Back to Journals » OncoTargets and Therapy » Volume 14
HSDL2 Acts as a Promoter in Pancreatic Cancer by Regulating Cell Proliferation and Lipid Metabolism
Authors Han A, Xu R, Liu Y, Yin X, Lin Z, Yang W
Received 21 October 2020
Accepted for publication 11 December 2020
Published 14 January 2021 Volume 2021:14 Pages 435—444
DOI https://doi.org/10.2147/OTT.S287722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alberto Bongiovanni
Anna Han,1,2 Ran Xu,1,2 Ying Liu,1,2 Xianglin Yin,3 Zhenhua Lin,1,2 Wanshan Yang1,2
1Department of Pathology and Cancer Research Center, Yanbian University Medical College, Yanji, People’s Republic of China; 2Key Laboratory of the Science and Technology Department of Jilin Province, Yanji, People’s Republic of China; 3Department of Pathology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Wanshan Yang
Department of Pathology and Cancer Research Center, Yanbian University Medical College, Gong Yuan Road No. 977, Yanji 133002, People’s Republic of China
Email [email protected]
Background: Pancreatic cancer (PC) is a leading cause of cancer mortality worldwide. Hydroxysteroid dehydrogenase like protein 2 (HSDL2) is overexpressed in a variety of malignant tumors and is might be closely related to the development of cancer. It also regulates different metabolism and signaling pathways.
Purpose: The purpose of this research was to find HSDL2 expression levels and investigate its underlying molecular mechanism in PC.
Patients and Methods: In the present study, a total of 66 PC samples and 54 normal tissues were used to examine the expression of HSDL2. In order to gain a broader insight into the molecular mechanism of HSDL2 in PC, the HSDL2 siRNA sequences were transfected into PC cell lines (Bxpc-3 and Panc-1), respectively. Cell proliferation was measured by MTT, colony formation assay and EdU assays. Furthermore, the lipid metabolism process was evaluated by triglyceride and phospholipid assay kits, BODIPY 493/503 staining and the expression of several pivotal lipid metabolic enzymes in PC.
Results: In this study, HSDL2 was highly expressed in PC and connected with shorter overall survival. When HSDL2 was silenced, the cell proliferation was significantly reduced, and the lipid metabolism was further inhibited.
Conclusion: High expression of HSDL2 plays an important role in the progression of PC and might be a potential new biomarker of poor prognosis as well as a therapeutic target in the future.
Keywords: HSDL2, proliferation, pancreatic cancer, lipid metabolism, prognosis
Introduction
Pancreatic cancer (PC) is one of the deadliest malignancies in the world and is arguably one of the most difficult to diagnose and cure. Because of the absence of reliable early biomarkers and efficient therapies, approximately 80% of patients with pancreatic cancer are diagnosed at an advanced stage,1 and the 5-year survival rate remains below 9%.2 Even worse, pancreatic cancer is not sensitive to both radiotherapy and chemotherapy. Thus, the development of effective therapeutic agents is urgently needed to overcome pancreatic cancer.
Previous studies have shown that short-chain dehydrogenases/reductases (SDRs) play important roles in many diseases through regulating different signal and metabolic pathways. SDRs are mostly NAD+ or NADP+ dependent oxidoreductases,3 which can catalyze the oxidation and reduction of a variety of substrates, including steroids, fatty acids, sugars, retinoids and isovitamins.4 SDRs are also involved in the regulation of many other diseases, such as Alzheimer’s disease, cancer and obesity.5–7
Hydroxysteroid dehydrogenase-like 2 (HSDL2), a member of the SDR family, is over 90 kb in length, has 11 exons and consists of 418 amino acids.8 HSDL2 has been identified as an effective fatty acid regulatory factor in lipid metabolism.9,10 Abnormal lipid metabolism is an established hallmark in various human cancers. HSDL2 abnormal expression was associated with many cancers. For example, the promotion effect of HSDL2 on the progression of lung adenocarcinoma cells depended on the AKT2 expression level.11 In bladder cancer HSDL2 plays an oncogenic role.12 And in human ovarian cancer HSDL2 knockdown inhibited cell proliferation, colony formation, motility and tumorigenesis.13 But the expression and functional roles of HSDL2 in PC have not been fully investigated.
