Back to Journals » ClinicoEconomics and Outcomes Research » Volume 11
HTA and innovative treatments evaluation: the case of metastatic castration-resistant prostate cancer
Authors Bretoni A, Ferrario L , Foglia E
Received 2 October 2018
Accepted for publication 13 March 2019
Published 17 April 2019 Volume 2019:11 Pages 283—300
DOI https://doi.org/10.2147/CEOR.S189436
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Samer Hamidi
Alberto Bretoni, Lucrezia Ferrario, Emanuela Foglia
Centre for Health Economics, Social and Health Care Management, LIUC – Università Cattaneo, Castellanza, Italy
Purpose: To investigate the implications of the introduction of two hormonal therapies, abiraterone acetate + prednisone (AA+P) and enzalutamide (ENZA), for the treatment of naïve patients with metastatic castration-resistant prostate cancer (mCRPC) in the Italian setting.
Methods: In 2017–2018, a Health Technology Assessment was conducted in Italy, considering the National Healthcare Service (NHS) perspective. Data were retrieved from literature evidence, economic evaluations, and qualitative questionnaires, considering the 9 EUnetHTA dimensions, and a final multi-criteria approach.
Results: On the basis of mCRPC prevalence and incidence rates in Italy, the analysis considered 11,212 males eligible to either AA+P or ENZA treatments. Both drugs led to an improvement of the patients’ overall survival, with respect to the standard of care, composed of docetaxel chemotherapy. However, AA+P showed a higher rate of drug-related moderate adverse events and a monitoring activities incidence superior to ENZA (+70%, p-value=0.00), which led to a major resources absorption (€ 1,056.02 vs € 316.25, p-value=0.00), whereas ENZA showed a better cost-effectiveness average value (CEV: 54,586.12 vs 57,624.15). Economic savings ranging from 1.46% to 1.61% emerged for the NHS, as well as organizational advantages, with fewer minutes required for the mCRPC management (AA+P: 815 mins vs ENZA: 500 mins). According to experts’ perceptions, based on a 7-item Likert scale (ranging from −3 to +3), similar results emerged on ethical and social impact (ENZA: 1.35 vs AA+P: 1.48, p-value>0.05), and on legal dimension (ENZA: 0.67 vs AA+P: 0.67, p-value>0.05), since both drugs improved the patients’ quality of life and received approval for use. High-level perceptions related to ENZA adoption emerged with regard to equity (ENZA: 0.69 vs AA+P: 0.25, p-value<0.05), since it is cortisone-free. Multi-criteria approach analysis highlighted a higher score of ENZA than comparator (0.79 vs 0.60, p-value=0.00).
Conclusion: The evidence-based information underlined the advantages of ENZA and AA+P treatments as therapeutic options for mCRPC patients. In the appraisal phase, the higher score than the comparator suggested ENZA as the preferred treatment for mCRPC.
Keywords: mCRPC, multidimensional assessment, economic evaluation, decision analysis, hormonal therapies, MCDA
Corrigendum for this paper has been published
Introduction
Prostate cancer (PC) is the most frequent neoplasia diagnosed among men worldwide.1
Castration-resistant prostate cancer (CRPC) is estimated to account from 10% to 27% of prostate cancer cases, depending on the regional and national area of reference, with over 70% of these cases diagnosed as metastatic CRPC (mCRPC).2,3 When localized, PC may have a good prognosis, whereas mCRPC may become lethal after a period of about 3–5 years, with an overall survival (OS) rate of about 29%.4,5
The “standard of care” for mCRPC is chemotherapy based on docetaxel.6,7 Despite docetaxel significant survival improvement, with respect to the “pre-docetaxel era”, mCRPC patients continue to have a poor prognosis.8
In this view, since 2010 a fundamental shift has been occurring in the mCRPC treatment landscape, in particular, for chemotherapy-naïve mCRPC patients, through the Food and Drug Administration/European Medicine Agency approvals and the consequent introduction of several novel androgen receptor signaling axis-targeting agents into the marketplace. Thus, abiraterone acetate plus prednisone (AA+P) and enzalutamide (ENZA) have been a breakthrough in the clinical management of advanced PC treatment, showing prolonged survival, when administered before docetaxel chemotherapy. Moreover, their oral formulations favor the compliance to the therapy.3,9–12
In phase III studies, both AA+P and ENZA have improved the radiographic progression-free survival (rPFS) and the OS rates of mCRPC patients, compared with placebo, when administered before docetaxel.13,14 The two therapeutic strategies differ in terms of the presence of prednisone, that is an essential component of AA+P, and whose long-term exposure could generate complications for patients.15–17 In particular, the most prevalent adverse events related to corticosteroids administration are hypertension, fracture or osteoporosis, nausea/vomiting/other gastrointestinal condition, cardiac condition, diabetes or hyperglycemia, and cataract.18
Published head-to-head clinical trials with a direct comparison between ENZA and AA+P for the treatment of chemotherapy-naïve patients with mCRPC are lacking. Only few studies tried to evaluate the relative efficacy, cost-effectiveness, and resources absorption, indirectly comparing the two oral agents.19,20
Since new therapeutic options can address the mCRPC patients, it is necessary to investigate not only cost and efficacy, but also the dimensions with emerging importance, such as safety, organizational, social and equity aspects, providing related evidence as required by the European EUnetHTA Core Model.21,22
The present study aims at evaluating the implications related to the introduction of ENZA, and AA+P therapies, into the Italian National Healthcare Service, in terms of clinical outcomes, economic efficiency, and safety profile. The study also evaluated both the potential organizational advantages for the clinical centers taking in charge the oncologic patients, and the accessibility to care, in order to guarantee a positive impact for patients, for related caregivers, and, more in general, for citizens and entire communities, having this specific healthcare need. This is important for the creation of evidence-based information useful for the rationalization of the expenditure devoted to mCRPC treatments, thus also optimizing the patient clinical pathway.
