been shown to suppress IL-17-mediated autoimmunity.25,55,56 In human clinical trials, blockage of IL-17 signaling by anti-IL-17 or anti-IL-17RA antibodies has been effective in treating psoriasis, rheumatoid arthritis, and uveitis, without increasing any adverse events, including infections.57–59 The findings from this study suggest that it is reasonable to conduct human clinical trials to prevent and/or treat human colon cancer, using the anti-IL-17 antibodies that have passed Phase II clinical trials.57–59
Acknowledgment
This work was supported by the Specialized Research Fund for Key Program of Hebei (grant number 20120196).
Disclosure
The authors report no conflicts of interest in this work.
References
Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. | ||
Novatchkova M, Leibbrandt A, Werzowa J, Neubüser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28(5):226–229. | ||
Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281(47):35603–35607. | ||
Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8(3):247–256. | ||
Maitra A, Shen F, Hanel W, et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104(18):7506–7511. | ||
Ho AW, Shen F, Conti HR, et al. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol. 2010;185(2):1063–1070. | ||
Liu C, Qian W, Qian Y, et al. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci Signal. 2009;2(92):ra63. | ||
Ge D, Dauchy RT, Liu S, et al. Insulin and IGF1 enhance IL-17-induced chemokine expression through a GSK3B-dependent mechanism: a new target for melatonin’s anti-inflammatory action. J Pineal Res. 2013;55(4):377–387. | ||
Zhu S, Pan W, Song X, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med. 2012;18(7):1077–1086. | ||
Hwang SY, Kim JY, Kim KW, et al. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res Ther. 2004;6(2):R120–R128. | ||
Bulek K, Liu C, Swaidani S, et al. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol. 2011;12(9):844–852. | ||
Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nat Immunol. 2011;12(9):853–860. | ||
Faour WH, Mancini A, He QW, Di Battista JA. T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: role of distal sequences in the 3′-untranslated region of COX-2 mRNA. J Biol Chem. 2003;278(29):26897–26907. | ||
Hata K, Andoh A, Shimada M, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282(6):G1035–G1044. | ||
Ma X, Reynolds SL, Baker BJ, Li X, Benveniste EN, Qin H. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J Immunol. 2010;184(9):4898–4906. | ||
Sylvester J, Liacini A, Li WQ, Zafarullah M. Interleukin-17 signal transduction pathways implicated in inducing matrix metalloproteinase-3, -13 and aggrecanase-1 genes in articular chondrocytes. Cell Signal. 2004;16(4):469–476. | ||
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Zeng S, Cai S. Colorectal cancer epidemiology and prevention study in China. Chin-Ger J Clin Oncol. 2003;2(2):72–75. | ||
Xu AG, Yu ZJ, Jiang B, et al. Colorectal cancer in Guangdong Province of China: a demographic and anatomic survey. World J Gastroenterol. 2010;16(8):960–965. | ||
Ahmed S, Johnson K, Ahmed O, Iqbal N. Advances in the management of colorectal cancer: from biology to treatment. Int J Colorectal Dis. 2014;29(9):1031–1042. | ||
Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematol Oncol Clin North Am. 2015;29(1):1–27. | ||
Tárraga López PJ, Albero JS, Rodríguez-Montes JA. Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol. 2014;7:33–46. | ||
Desai D, Desai N. Colorectal cancer surveillance in inflammatory bowel disease: A critical analysis. World J Gastrointest Endosc. 2014;6(11):541–548. | ||
Liu S, Ren J, Li J. IL-17RA in intestinal inflammation: structure, signaling, function, and clinical application. Inflamm Bowel Dis. 2015;21(1):154–166. | ||
Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–1022. | ||
Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2010;107(12):5540–5544. | ||
Chae WJ, Bothwell AL. IL-17F deficiency inhibits small intestinal tumorigenesis in ApcMin/+ mice. Biochem Biophys Res Commun. 2011;414(1):31–36. | ||
Tong Z, Yang XO, Yan H, et al. A protective role by interleukin-17F in colon tumorigenesis. PLoS One. 2012;7(4):e34959. | ||
Guo X, Jiang X, Xiao Y, et al. IL-17A signaling in colonic epithelial cells inhibits pro-inflammatory cytokine production by enhancing the activity of ERK and PI3K. PLoS One. 2014;9(2):e89714. | ||