Energy is essential for the growth of tumor cells, and metabolic alterations are required to maintain the proliferative potential of growing tumors.14,15 More and more evidences have shown that aberrant lipogenesis may cause cancer, and, with unlimited adipogenesis, sufficient lipid building blocks are provided for cancer cells to support their uncontrolled proliferation.16–18 Recently, Lee et al observed that branched-chain amino acids sustain metabolism and thus play a main role in pancreatic ductal adenocarcinoma (PDAC) growth by adjusting lipogenesis.19 HSDL2 has received widespread attention for its possible involvement in lipid metabolism, and it is also well known as one of the major tumor markers when lipid metabolism is abnormal. However, the expression of HSDL2 in cell growth and lipid metabolism of PC remains unclear.
Herein, we first investigated that HSDL2 was upregulated in PC tissues and indicated exacerbated prognosis in PC. More essentially, functional experiments validated that the expression of HSDL2 significantly correlated with PC cell proliferation. Additionally, we also newly revealed that HSDL2 triggered the tumorigenesis through lipid metabolism in PC. In conclusion, HSDL2 plays a fatal role in a variety of PC progression, which indicates that HSDL2 may become a novel target in biotherapy and prognosis of PC.
Patients and Methods
Ethical Statement
This study was carried out in accordance with the principles of Helsinki Declaration and was approved by Human Ethics and Research Ethics Committee of Medical School of Yanbian University. Patients were acquainted that the excised samples stored by the hospital were possibly utilized to identify information/images (if applicable) for scientific researches and publication, and their privacies were guaranteed to be protected. All patients provided informed consent. The follow-up survival data were collected retrospectively through medical-record analyses.
Clinical Specimens
PC tissue specimens were randomly selected from Shanghai Outdo Biotech Co. Ltd. The ratio of normal pancreatic tissue in PC tissue was 54:66. The samples included patients who underwent surgery from 2009 to 2014, and followed up on their survival status. Clinical pathological parameters containing gender, age, tumor size, clinical stage, location of tumor, lymphatic metastasis and survival data were meticulously inspected. The male-to-female ratio was 39:29. Ages of patients ranged from 38 to 90. A total of 37 of 66 PC specimens were staged as I–IIA and 25 as IIB-IV, and others were not specified. None of these patients had received preoperative adjuvant chemotherapy or radiotherapy.
Immunohistochemistry (IHC)
Sample sections were dewaxed and hydrated with xylene and ethanol, then repaired with citrate antigen. The endogenous peroxidase activity of tissue sections was cooled with 3% H2O2. HSDL2 protein antibody was diluted (Proteintech, USA, 1:200) and used for incubation at 4°C overnight. Tissues were stained with DAB after incubating with secondary antibodies at room temperature and then dipped with hematoxylin on the second day. Finally, after dehydration with ethanol and permeation with xylene, the tablets were sealed with neutral gum. Samples were classified according to the degree of staining and the percentage of positive cells. The dyeing intensity was 0 (no staining), 1 (light yellow), 2 (brown yellow) and 3 (tan). Percentage of positive cells was 0 (<10%), 1 (10–25%), 2 (26–50%), 3 (51–75%), 4 (76–100%). The multiplication of the staining intensity and the percentage of stained positive cells is the positive grade, 0 (-), 1–4 (+), 5–8 (++), 9–12 (+++). And (-) is regarded as negative, (+) is regarded as weak positive, (++) and (+++) are regarded as strong positive expression.
Cell Culture
PC cell lines including Bxpc-3, and Panc-1 from the Cell Bank in the Chinese Academy of Medical Sciences (Shanghai, China) were stored at the Cancer Research Center of Yanbian University. Cells were cultured with DMEM (Gibco, Gaithersburg, MD, USA) and exposed to 10% fetal bovine serum, 100 U/mL penicillin and 100 μg streptomycin, incubated at 37°C and 5% CO2.