Methods
A Health Technology Assessment (HTA) analysis was implemented in 12 months (from 2017 to 2018) in Italy, comparing AA+P and ENZA therapies, as innovative therapeutic strategies, currently available for the mCRPC treatment.
Besides the assessment of the HTA dimensions, an appraisal phase was developed, thanks to the support of a Multiple Criteria Decision Analysis (MCDA) approach.22–24
Assessment of EUnetHTA dimensions
Due to the multi-dimensional and multi-disciplinary nature of HTA, several aspects of the medical technologies (drugs) were analyzed as stated in the EUnetHTA Core Model: i) general relevance; ii) safety; iii) efficacy; iv) economic and financial impact; v) equity; vi) legal aspects; vii) social and ethical impact; and viii) organizational implications.22
The above dimensions were deployed, taking into account scientific evidence, economic evaluations, and qualitative approaches.
Literature review
Before starting the assessment of the dimensions, the PICO approach (Problem/population, Intervention, Comparator and Outcome) for the literature validation, was identified, thus defining the research question.25,26 In particular, the following PICO was discussed: i) P (population): mCRPC naïve population; ii) I (intervention): enzalutamide; iii) C (comparator): abiraterone acetate plus prednisone; iv) O (outcome): clinical effectiveness, in terms of overall survival and rPFS.
Literature evidence came from the systematic search of literature databases (Pubmed, Embase and Cochrane Library), from 2011, up to December 2017. The search terms were the followings: “Enzalutamide”, “Abiraterone acetate”, “hormone therapy”, “Metastatic Castration–Resistant Prostate Cancer”, “clinical effectiveness”, “overall survival”, “radiographic progression-free survival”. It should be noted here, that in accordance with the use of GRADE, within an HTA exercise, only RCTs have been taken into consideration, focusing only on mCRPC naïve population, thus leading to high-quality evidence. Furthermore, besides the RCT study design, and its focus on a specific population, the defined inclusion criteria were the following: i) number of patients enrolled in the study (>1,000) and ii) journal impact-factor (≥16). Peer-reviewed papers that explicitly described the clinical effectiveness of ENZA and AA+P within the naïve population affected by mCRPC, were consequently included, and synthetized according to a PRISMA flow diagram, thus mapping out the number of records (in terms of papers) identified, included and/or excluded, and the reasons for exclusion.27
The validation of the scientific evidence available on the topic was performed through the New Castle–Ottawa Evaluation scale.28 A “star system” ranking scale has been developed, in which a study or evidence is judged, considering three broad perspectives: i) the selection of the study groups; ii) the comparability of the groups; and iii) the ascertainment of either the exposure or outcome of interest, for case-control or cohort studies, respectively.
Besides the qualitative assessment of evidence deployed by means of the above mentioned New Castle–Ottawa Evaluation scale, the papers included in the HTA have been also evaluated, following a quantitative approach, proposed by the IMPAQHTA model.29 In particular, literature evidence was briefly evaluated, with regard to the quality, completeness, and replicability, of the results, all of which were measured based on a 4-level evaluation scale.
Literature was used for highlighting efficacy profile in terms of OS, rPFS and safety profile (measured as drug-related adverse events rate). Since only primary evidence have been considered, the literature review proposed in the present paper collected high-quality efficacy and safety information.