Wang YL, Fang M, Wang XM, et al. Proinflammatory effects and molecular mechanisms of interleukin-17 in intestinal epithelial cell line HT-29. World J Gastroenterol. 2014;20(47):17924–17931. | ||
Hölttä V, Klemetti P, Salo HM, et al. Interleukin-17 immunity in pediatric Crohn disease and ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;57(3):287–292. | ||
Wedebye Schmidt EG, Larsen HL, Kristensen NN, et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm Bowel Dis. 2013;19(8):1567–1576. | ||
Hyun YS, Han DS, Lee AR, Eun CS, Youn J, Kim HY. Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis. 2012;33(4):931–936. | ||
Qi H, Yang H, Xu G, et al. Therapeutic efficacy of IL-17A antibody injection in preventing the development of colitis associated carcinogenesis in mice. Immunobiology. 2015;220(1):54–59. | ||
De Simone V, Pallone F, Monteleone G, Stolfi C. Role of TH17 cytokines in the control of colorectal cancer. Oncoimmunology. 2013;2(12):e26617. | ||
Le Gouvello S, Bastuji-Garin S, Aloulou N, et al. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut. 2008;57(6):772–779. | ||
Laan M, Lötvall J, Chung KF, Lindén A. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol. 2001;133(1):200–206. | ||
Andoh A, Shimada M, Bamba S, et al. Extracellular signal-regulated kinases 1 and 2 participate in interleukin-17 plus tumor necrosis factor-alpha-induced stabilization of interleukin-6 mRNA in human pancreatic myofibroblasts. Biochim Biophys Acta. 2002;1591(1–3):69–74. | ||
Li Q, Liu L, Zhang Q, Liu S, Ge D, You Z. Interleukin-17 Indirectly Promotes M2 Macrophage Differentiation through Stimulation of COX-2/PGE2 Pathway in the Cancer Cells. Cancer Res Treat. 2014;46(3):297–306. | ||
Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998;273(42):27467–27473. | ||
Martel-Pelletier J, Mineau F, Jovanovic D, Di Battista JA, Pelletier JP. Mitogen-activated protein kinase and nuclear factor kappaB together regulate interleukin-17-induced nitric oxide production in human osteoarthritic chondrocytes: possible role of transactivating factor mitogen-activated protein kinase-activated proten kinase (MAPKAPK). Arthritis Rheum. 1999;42(11):2399–2409. | ||
Li J, Lau GK, Chen L, et al. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 2011;6(7):e21816. | ||
Xu B, Guenther JF, Pociask DA, et al. Promotion of lung tumor growth by interleukin-17. Am J Physiol Lung Cell Mol Physiol. 2014;307(6):L497–L508. | ||
Zhang Q, Liu S, Ge D, et al. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 2012;72(10):2589–2599. | ||
Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. | ||
Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13(8):1016–1023. | ||
Masuelli L, Di Stefano E, Fantini M, et al. Resveratrol potentiates the in vitro and in vivo anti-tumoral effects of curcumin in head and neck carcinomas. Oncotarget. 2014;5(21):10745–10762. | ||
Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70(24):10112–10120. | ||
Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor-alpha-induced elaboration of proangiogenic factors from fibroblasts. Immunol Lett. 2004;93(1):39–43. | ||
Honorati MC, Cattini L, Facchini A. IL-17, IL-1beta and TNF-alpha stimulate VEGF production by dedifferentiated chondrocytes. Osteoarthritis Cartilage. 2004;12(9):683–691. | ||
Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol Lett. 2005;98(2):189–193. | ||
Zhang Q, Liu S, Zhang Q, et al. Interleukin-17 promotes development of castration-resistant prostate cancer potentially through creating an immunotolerant and pro-angiogenic tumor microenvironment. Prostate. 2014;74(8):869–879. | ||
Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014;41(2):235–251. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Xiao M, Wang C, Zhang J, Li Z, Zhao X, Qin Z. IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res. 2009;69(5):2010–2017. | ||
Solt LA, Kumar N, Nuhant P, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472(7344):491–494. | ||
Hueber W, Patel DD, Dryja T, et al; Psoriasis Study Group; Rheumatoid Arthritis Study Group; Uveitis Study Group. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. | ||
Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–1199. | ||
Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.