Western Blot Analysis
RIPA lysate containing protease inhibitors and phosphatase inhibitors was added to collected cells to extract the total protein of the cells. The protein was separated by SDS-polyacrylamide gel and transferred onto the PVDF membrane. HSDL2 antibody was incubated overnight at 4°C. The secondary antibody incubation was carried out at room temperature for 1 h, and the PVDF membrane was detected by enhanced chemiluminescence.
MTT Assay
Each cell suspension was diluted and seeded in a 96-well plate (5000 cells/well), cultured at 37°C and 5% CO2. After 24, 48, 72 and 96 h of incubation, the supernatant was discarded, and 20 μL of MTT (5 mg/mL) was added to each well and incubated about 4 h, then DMSO was used to mix thoroughly for 10 min. Cell absorbance was detected at 570 nm.
Colony Formation Assay
The cells were seeded in a 6-well plate (1000 cells/well) and cultured in 37°C, 5% CO2 for 20 days. After discarding the supernatant, cells were fixed by 4% paraformaldehyde for 15 min, and hematoxylin was used for staining for 30 min. Finally, the colonies were pictured and counted correctly.
5-Ethynyl-2ʹ-Deoxyuridine (EdU) Incorporation Assay
Cell-Light™ EdU Apollo®488 In Vitro Imaging Kit (RiboBio) was used according to the manufacturer’s instructions. Cells were incubated with 50 μM EdU at room temperature for 2 h, then fixed with 4% paraformaldehyde for 15 min and soaked with 0.5% Triton X-100 for 20 min. Hoechst 33,342 stain was used for 15 min. Finally, the cells were observed under the Leica Sp5II-CLSM microscope.
Transfection
According to the manufacturer’s instructions, siRNA targeting HSDL2 gene (si-HSDL2) was transfected with Lipofectamine 3000 (Invitrogen, CA, USA). All sequences (Table 1) were synthesized by RiboBio (Guangzhou, China). Nonspecific siRNA sequence was used as negative control (si Control).
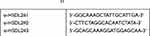 |
Table 1 siRNA Sequence21 |
Detection of Triglyceride and Phospholipid Content
Each well was seeded with 1×106 cells in a 6-well plate, and the cells were cultured at 37°C, 5% CO2. The triglyceride and phospholipid kit were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The triglyceride and phospholipid levels of collected cells were detected using the corresponding kit in strict accordance with the kit instructions.
BODIPYTM 493/504 Staining
Cells grown on 6-well culture slides were fixed with 4% paraformaldehyde. After washing with PBS, BODIPYTM 493/504 staining solution was used for incubation for 15 min in the dark. At the end, the plate was mounted by film and quickly observed under the microscope.
Statistical Analysis
SPSS 20.0 software and Graph Pad Prism 8.0 were used for data analysis. Kaplan–Meier method was used to analyze survival curve, and logarithmic rank test was used to analyze the difference of survival curve. The prognostic significance of univariate and multivariate models was analyzed. All the results were confirmed by three independent biochemical experiments, and the results were expressed as mean ± SD. The value of P < 0.05 was considered to be statistically significant.
Results
HSDL2 Was Overexpressed in PC
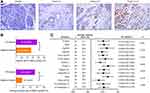
The expression of HSDL2 in normal tissues and PC tissues was detected by IHC. IHC staining showed that HSDL2 expression was negative in normal adjacent tissues, while positive in PC tissues. In the meantime, HSDL2 was mainly expressed in cytoplasm, and there is also a small amount of expression in nucleus (Figure 1A). In addition, the positive rate of HSDL2 expression in PC tissues was 74.2% (49/66), which was significantly higher than in adjacent tissues (53.7%, 29/54; P<0.05). Similarly, the strongly positive rate of HSDL2 in PC tissues was 53.0% (35/66), which was markedly higher than inadjacent tissues (16.7%, 9/54; P<0.01). (Figure 1B). The clinicopathological characteristics of patients with PC was shown in forest plots (Figure 1C). We observed that HSDL2 expression was significantly associated with histological grade (P=0.038) and location of tumor (P=0.015). However, the expression of HSDL2 was irrelevant to gender, age, tumor size, clinical stage and LN metastasis.