In order to verify the efficacy and the safety profiles replicability and scalability, from the international to the Italian “real-life” setting, information derived through literature were also validated with the involvement of 10 experts (referring to 5 Italian hospitals) taking in charge mCRPC patients, thus presenting an Italian country-oriented approach. In this view, the personal perceptions of the professionals using the drugs were collected, following specific items of relevance, according to the EUnetHTA Core Model.22
Economic evaluations
For economic dimension evaluation, pharmaco-economics tools, and budget impact analysis were used (Table 1). Information was gathered according to the standard clinical pathway performed in the 5 Italian hospitals involved in the study.
 | Table 1 Methods used for the assessment of each HTA dimension |
The clinical pathway of mCRPC patients was described and standardized. The clinicians and pharmacists of reference approved the clinical pathway, with a Delphi method approach.30 The pathway analysis leads to the identification of the following five phases: 1) urology clinic; 2) radiological staging; 3) medical oncology; 4) hormonal pharmacological treatment; and 5) follow-up, in a time horizon of 12 months, and related activities, procedures, and professionals involved. In this view, the economic impact of a patient following this pathway, and receiving mCRPC treatments was determined, using these components.
- Drugs costs: the dosing schedules of ENZA and AA+P, were based on the Italian product labels;31,32 the cost of each drug was based on the Italian ex-factory price, considering the mandatory discounts required by law and the V.A.T. The recommended daily dose was determined according to the Summary of Product Characteristics, of each drug;33,34 the drugs costs were derived from the officially published NHS price list.
- Non-drug related costs: medical costs for mCRPC management, including the total amount of hematologic and cultural tests, diagnostic procedures, outpatient visits, medical examinations, hospital admissions.
- Cost of side effects management (laboratory tests, diagnostic procedures, clinician visits, hospital admissions): depending on the incidence and the applied therapeutic strategy, according to the literature.13,14
The clinical pathway economic analysis was performed considering a time horizon of 12 months, and according to the 2017 Italian reimbursement tariffs of outpatients and hospital admissions.
The economic evaluation of an mCRPC patient pathway was completed with a cost-effectiveness and a budget impact analyses, taking into consideration efficacy and safety parameters, derived from phase III related studies.13,14 On one hand, the cost-effectiveness value indicator (CEV), in average terms, was determined to choose the technology that shows a better cost-effectiveness trade-off (calculated as cost per patient divided for the OS indicator, in terms of percentage of survived patients, considering a 12-month time horizon).35,36 Furthermore, the cost-effectiveness incremental value (ICER) was investigated, useful for the determination of the ratio between costs and the OS gained.35,36
On the other hand, the budget impact analysis (BIA) compared a baseline scenario (in which all the eligible patients were treated with AA+P), with an innovative scenario (in which eligible patients were treated with either AA+P – 66% or ENZA – 34%). The above market shares used for defining the two scenarios were retrieved in accordance with real-life data, carried out in the 5 hospitals involved in the analysis, with a Delphi approach.30
Two different projections were hypothesized, depending on the median treatment lengths, equal to 13.8 and 16.6 months, respectively.13,14 In particular, the healthcare expenditure evolution up to 3 years was estimated assuming the NHS perspective. The number of eligible patients was determined based on the Italian epidemiological prevalence and incidence rates, regarding the mCRPC pathology, of the male population.3,37–39 In this view, 41,221 incident Italian males (being chemotherapy naïve) received a diagnosis of prostate cancer, with 33.5% could be classified as CRPC.40,41 Out of them, 80.50% could potentially progress to mCRPC, thus being considered eligible for therapy. It emerged that 11,116 naïve males would suffer from mCRPC in the Italian setting.
Qualitative approach
Qualitative questionnaires derived from the EUnetHTA Core Model were administered to 10 mCRPC experts (6 medical oncologists and 4 experts from the Pharmaceutical Department), referring to 5 medium-size hospitals (at least, 1,140 ordinary and 59 day-hospital beds), treating oncological patients, who completed the questionnaire according to their own experience and perceptions.
The qualitative questionnaires were used for examining equity, social, legal, and organizational aspects, considering a comparative approach of the two technologies under assessment (ENZA and AA+P), in accordance with a 7-item Likert scale ranging from – 3 to +3.
Detailed information with regard to the specific items related to each dimension is shown in Table 1.
The appraisal phase
After the assessment of the dimensions, the appraisal phase was conducted, in order to identify the drug presenting a higher final value, thus directly comparing AA+P and ENZA.
