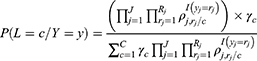Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
How to Define Mild to Severe Hidradenitis Suppurativa? A Simple New Tool Based on Latent Class Analysis of EPIVER Data Study
Authors Perrot JL, Maccari F, Guillem P, Fougerousse AC , Nassif A, Beneton N, Cinotti E, Girard C, Binois R, Reguiaï Z
Received 15 March 2022
Accepted for publication 31 May 2022
Published 16 June 2022 Volume 2022:15 Pages 1091—1103
DOI https://doi.org/10.2147/CCID.S362622
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Jean-Luc Perrot,1,2 François Maccari,2,3 Philippe Guillem,2,4– 6 Anne-Claire Fougerousse,2,7 Aude Nassif,2,8 Nathalie Beneton,2,9 Elisa Cinotti,2,10 Céline Girard,2,11 Raphaelle Binois,2,12 Ziad Reguiaï2,13
On behalf of the ResoVerneuil group
1Department of Dermatology, University Hospital of Saint-Etienne, Saint-Etienne, France; 2ResoVerneuil, Ville, France; 3Private Office, La Varenne St Hilaire, France; 4Service de chirurgie adulte, clinique du Val-d’Ouest, Ecully, 69130, France; 5European Hidradenitis Suppurativa Foundation, Dessau, Germany; 6Groupe de Recherche En proctologie de la Société Nationale Francophone de Colo-Proctologie, Paris, France; 7Military Teaching Hospital Bégin, Saint Mandé, France; 8Centre Médical de l’Institut Pasteur, Paris, 75015, France; 9Dermatology Department, Centre Hospitalier du Mans, Le Mans, France; 10Department of Medical, Dermatology Unit, Surgical and Neuro.Sciences, University of Siena, Siena, Italy; 11Dermatology Department, Centre Hospitalier Universitaire de Montpellier, Montpellier, France; 12Dermatology Department, Centre Hospitalier Régional d’Orléans, Orléans Cedex, 45067, France; 13Service de dermatologie, polyclinique Courlancy, Reims, 51100, France
Correspondence: Jean-Luc Perrot, Department of Dermatology, University Hospital of Saint-Etienne, Saint-Etienne, France, Email [email protected]
Purpose: Hidradenitis suppurativa (HS) is an inflammatory skin disease characterized by recurrent or chronic painful and suppurating lesions in the apocrine gland-bearing regions. The lack of knowledge about HS and its extremely heterogeneous clinical presentation, in terms of both lesion appearance and sites of involvement, frequently delay its diagnosis for several years. Objectives: in this study, using the latent class analysis, it was demonstrated that severity of HS could be evaluated not only with clinical or surgical characteristics but also with gender specificities.
Patients and Methods: Clinical and sociodemographic data of HS patients were retrospectively analysed with the latent class method in order to create a classification tool of disease severity.
Results: From the study of 1428 HS patients (544 men and 884 women), two classification models, depending on gender, were developed. Each classification model was composed of three distinct latent classes clearly identified and defined from mild-to-severe cases of HS. These classification models of HS severity were not distorted by patient ages and were coherent with Hurley stages but were more clinically precise.
Conclusion: In this study, a convenient classification tool, useful for facilitating decision support in routine practice, has been developed. This tool could be used to define clinical subgroups within a study population.
Keywords: hidradenitis suppurativa, classification, severity, latent class, Hurley stage
Introduction
Hidradenitis suppurativa (HS) concerning 0.10% of population, is an uncommon but not rare inflammatory skin disease,1 characterized by recurrent or chronic painful and suppurating lesions in the apocrine gland-bearing regions.2 The lack of knowledge about HS and its extremely heterogeneous clinical presentation, in terms of both lesion appearance and sites of involvement, frequently delay its diagnosis for several years.3
Considerable variability occurs in disease severity. As described by Ingram et al,4 many instruments were used to evaluate HS severity in randomized controlled trials (RCTs).4 In 12 RCTs included in an HS Cochrane review,5 30 outcome measure instruments were described; HS severity can be assessed by lesion count, physician’s global assessment, or patient’s global assessment using different tools alone or combined, which may be completed by ultrasound exams6,7, (Supplementary Material 1).
Heterogeneity is a hindrance for HS research and will become an increasing problem if an outcome consensus process is not undertaken soon. More recently, Canoui-Poitrine et al8 described an empirical classification of HS severity based on clinical variables and using a latent class (LC) method. Their results indicated the existence of three classes with clinical coherence.8 However, these tools do not include all disease aspects (clinical presentation, evolution, comorbidities, and response to treatment) or patients’ characteristics such as gender. Another important question would be whether there are predictors of severe disease that might justify early treatment to arrest disease progression, to prevent subsequent scarring and increase quality of life. Therefore, the ability to classify HS severity is an essential step towards conducting studies aimed at elucidating the etiology of HS, and at developing effective treatments and personalized therapy.