Upregulation of HSDL2 Was Associated with the Poor Prognosis of PC
We further investigated the prognosis value of HSDL2 expression in PC. Forest plots showed the results of univariate Cox regression analysis (Figure 2A): we observed that high-level expression of HSDL2 related to patients’ stage (P=0.029, HR=1.857, 95% CI=1.067 to 3.231) and LN metastasis (P=0.045, HR=1.764, 95% CI=1.013 to 3.073). Furthermore, multivariate Cox regression analysis was followed to reveal that HSDL2 expression was validated as an independent indicator of overall survival in PC (P=0.014, HR=2.14, 95% CI=1.17 to 3.916). By interrogating publicly available data we found that HSDL2 expression was strongly correlated with PC patients’ overall survival (Figure 2B) in The Human Protein Atlas (https://www.proteinatlas.org/). Similarly, Kaplan–Meier analysis showed that the overall survival rates were significantly higher in patients with HSDL2-negative expression than HSDL2-positive expression (Figure 2C). Additionally, compared with negative HSDL2 expression patients, patients with well-differentiated status (both P=0.018), early clinical stages (P=0.025) and LN metastasis (-) (P=0.034), had significantly curtailed OS (Figure 2D, F and H). However, HSDL2-positive expression patients with moderate differentiation, late stage and LN metastasis (+) is not obvious (Figure 2E, G and I). These results suggested that HSDL2 might be a novel prognostic biomarker of PC.
HSDL2 Promoted the Proliferation of PC Cells
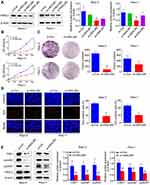
To further investigate the role of HSDL2 in PC cells, we designed two different siRNAs to reduce the expression of HSDL2 in PC cell lines, including Bxpc-3 and Panc-1 cells. We observed the expression level of HSDL2 was significantly suppressed in si-HSDL2#1 group, si-HSDL2#2 group and si-HSDL2#3 group compared to si-Control group by Western blot; especially, the inhibitory effect of si-HSDL2#2 group was the most obvious. Therefore, si-HSDL2#2 was selected for the follow-up experiments (Figure 3A). Next, in order to observe the function of HSDL2 in proliferation of PC cells, MTT assays were utilized to show that proliferative capability of PC cells was obviously suppressed among si-HSDL2 groups (Figure 3B). In the meantime, after silencing HSDL2, the colony formation ability of PC cells was also inhibited (Figure 3C). Additionally, EdU cell proliferation staining showed that immunofluorescence intensity was significantly reduced in si-HSDL2 groups (Figure 3D). Western blot was used to analyze the expression of cell cycle-related proteins (CDK1, cyclinB1 and cyclinD1), and the results showed that the levels of CDK1, cyclinB1 and cyclinD1 decreased slightly after HSDL2 silencing (Figure 3E). Taken together, these results indicated that HSDL2 promoted the proliferation of PC cells.
HSDL2 Was Associated with Lipid Metabolism in PC
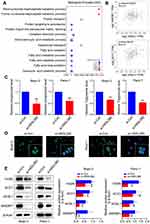
To determine the function of HSDL2 in lipid metabolism, we found the genes by GO biological process analysis, and the bubble chart showed that HSDL2 was closely related to the fatty acid metabolic process (Figure 4A). In addition, GEPIA database (http://gepia.cancer-pku.cn/) showed significant correlation between HSDL2 and key factors of fatty acid synthesis (SRBEF1, ACC1) (Figure 4B). Next, the phospholipid and triglyceride assay kits showed that, after silencing HSDL2, the expression of phospholipid and triglyceride was significantly reduced. Simultaneously, we performed BODIPY 493/503 staining and found that HSDL2 knockdown reduced the intracellular contents of neutral lipids, and the results of BODIPY 493/503 staining were paralleled with the phospholipid and triglyceride assay kits (Figure 4C and D). Simultaneously, we utilized Western blotting to probe the effects of HSDL2 on several pivotal lipid metabolic enzymes (FASN, ACCI, ACSL1 and SREBP1). The result showed that FASN, ACCI ACSL1 and SREBP1 were all fairly down-regulated in si-HSDL2 groups of PC cells (Figure 4E). In conclusion, these results showed that HSDL2 may promote progression of PC through lipid metabolism.