At first, as required by the operative implementation of MCDA approach at the institutional level, the 10 mCRPC experts who completed the qualitative HTA questionnaire prioritized the above dimensions by a rating scale ranging from 8 (more important dimension) to 1 (less important dimension).42 Then, an MCDA approach was applied.23,24 In particular, three evaluators (different from the ones performing the prioritization and the assessment) – 2 Hospital Health Directors, and 1 health economist – with an HTA, decision-making and organizational background, assigned to each sub-dimension (listed in Table 1) a five-level mark (ranging from a minimum of 0 to a maximum of 4). Thanks to this approach, the experts suggested a final and synthetic mark to the quality and completeness of the information retrieved for both AA+P and ENZA, in the HTA report.
In conclusion, the identification of the final score for each drug was obtained by multiplying the standardized score, calculated for each dimension (average value derived from sub-dimensions), by the normalized value of priority. Thus, the higher the score acquired, the more preferable is the technology.
Statistical analysis
Economic and qualitative data were first analyzed, considering descriptive statistics. Differences between AA+P and ENZA were evaluated, according to a significance level lower than 0.05 (p-value), thus using the Independent sample T-test.
Furthermore, with regard to the economic dimension, both a scenario and a sensitivity analyses were conducted, in order to verify the robustness of the results. At first, a scenario analysis was performed, on the basis of the uncertainty existing with regard to PC and CRPC incidence rate (the first ranging from 35,300 patients, to 44,525 patients; the second ranging from 33% to 34%), and the mCRPC prevalence rate (ranging from 85% to 75%).40,41 Starting from the above information, three different hypotheses of population were developed, thus defining an average population equal to 11,116 naïve mCRPC patients, ranging from a minimum scenario composed of 9,902 Italian males, and a maximum scenario composed of 11,354 Italian males, potentially eligible to oral treatment.
Second, a sensitivity analysis was performed, in order to verify the robustness of the results, in terms of cost and efficacy data, on the basis of the different OS percentages, within different time horizons, as revealed in the phase III studies, and the related clinical pathway cost.13,14 In addition, Bayesian statistics was performed, since Bayesian methods provide a complete paradigm for both statistical inference and decision-making under uncertainty. Beta and Gamma distributions were accordingly developed, in order to verify the robustness of the results, in presence of uncertainty factors (efficacy, clinical pathway cost, and CEV parameters were analyzed). The probability to have: i) any OS average value of the beta distribution of ENZA, higher with respect to AA+P; ii) any cost average value of the gamma distribution of ENZA, lower with respect to AA+P; and iii) any average CEV of the gamma distribution of ENZA lower with respect to AA+P, was evaluated.
Also for the appraisal phase results, an uncertainty analysis was conducted. With regard to the MCDA analysis, the prioritization phase consistency and the Beta distributions of the final results obtained for AA+P and ENZA were performed, in order to test their reliability.
Results
Results from the assessment of EUnetHTA dimensions
Results from literature review
The search for Mesh terms resulted in 261 records. Out of them, only 32 were assessed for eligibility. In accordance with the above-mentioned search strategy, only 3 articles met the inclusion criteria defined in the methodology section and focused on mCRPC naïve population, assuming either ENZA or AA+P.13,14,43 The other 29 articles had different aims, without focusing the attention on efficacy/safety data, nor reporting ongoing studies, nor presenting observational studies (without being high-quality RCTs, as presented in Figure 1).
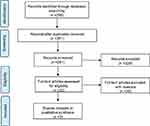 | Figure 1 Prisma flow chart. |
The literature review revealed the lack of scientific evidence, concerning the head-to-head comparison between AA+P and ENZA in terms of safety and clinical efficacy. Despite the above missing information, the articles included in the analysis, presented quality and reliable data assessed, being RCTs. In particular, in accordance with the New Castle–Ottawa Evaluation Scale, the risk of bias was not high (Table 2). The control group was accordingly determined, the outcomes measurement proved to be relevant in most cases, and both positive and negative outcomes were determined and explained in the evidence. The quantitative assessment of literature confirmed the quality, the completeness and the replicability of the results (Table 2).
 | Table 2 Qualitative and quantitative assessment of scientific evidence included in the HTA report |
The clinical efficacy was derived from the above randomized clinical trials that enrolled the naïve patients’ population.13,14 Both drugs resulted in an improved OS versus placebo (AA+P: 34.7 months; ENZA: 35.3 months), whereas the increase of rPFS was 16.5 months for AA+P and 20 months for ENZA. In particular, focusing on the therapeutic success within a 12-month time horizon, it emerged an OS rate equal to 91% for ENZA and equal to 88% for AA+P. For the safety profile, Table 3 shows the rate of adverse events and the economic evaluation of the related clinical pathway management, in a 12-month time horizon.13–19 AA+P was associated with a higher development of adverse events, with consequent economic resources absorption for the patient’s management and care, which is +234% higher than ENZA (p-value=0.000).