Given the heterogeneity of HS, it was hypothesized that underlying subclasses could be identified based on patient characteristics (gender, socio-demographic data, medical history, risk factors and hormonal influence). The aim of this study is to build a classification scheme based on clinical presentation, risk factors, treatment, and hormonal description to evaluate the severity of HS using the LC method.
Patients and Methods
Study Design and Data Collection
EpiVer was a prospective international multicentre cohort study conducted by an international network of 150 physicians involved in the management of HS, “ResoVerneuil” (Dermatologists, general surgeons, infectiologists, and geneticists). It included all consecutive patients with HS seen in consultations by ResoVerneuil members from 2016 to 2017. Anonymously recorded clinical examination data was collected using standardized case report forms (CRF) (Supplementary Material 2) for all HS patients who agreed to participate. According to the French law, this study requires the information of the patient and the collection of non-opposition to the treatment of his health data. In our study, this process was respected.
Statistical Analysis
The management and statistical analysis of the data was performed using SAS software (Version 9.4, SAS Institute, North Carolina USA). The risk of the first species (α) was fixed at 0.05.
Latent Class Analysis
The latent class method required a limited number of variables.9 The CRF contained 40 questions (Supplementary Material 2), and none of them could be integrated into the model. Thus, selection and aggregation of variables to create 12 items was made and validated by the opinions of HS experts (based on factors associated with the disease and literature) and the actual impact of the variable on the disease was considered (cardiovascular history, other history, family history of HS, other family history, BMI, psychotropic, therapy and duration of treatment, number of attacks and multiple treatment, scores, pregnancy, menopause and contraception and hormonal influence). Among these 12 items, other history and other family history were excluded because of low impact on disease severity. For the model of male subjects, 7 items were selected, and 10 items were selected for the model of female subjects (Tables 1 and 2). Compared to the model of male subjects, the model of female subjects included the addition of hormonal data. The two models were generated separately using the SAS 9.4 LCA procedure. The missing data was handled as randomly missing (Missing At Random [MAR]). Latent classes were identified as “Mild”, “Moderate” and “Severe” for both models, following HS expert analyses of modalities associated, clinical characteristics of population, in line with the literature and the clinical practice.
 |
Table 1 Conditional Probabilities of Baseline Characteristics Based on Latent Class Model Defined from 544 Men Patients with Hidradenitis Suppurativa (HS) |
 |
Table 2 Conditional Probabilities of Baseline Characteristics Based on Latent Class Model Defined from 884 Women Patients with Hidradenitis Suppurativa (HS) |
Definition of Latent Class for Both Models
The selection of the modalities, defining the latent class, was made by comparing the probability that each modality had of appearing in each of the different classes. When the modality was sufficiently discriminating, for one class compared to the others, it was selected (Figures 1 and 2).
 |
Figure 1 Probability of modalities numbers 1 (A), 2 (B) and 3 (C) (defined in Table 1) for each item depending on latent class for the male subject’s model. |
 |
Figure 2 Probability of modalities numbers 1 (A), 2 (B) and 3 (C) (defined in Table 2) for each item depending on latent class for the female subject’s model. |
Model Validation
The internal and external validation was made by comparisons of age and Hurley stage distribution in clusters defined by the LC method. These analyses were conducted to assess the reliability and the added value of the model.
Results
Study Population
Between March 2016 and May 2017, 1428 consecutive HS patients were included in this study: 544 men and 884 women with a mean age of 33.5 (SD ± 11.31) years; 33 (± 11.29) years for men and 31 (± 11.31) years for women. Among them, 76.7% were smokers, 65.7% of the studied population had HS disease since less than fifteen years, and the mean age for the diagnosis was 29.1 years old (± 9.95). Of the subjects, 43.70% matched Hurley stage I, 39.85% Hurley stage II, and 15.76% Hurley stage III (0.70%, 10 patients could not be classified).
Two different models based on gender-specificities and using latent class analysis were created.
Model for the Male Subjects
Three classes ordering HS patients according to disease severity were defined: mild (LCmi), moderate (LCmo), and severe (LCse) HS (Table 1).
Among the 544 male patients, the probabilities a priori of belonging to the LCmi, LCmo, and LCse classes were 28.0%, 51.8%, and 20.2%, respectively.