Discussion
Hydroxysteroid dehydrogenase like 2 (HSDL2), a member of SDRs family, consists of a C-terminal SCP2-like domain and an N-terminal SDR domain. HSDL2 was located in 32 regions of the long arm of chromosome 9, and its cDNA length was 3211 bp.8 The protein is composed of 418 amino acids and contains a SCP2 domain, which is involved in many diseases, such as cancer and obesity.6 However, the role and molecular mechanism of HSDL2 in tumor progression seem to be diverse and complex, and further research is needed.
Previous study reported that HSDL2 was highly expressed in ovarian cancer and correlated with poor outcomes.13 Hence, we performed a series of assays to demonstrate the deduction. We firstly found that HSDL2 was obviously overexpressed in PC tissues and located in cytoplasm. The overexpression of HSDL2 was significantly correlated with clinical stage and the location of tumor. Of note, HSDL2 overexpression was significantly related to poor prognosis of PC patients, even including patients with early stage and without LN metastasis. We considered that high expression of HSDL2 may be involved in early tumor formation. These results were in agreement with the study of Zeng et al which showed that down-regulated HSDL2 expression suppresses cell proliferation and promotes apoptosis in papillary thyroid carcinoma.20 Dong et al21 reported that HSDL2 was strongly positive staining in breast cancer, and furthermore that knockdown of HSDL2 inhibited the proliferation and induced cell cycle arrest of breast cancer cells. Therefore we considered that HSDL2 may take part in the proliferation in PC cells and performed MTT, colony and EdU assays which confirmed this hypothesis. The above results showed that HSDL2 promoted the capability of cell growth in vitro and might be a new marker of early diagnosis of PC.
Abnormal lipid metabolism is one of the main signs of tumor development.22 The increased production of lipids promoted lipid synthesis in cell membranes, thereby facilitating the rapid growth of cancer cells. SCP2, a domain of HSDL2, could not only regulate cholesterol in organelles and affect liver cholesterol accumulation,23 but also regulated isoprenoid and cholesterol metabolism, steroid production, peroxisome oxidation of branched and long-chain fatty acids, and absorption of cholesterol.24–30 In addition, HSDL2 was located in peroxisomes and mitochondria which are especially pivotal for the lipid activity. But whether there is a correlation between HSDL2 and lipid metabolism in PC remains unclear. GO analysis showed that HSDL2 was intimately related to the fatty acid process. Accordingly, we observed that triglyceride and phospholipid were down-regulated in the HSDL2-depleted pancreatic cancer cells through triglyceride, phospholipid assay kits and BODIPY 493/503 staining. In addition, Western blotting experiments were carried out to confirm that HSDL2 plays an important role in fatty acid metabolism. The critical enzymes of lipid metabolism such as FASN, ACC1, ACSL1 and SREBP1 were all down-regulated after depletion of HSDL2. The results above reminded us that HSDL2 functions as the momentous factor in altered lipid metabolism promoting cancer progress. It highlights the applicability of HSDL2 as a potential target of a wide range of therapeutic methods.