 | Table 3 Rate and economic evaluation of drug-related adverse events |
The validation of the above theoretical efficacy and safety profiles, derived from international literature evidence, confirmed their replicability also for the Italian clinical practice, as suggested by the perceptions of the clinicians and pharmacists involved in the study (please see Table 4).
 | Table 4 Qualitative dimensions evaluation |
Results from the economic evaluations
The annual cost to treat an mCRPC patient was estimated of 50,709.25 € for AA+P and of 49,673.37 € for ENZA (p-value=0.000), as showed in Table 5, according to the five different phases of the patient clinical pathway, and in a time horizon of 12 months. In particular, differences emerged regarding the drug and the follow-up costs, since AA+P required a higher number of medical accesses compared with ENZA (+70% monitoring activities, p-value =0.000), in the same time horizon of treatment. Gamma distributions for costs confirmed that considering a time horizon of 13.8 months, 16.6 months and 18 months, ENZA clinical pathway presented a probability to absorb lower economic resources equal to 66.63%, 50.00%, and 61.34%, respectively (Figure 2).
 | Table 5 Economic evaluation of mCRPC patient clinical pathway |
 | Figure 2 Gamma distributions of AA+P and ENZA in terms of clinical pathway cost |
Due to lower cost and higher efficacy, ENZA could be considered the “dominant” technology, thus being preferable from a cost-effectiveness point of view (Table 6).44 In particular, focusing only on efficacy data, and considering a time horizon of 13.8 months, 16.6 months, and 18 months, ENZA presented a probability equal to 99.9%, 98.7%, and 99.7%, respectively, to achieve a higher OS rate (Figure 3).
 | Table 6 Cost-effectiveness analysis |
 | Figure 3 Beta distributions of AA+P and ENZA in terms of overall survival. |
Furthermore, the innovative hormonal treatment presented a 84.82%, 99.80%, and 98.7% possibility to have the better trade-off between cost and effectiveness, with respect to AA+P, assuming a time horizon of 13.8 months, 16.6 months, and 18 months, respectively (Figure 4).
 | Figure 4 Gamma distributions of AA+P and ENZA in terms of cost-effectiveness value |
Results from the budget impact analysis revealed that the NHS would benefit from ENZA adoption into the clinical practice, with economic savings, ranging from 1.46% to 1.61%, depending on the therapeutic strategy duration (Table 7), for the treatment of 11,126 naïve males with mCRPC (average population). The same trend emerged considering both the minimum and the maximum population eligible to oral treatment.
 | Table 7 Budget impact analysis results |
This cost saving could not be relegated only to the economic sphere. Table 8 shows that ENZA could be the preferable drug with a 41.18% reduction of specialist visits and blood examinations versus comparator (p-value=0.000), thus leading to a significant time saving per mCRPC patient management of about 38.65% mins (p-value=0.000).
 | Table 8 Follow-up monitoring activities |
Results from the qualitative approach
Focusing on the qualitative aspects (Table 4), ENZA and AA+P could be considered super-imposable in the measurement concerning the ethical and social impact (ENZA: 1.35 vs AA+P: 1.48, p-value >0.05) and the legal dimension (ENZA: 0.67 vs AA+P: 0.67, p-value >0.05). The emerged difference in the average value of the ethical and social impact perceptions is particularly due to the swallowing difficulty of ENZA pills, given its big dimensions. High-level perceptions related to ENZA adoption surged about the equity (ENZA: 0.69 vs AA+P: 0.25, p-value <0.05) and the organizational impact (ENZA: 0.83 vs AA+P: 0.45, p-value <0.05).
Results from the appraisal phase
The appraisal phase required both a prioritization of the dimensions and the implementation of a multi-criteria decision approach.
According to the opinions of the 10 mCRPC experts involved, the prioritization revealed that the most important aspect was the efficacy profile followed by safety and economic impact. The less relevant dimension appeared the legal impact since both drugs have received approval for use in the clinical practice. No statistically significant “between-professional” differences emerged regarding the prioritization of the dimensions: clinicians and pharmacists perceived value of importance was thus consistent to each other.
The results from the MCDA (Table 9), highlighted a superior score for ENZA than the comparator (0.79 vs 0.60, p-value=0.000), thus suggesting the preferable treatment for mCRPC patients.
 | Table 9 Multi-criteria decision analysis |
Figure 5 depicts that the final value related to ENZA, is always higher than the one related to AA+P, confirming the preference in adopting ENZA, during the decision-making process.