Many differences were clearly identified between these three classes. LCse patients presented a higher probability of cardiovascular history than LCmo and LCmi patients (59.50% vs 0.0% and 8.31%, respectively) (Table 1). The family history probability of HS increased from the LCmi to the LCmo class and from the LCmo to the LCse class (12.44%, 21.35%, and 33.79%, respectively). Obesity was more present in LCse patients (57.25%) than in LCmi and LCmo patients (17.34% and 17.12% respectively). LCse patients had a higher probability of being smokers than LCmi and LCmo patients (79.32% ±5.76%, 66.69% ±4.06%, and 68.47% ±3.14%, respectively). In LCse and LCmo classes, the probability of having a heavy or long treatment (Antibiotic combination (2 or 3 antibiotics together) or treatment duration <3 months) was higher than in the LCmi class (49.92%, 59.35% vs 18.49%, respectively). The impact of HS (Analogical visual scale (EVA) or Dermatology life quality index (DLQI) high score (>6 or >11 respectively)) was higher for patients from the LCse and LCmo classes than for patients from the LCmi class (75.18%, 77.65% vs 64.17% respectively). Similarly, the number of affected areas and treatments was higher in the LCse and LCmo classes than in the LCmi class (100%, 76.71% vs 0.01% respectively) (Table 1).
To summarize (Figure 1), latent classes identified by the algorithm for HS severity classification of the male population could be defined by:
LCmi: Mild HS
- No previous cardiovascular history (91.69%),
- No obesity (82.66%),
- No antibiotic combination (2 or 3 antibiotics together), or treatment duration <3 months (81.51%),
- Less than 2 treatment modalities (medical or surgical treatment), and number of affected areas ≤3 (99.99%).
LCmo: Moderate HS
- No previous cardiovascular disease history (100%),
- No obesity (82.88%),
- More than 2 treatment modalities (medical or surgical treatment), or number of affected areas >3 (100%).
LCse: Severe HS
- Cardiovascular history (59.50%),
- More than 2 treatment modalities (medical or chirurgical treatment), or number of affected areas >3 (76.71%).
Model for the Women (Table 2)
Three classes were established to characterize HS severity in women population: mild (LCmi), moderate (LCmo), and severe (LCse) HS. Among the 884 women, the probabilities a priori of belonging to the LCmi, LCmo, and LCse classes were 34.4%, 36.1%, and 29.5%, respectively.
Unlike in the model for the male subjects, cardiovascular and family histories were not selected to define disease severity (Table 2). LCse and LCmo patients had a higher probability of presenting with risk factors (Obesity: 32.15% (severe HS), 44.52% (moderate HS) vs 23.27% (mild HS), Smoker: 65.51% (severe HS), 65.47% (moderate HS) vs 34.18% (mild HS)), and a lower quality of life (a DLQI score > 11 and an EVA score >6:73.55% (severe HS), 82.92% (moderate HS) vs 73.27% (mild HS)) than LCmi patients. LCse patients presented with a higher probability of having heavy or long treatment (multiple treatments and number of affected areas > 3: 99.94% (severe HS) vs 46.75% (moderate HS) and 47.79% (mild HS), and antibiotic combination (2 or 3 antibiotics together) or treatment > 3 months: 94.82% (severe HS) vs 37.42% (mild HS) or 21.24% (moderate HS)) along with more than 3 affected areas than the other classes.
In the model for female patients, hormonal influence, pregnancy, and menopause impact were analyzed. The LCse and LCmo patients showed a higher probability of a previous pregnancy (64.52% and 89.78% respectively) or menopause (12.49% and 11.09%, respectively) than LCmi patients (2.77% and 0.01%, respectively). In LCmo and LCse patients, hormonal influence had a higher impact on the severity stage by at least one aggravation (HS during pregnancy, after delivery, and in link with menopause or the menstrual cycle) than in LCmi patients (56.43% and 51.66% vs 30.70%).
To summarize (Figure 2), latent classes identified by the algorithm for HS severity classification of the women population could be defined by:
LCmi: Mild HS:
- Fewer smokers (46.54%),
- -No antibiotic combination (2 or 3 antibiotics together) or treatment duration <3 months (62.58%, but less than in LCmo),
- With a history of pregnancy (97.23%),
- No aggravation due to hormonal influence (69.30%).
LCmo: Moderate HS:
- Mostly smokers (65.51%),
- No antibiotic combination (2 or 3 antibiotics together) or treatment duration <3 months (78.76%; but more than in the LCmi class),
- With a history of pregnancy (but more than LCse) (89.78%),
- At least one aggravation due to hormonal influences (56.43%).
LCse: Severe HS:
- Mostly smokers (65.47%),
- With antibiotic combination (2 or 3 antibiotics together) or treatment duration ≥3 months (94.82%),
- With a history of pregnancy (but less than LCmo) (64.52%),
- At least one aggravation due to hormonal influences (57.56%),
- More than 2 treatment modalities (medical or surgical treatment) or number of affected areas >3 (99.94%).