Currently, many studies have reported that HSDL2 participates in several signaling pathways in tumor cells and plays facilitating roles in cancer progression, metastasis and invasion. However, Zhang et al found that HSDL2 is mainly expressed in the nucleus and overexpression of HSDL2 inhibited cell proliferation and activated apoptosis in cholangiocarcinoma.31 Therefore, we speculated that the “duality” of HSDL2 may be related to the following aspects: 1) Different localization of HSDL2 in different tumor cells leads to different biological processes and signal pathways involved in HSDL2. 2) Tissue specificity or/and the microenvironment of different tumors may also lead to the differential expression of HSDL2. 3) Related oncogene/tumor suppressor genes may also influence the biological action of HSDL2. Hence further study is also necessary to explicate the mechanism underlying HSDL2 overexpression in pancreatic cancers.
In summary, we confirmed for the first time that the expression of HSDL2 is upregulated in PC tissues and cell lines, and the expression of HSDL2 is closely related to the survival of patients. In addition, we found that HSDL2 gene knockout can inhibit the proliferation of PC cells. Most importantly, these findings gave new insights into the role of HSDL2 in the lipid metabolism of PC, but the specific mechanisms need further research to confirm. Hence, our study linked HSDL2 to PC progression and established HSDL2 as a promising biomarker of clinical prognosis and a novel therapeutic target in PC.
Acknowledgments
This research was supported by the The “13th Five-Year” Science Technology Project of Jilin Provincial Education Department (JJKH20200524KJ), The Special Funds for Local Science and Technology Development Guided by the Central Committee, Basic Research Project of Jilin Province (202002021JC), and The thirteenth Five-Year Plan The Funds of Application Basic Foundation of Yanbian University.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors confirm that they have no conflicts of interest.
References
1. Long J, Luo GP, Xiao ZW, et al. Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett. 2014;346(2):273–277. doi:10.1016/j.canlet.2014.01.004
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
3. Duax WL, Ghosh D. Structure and function of steroid dehydrogenases involved in hypertension, fertility, and cancer. Steroids. 1997;62(1):95–100. doi:10.1016/S0039-128X(96)00166-3
4. Oppermann UC, Salim S, Tjernberg LO, et al. Binding of amyloid beta-peptide to mitochondrial hydroxyacyl-CoA dehydrogenase (ERAB): regulation of an SDR enzyme activity with implications for apoptosis in Alzheimer’s disease. FEBS Lett. 1999;451(3):238–242. doi:10.1016/S0014-5793(99)00586-4
5. Gallegos AM, Atshaves BP, Storey SM, et al. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res. 2001;40(6):498–563. doi:10.1016/S0163-7827(01)00015-7
6. Seedorf U, Ellinghaus P, Nofer JR. Sterol carrier protein-2. Biochim Biophys Acta. 2000;1486(1):45–54. doi:10.1016/S1388-1981(00)00047-0
7. Chanderbhan RF, Kharroubi AT, Noland BJ, et al. Sterol carrier protein 2: further evidence for its role in adrenal steroidogenesis. Endocr Res. 1986;12(4):351–370. doi:10.3109/07435808609035445
8. Dai J, Xie Y, Wu Q, et al. Molecular cloning and characterization of a novel human hydroxysteroid dehydrogenase-like 2 (HSDL2) cDNA from fetal brain. Biochem Genet. 2003;41(5–6):165–174. doi:10.1023/A:1023377627138
9. Ruokun C, Yake X, Fengdong Y, et al. Lentivirus-mediated silencing of HSDL2 suppresses cell proliferation in human gliomas. Tumour Biol. 2016;37(11):15065–15077. doi:10.1007/s13277-016-5402-6