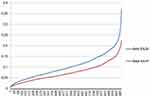 | Figure 5 Gamma distributions of AA+P and ENZA in terms of MCDA final value |
Discussion
During recent years, hormonal therapies have significantly changed the landscape of the mCRPC management. Clinical decisions about the mCRPC therapies remain largely consensus-based than evidence-based, given the lack of prospective head-to-head trials assessing the efficacy of the treatments.
The present study partially tried to overcome this knowledge gap by applying a multi-dimensional evaluation and showing the strengths and weaknesses of the two hormonal therapies currently available.
Thus, the appraisal phase revealed to decision makers that ENZA could be a technological option to be administered for this specific setting, with important benefits in terms of economic and organizational savings, safety profile and accessibility improvement. In particular, ENZA is related to less economic resources absorption than AA+P, resulting in a better cost-effectiveness indicator, and a feasible and sustainable 12-month budget impact, with economic savings ranging from 1.46% to 1.61%. This is partly due to the cost offset of a moderate incidence of adverse events and the lack of additional monitoring required.45,46
Moreover, despite literature provides information about the economic impact approaching the topic with different methods and input data,20,45–48 the present study revealed significant benefits of ENZA treatment from the patients management. A positive impact has emerged regarding the follow-up visits (ENZA: 2.25 vs AA+P: −0.75, p-value=0.000), and the entire patient clinical pathway (ENZA: 1.25 vs AA+P: 0.75, p-value=0.000).
A positive impact was also generated at the organizational level, in terms of improvement and optimization of mCRPC care and treatment, with the consequent possibility of freeing up resources, reducing hospital waiting lists and enhancing the accessibility to health care services. This aspect is linked to the fact that the therapeutic strategy AA+P includes prednisone, whose long-term exposure could generate side effects,15–18,49–51 thus requiring a higher number of follow-up monitoring steps and reducing the number of patients that could benefit from a mCRPC oral treatment (for example, diabetic population). In this view, the administration of ENZA could improve the accessibility to care for patients, who, given other diagnosed comorbidities (such as diabetes) could not assume AA+P, because of the presence of prednisone.
Furthermore, ENZA administration, characterized by fewer follow-up monitoring activities, could positively impact on the organizational aspects and on patients’ social costs, in terms of reduction in productivity loss. Focusing on patients’ perspective, clinicians declared that the investigated drugs improve patients’ quality of life, due to their capability to prolong the OS.13,14 Previous studies strengthened this consideration: both ENZA and AA+P are associated with reduced risk for degradation in all the Functional Assessment of Cancer Therapy-Prostate subscales-FACT-P –compared to placebo.43,52–54
To the best of the authors’ knowledge, this study could be considered the first attempt to fully evaluate the implications derived from ENZA and AA+P administration, into the Italian clinical practice, thus also paving the way to the possibility to perform an HTA exercise on drugs, whose efficacy and safety profiles have been already validated and approved with the development of proper randomized control trials. Moving on from these premises, it should be noted that Italy is an HTA user, without being an HTA doer: this is the rational behind the use of evidence-based information derived from RCTs, even if performed in other Countries, avoiding the duplication principle of scientific evidence. In this view, the value of drugs is strictly related to the strength, level, and reliability of the evidence-based information coming from literature review, in particular from primary evidence (such as RCTs or series of RCTs). With regard to the full assessment, with the exception of the efficacy and safety dimensions, all the information presented a country-oriented approach, with the involvement of 5 Italian hospitals taking in charge mCRPC patients. The health care services may consider the results provided by the present study as an opportunity to insert mCRPC patients in the most adequate oral treatment arm, guaranteeing a personalized clinical pathway, thus becoming more efficient and effective, and supporting the decision-making process with the deployment of innovative aspects, such as the organizational impact and the accessibility measure, particularly interesting in the drug landscape. In terms of limitations, despite the results of the present study could provide solutions to scholars and practitioners on the topic, it should be noted that evidence-based information about safety and efficacy, did not rely on prospective, randomized, controlled, head-to-head trials comparing ENZA and AA+P since it was based on accepted methodology for indirect comparison.55 In this view, further analysis may complement the present HTA analysis, in order to understand costs and advantages of clinical outcome, derived from the real-life clinical practice, and concerning the specific Italian setting.
Conclusion
As the innovative ENZA and AA+P treatments have been introduced in the market and approved for a broader mCRPC population, public and health care services concern has grown, regarding the impact of the drugs-related costs, which are most expensive compared to the standard of mCRPC care.56
The results of this study provide helpful evidence-based information to policy-makers through the examinations of the relative values of intervention, for deciding on the efficient and equitable allocation of health care resources, since it emerged that compliance, patients profile and cost could play an important role in the clinical practice decision-making process.
Abbreviations list
AA+P, Abiraterone Acetate + Prednisone; CEV, Cost-Effectiveness Value; CRPC, Castration-resistant prostate cancer; EMA, European Medicine Agency; ENZA, Enzalutamide; FDA, Food and Drug Administration; HTA, Health Technology Assessment; ICER, Incremental cost-effectiveness ratio; MCDA, Multi-Criteria Decision Analysis; mCRPC, metastatic castration-resistant prostate cancer; NHS, National Healthcare Service; OS, overall survival; PC, prostate cancer; rPFS, radiographic progression-free survival.
Acknowledgments
The Authors would like to thank all the professionals involved (Laura Bistocchi, Maria Cossu Rocca, Paola Salvaderi, Elena Verri, Paolo Andrea Zucali), for their valuable support to complete the questionnaire and to produce the mCRPC standard clinical pathway, leading to the success of the present research activity.
The editing of the manuscript and the editorial support was provided by Rossella Ferrari.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
2. Kirby M, Hirst C, Crawford ED. Characterizing the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180–1192. doi:10.1111/j.1742-1241.2011.02799.x
3.
4. Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. JCO. 2014;32(7):671–677. doi:10.1200/JCO.2013.52.3696
5.
6. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. NEJM. 2004;351(15):1513–1520. doi:10.1056/NEJMcp041956
7. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. NEJM. 2004;351(15):1502–1512. doi:10.1056/NEJMoa040720
8. Lorente D, Mateo J, Perez-Lopez R, de Bono JS, Attard G. Sequencing of agents in castration-resistant prostate cancer. Lancet Oncol. 2015;16(6):e279–e292. doi:10.1016/S1470-2045(15)70033-1
9. Tran C, Ouk S, Clegg NJ, et al. Development of a second – generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi:10.1126/science.1168175
10. Lorente D, Fizazi K, Sweeney C, de Bono JS. Optimal treatment sequence for metastatic castration – resistant prostate cancer. Eur Urol Focus. 2016;2(5):488–498. doi:10.1016/j.euf.2016.10.008
11. Ritch CR, Cookson MS. Advances in the management of castration resistant prostate cancer. BMJ. 2016;355:i4405. doi:10.1136/bmj.i4405
12. Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2017;73(2):178–211.
13. Ryan CJ, Smith MR, Fizazi K, Al E. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–160. doi:10.1016/S1470-2045(14)71205-7
14. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71(2):151–154. doi:10.1016/j.eururo.2016.07.032
15. Clore JN, Thurby-Hay L. Glucocorticoid – induced hyperglycemia. Endocr Pract. 2009;15:469–474. doi:10.4158/EP08331.RAR
16. Bonomio M. Iperglicemia da steroidi: meccanismi e trattamento. [Steroid hyperglycemia: mechanisms and treatment]. G IT Diabetol Metab. 2015;35:8–15. Italian.
17. De Micheli A. Il diabete steroideo: inquadramento e gestione. [Steroid diabetes: classification and management]. G Ital Nefrol. 2016;33:S68. Italian.
18. Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39(11):2216–2229. doi:10.1016/j.clinthera.2017.09.011
19. Massoudi M, Balk M, Yang H, et al. Number needed to treat and associated incremental costs of treatment with enzalutamide versus abiraterone acetate plus prednisone in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer. J Med Econ. 2017;20(2):121–128. doi:10.1080/13696998.2016.1229670
20. Restelli U, Ceresoli GL, Croce D, et al. Economic burden of the management of metastatic castrate-resistant prostate cancer in Italy: a cost of illness study. Cancer Manag Res. 2017;7(9):789–800. doi:10.2147/CMAR.S148323
21. Drummond MF, Schwartz JS, Jönsson B, et al. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int J Technol Assess Health Care. 2008;24(3):244–258. doi:10.1017/S0266462308080343
22.
23. Thokala P, Duenas A. Multicriteria decision analysis for health technology assessment. Value Health. 2012;15(8):1172–1181. doi:10.1016/j.jval.2012.06.015
24. Thokala P, Devlin N, Marsh K, et al. Multiple criteria decision analysis for health care decision making — an introduction: report 1 of the ISPOR MCDA emerging good practices task force. Value Health. 2016;19(1):1–13. doi:10.1016/j.jval.2015.12.003
25. Huang X, Lin J, Demner-Fushman D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Ann Symp Proc. 2006;359–363.
26. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi:10.1186/1472-6947-7-16
27. Moher D, Liberati A, Tetzlaff J, Altman DG. The prisma group preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
28. Wells GA, Shea B, O’Connell D, et al., The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. 2000 Available from:
29. Foglia E, Lettieri E, Ferrario L, et al. Technology assessment in hospitals: lessons learned from an empirical experiment. Int J Technol Assess Health Care. 2017;33(2):288–296. doi:10.1017/S0266462317000356
30. Quinn Patton M. Qualitative Research & Evaluation Methods.
31.