10. Kowalik D, Haller F, Adamski J, Moeller G. In search for function of two human orphan SDR enzymes: hydroxysteroid dehydrogenase like 2 (HSDL2) and short-chain dehydrogenase/reductase-orphan (SDR-O). J Steroid Biochem Mol Biol. 2009;117(4–5):117–124. doi:10.1016/j.jsbmb.2009.08.001
11. Shi Y, Mao Z, Huang Y, et al. Knockdown of HSDL2 inhibits lung adenocarcinoma progression via down-regulating AKT2 expression. Biosci Rep. 2020;40(4):BSR20200348. doi:10.1042/BSR20200348
12. Jia LH, Hu MD, Liu Y, et al. HSDL2 promotes bladder cancer growth in vitro and in vivo. Int J Med Sci. 2019;16(5):654–659. doi:10.7150/ijms.31288
13. Sun Q, Zhang Y, Su J, et al. Role of hydroxysteroid dehydrogenase-like 2 (HSDL2) in human ovarian cancer. Med Sci Monit. 2018;24:3997–4008. doi:10.12659/MSM.909418
14. Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(3):230–234. doi:10.1038/sj.pcan.4500879
15. Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13(6):472–482. doi:10.1016/j.ccr.2008.05.005
16. Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16(3):202–208. doi:10.1016/S0899-9007(99)00266-X
17. Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9(4):358–365. doi:10.1097/01.mco.0000232894.28674.30
18. Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–777. doi:10.1038/nrc2222
19. Lee JH, Cho YR, Kim JH, et al. Branched-chain amino acids sustain pancreatic cancer growth by regulating lipid metabolism. Exp Mol Med. 2019;51(11):1–11. doi:10.1038/s12276-019-0299-y
20. Zeng J, Ma X, Wang J, et al. Down-regulated HSDL2 expression suppresses cell proliferation and promotes apoptosis in papillary thyroid carcinoma. Biosci Rep. 2019;39(6):BSR20190425. doi:10.1042/BSR20190425
21. Dong B, Yang Y, Han A, et al. Ectopic expression of HSDL2 is related to cell proliferation and prognosis in breast cancer. Cancer Manag Res. 2019;11:6531–6542. doi:10.2147/CMAR.S205316
22. Howard BV, Morris HP, Bailey JM. Ether-lipids, -glycerol phosphate dehydrogenase, and growth rate in tumors and cultured cells. Cancer Res. 1972;32(7):1533–1538.
23. Falomir Lockhart LJ, Burgardt NI, Ferreyra RG, et al. Fatty acid transfer from Yarrowia lipolytica sterol carrier protein 2 to phospholipid membranes. Biophys J. 2009;97(1):248–256. doi:10.1016/j.bpj.2009.03.063
24. Hillard CJ, Huang H, Vogt CD, et al. Endocannabinoid transport proteins: discovery of tools to study sterol carrier protein-2. Methods Enzymol. 2017;593:99–121.
25. Wüstner D, Solanko K. How cholesterol interacts with proteins and lipids during its intracellular transport. Biochim Biophys Acta. 2015;1848(9):1908–1926. doi:10.1016/j.bbamem.2015.05.010
26. Singarapu KK, Ahuja A, Potula PR, Ummanni R. Solution nuclear magnetic resonance studies of sterol carrier protein 2 like 2 (SCP2L2) reveal the insecticide specific structural characteristics of SCP2 proteins in aedes aegypt mosquitoes. Biochemistry. 2016;55(35):4919–4927. doi:10.1021/acs.biochem.6b00322
27. Seidel G, Prohaska R. Molecular cloning of hSLP-1, a novel human brain-specific member of the band 7/MEC-2 family similar to Caenorhabditis elegans UNC-24. Gene. 1998;225(1–2):23–29. doi:10.1016/S0378-1119(98)00532-0
28. Oppermann UC, Filling C, Jörnvall H. Forms and functions of human SDR enzymes. Chem Biol Interact. 2001;130–132(1–3):699–705. doi:10.1016/S0009-2797(00)00301-X
29. Aube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi:10.1158/1078-0432.CCR-13-3271
30. Baker ME. Evolution of 17beta-hydroxysteroid dehydrogenases and their role in androgen, estrogen and retinoid action. Mol Cell Endocrinol. 2001;171(1–2):211–215. doi:10.1016/S0303-7207(00)00414-7
31. Zhang DY, Liu Z, Lu Z, et al. Lentivirus-mediated overexpression of HSDL2 suppresses cell proliferation and induces apoptosis in cholangiocarcinoma. Onco Targets Ther. 2018;11:7133–7142. doi:10.2147/OTT.S176410
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.