32.
33.
34.
35. Porter M. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi:10.1056/NEJMp1011024
36. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston: Harvard Business School Press; 2006.
37.
38. Buzzoni C, Auvinen A, Roobol MJ, et al. Metastatic prostate cancer incidence and prostate – specific antigen testing: new insights from European randomized study of screening for prostate cancer. Eur Urol. 2015;68(5):885–890. doi:10.1016/j.eururo.2015.02.042
39. Carteni G, Pappagallo G. Carcinoma della prostata resistente alla castrazione in Italia: un unmet in via di risoluzione. [Castrate Resistant Prostate CANCER IN Italy: an unmet medical need about to be solved]. Ital J Public Health. 2011;8(Suppl 4):S3–S8. Italian.
40. Marteau F, Gimonet G, Gabriel S, Dinet J, Flinois A, LE Cleac’h JY. Epidemiology of patients with metastatic castrate resistant prostate cancer in Europe and Australia. Value Health. 2017;17(7):a619. doi:10.1016/j.jval.2014.08.2188
41. Sternberg C, De Bono J, Chi K, et al. Outcomes in elderly patients with metastatic castration-resistant prostate cancer (mCRPC) treated with the androgen receptor inhibitor enzalutamide: results from the phase III AFFIRM trial. J Clin Oncol. 2013;31(Suppl. 6):abstract 16. doi:10.1200/JCO.2013.49.0219
42. Radaelli G, Lettieri E, Masella C, Merlino L, Strada A, Tringali M. Implementation of Eunethta core model® in Lombardia: the VTS framework. Int J Technol Assess Health Care. 2014;30(01):105–112. doi:10.1017/S0266462313000639
43. Loriot Y, Miller K, Sternberg CN, et al. Effect of enzalutamide on health-related quality of life, pain, and skeletal-related events in asymptomatic and minimally symptomatic, chemotherapy-naive patients with metastatic castration-resistant prostate cancer (PREVAIL): results from a randomised, phase 3 trial. Lancet Oncol. 2015;16(5):509–521. doi:10.1016/S1470-2045(15)70113-0
44. Gold MR. Cost-Effectiveness in Health and Medicine. Oxford, UK: Oxford University Press; 1996.
45. Bui C, O’Day K, Flanders S, et al. Budget impact analysis of enzalutamide for the treatment of metastatic castration-resistant prostate cancer from a US payer perspective. Value Health. 2015;18(3):A196. doi:10.1016/j.jval.2015.03.1135
46. Bui C, O’Day K, Flanders S, et al. Budget impact of enzalutamide for chemotherapy-naïve metastatic castration-resistant prostate cancer. J Manag Care Spec Pharm. 2016;22(2):163–170. doi:10.18553/jmcp.2016.22.2.163
47. Pollard ME, Moskowitz AJ, Diefenbach MA, Hall SJ. Cost-effectiveness analysis of treatments for metastatic castration resistant prostate cancer. Asian J Urol. 2017;4(1):37–43. doi:10.1016/j.ajur.2016.11.005
48. Pilon D, Queener M, Lefebvre P, Ellis LA. Cost per median overall survival month associated with abiraterone acetate and enzalutamide for treatment of patients with metastatic castration-resistant prostate cancer. J Med Econ. 2016;19:777–784. doi:10.3111/13696998.2016.1173042
49. Khaleeli AA, Edwards RH, Gohil K, et al. Corticosteroid myopathy: a clinical and pathological study. Clin Endocrinol (Oxf). 1983;18(2):155–166.
50. Pagano G, Cavallo-Perin P, Cassader M, et al. An in vivo and in vitro study of the mechanism of prednisone – induced insulin resistance in healthy subjects. J Clin Invest. 1983;72(15):1814–1820. doi:10.1172/JCI111141
51. Blackburn D, Hux J, Mamdani M. Quantification of the risk of corticosteroid – induced diabetes mellitus among the elderly. J Gen Intern Med. 2002;17(9):717–720.
52. Cella D, Ivanescu C, Holmstrom S, et al. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol. 2015;26(1):179–185. doi:10.1093/annonc/mdv383
53. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
54. Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815–825. doi:10.1016/j.eururo.2014.01.003
55. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
56. Pollack A. New drug for prostate cancer gets F.D.A. Nod. The New York Times. 2012. The New York Times Company, New York.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