Validation
The mean age for the model of men patients was 32 years (min 13– max 63) in LCmi class, 33 years (min 10– max 67) in LCmo class, and 45 years in LCse class (min 15–max 67) (p < 0.001). In the model of female subjects, the mean age was 26 years (min 9–max 51) in the LCmi class, 38 years (min 13– max 72) in the LCmo class, and 37 years (min 14–max 96) in the LCse class (p < 0.001) (Figure 3).
 |
Figure 3 Mean age of men (p<0.001) and women (p<0.001) depending on HS severity classes. |
In this classification, Hurley stage distribution increased along with the HS severity, but it was not exactly the same while remaining consistent. The majority of male patients with Hurley stage I were present in LCmi class (53.8%) and to a lesser extent, in LCmo and LCse classes (33.9% and 23.5% respectively). Similarly, patients with Hurley stage III were more prevalent in LCse class (36.8%) than in LCmo or LCmi classes (22.2% and 11.9% respectively) (p < 0.001) (Figure 4).
 |
Figure 4 Description of patients’ distribution in our models, depending on Hurley stage, for men (A) and women (B). |
Most female patients with Hurley stage I were present in LCmi class (60.1%) and, to a lesser extent, in LCse and LCmo classes (52.7% and 28.8% respectively). Hurley stage III patients were more prevalent in LCse class (23.2%) than in LCmo or LCmi classes (7.1% and 8.1% respectively) (p < 0.001) (Figure 4).
Patient’s Classification
The a posteriori probability belonging to each class is calculated with the following formula (See details on Supplementary Material 3):
An Excel tool was developed and proposed to easily classify individuals into an HS severity class using patient characteristics and based on calculations of a posteriori probability presented here (This tool is available from request to the corresponding author).
Discussion
Exploitation of this large cohort of patients (n = 1428), composed of 544 men and 884 women, has enabled two classification systems of HS severity to be created based on gender characteristics and using latent class analysis. The results of this study demonstrate the existence and precise definition of three classes for each gender: LCmi, LCmo, and LCse HS identified as mild, moderate, and severe HS. This severity classification makes clinical sense. Model validation was performed by internal and external evaluation and proved that the models were not based on patient ages. The model validation also proved that the models were coherent with the Hurley stages but were more precise because it considers clinical, socio-demographic, lifestyle and gender aspects. Sartorius method was too complex to be used in an outpatient context and to be used to validate this tool.
Baseline disease severity in each skin region is often measured using the Hurley staging system, which is relatively insensitive to change. Although it successfully provides an overall staging of disease severity, it is lacking in detail; indeed, it does not take into account treatment response and the other aspects of the disease.3 The Hurley staging system is very useful for the overall classification of cases and may form the basis for the selection of appropriate treatment. Among patients with HS who seek help from dermatologists, cases graded as Hurley II form the majority. Within this group, there is a wide variety of clinical findings and symptoms.10 Other instruments are used to measure treatment efficacy for HS disease, such as Canoui-Poitrine classification. These instruments are based on clinicopathological characteristics and do not include gender specificities or lifestyle information.8 As described before, several physician-reported or patient-reported instruments are available in the literature. However, most have not undergone robust validation5, and do not allow all disease specificities to be considered. Moreover, all classification systems made before now have not used gender differentiation, despite HS being a predominantly female disease1. These tools need to be combined to be more efficient in HS severity evaluation. Therefore, it is important to develop a more detailed scoring system to evaluate HS severity.
The classification system in this study considers all factors could affect the disease severity. It details the impact of gender characteristic specificities, comorbidities, and lifestyle on disease severity. This classification process confirmed the starting hypothesis that men’s and women's HS severity need to be determined following different modalities. This study showed that the variables influencing the severity of the disease vary among men and women. It should be noted that the items included in the composition of the aggregated variables used for our classification have also been selected by other authors. Sartorius et al described the impact that obesity and tobacco smoking10 have on HS severity. Their results were in line with the results of this study, which show that obesity and smoking are associated with the LCse class. Garg et al showed that HS was more prevalent among female patients than among male patients.1 The role of hormones in HS remains unclear,11 but the observation of premenstrual flares, female predominance, and improvement during pregnancy suggest a hormonal/metabolic background.12 These results are also in line with the effect of menopause on symptom attenuation that has been reported previously.13 The reported positive effects of antiandrogen therapy may support the possibility that androgens play a negative role in HS.”12 However, a new study about the role of gender in HS in a Korean population found the female-to-male ratio was 1:1.6. They described “a remarkable predisposition for HS among males” and noted that this finding was contrary to the findings usually reported in European studies,14 but was in line with results shown in recent Japanese15 and Turkish studies.16 Two hypotheses based on genetic and environmental factors were developed to explain “Asian” vs “European” difference. First, the discrepancies in gamma-secretase gene mutations occurring between Asian and European studies could contribute to this difference, as they indicate a different genetic background, and second, the divergent smoking habits in Eastern and Western countries could also be a contributing factor.17 Obesity contributes significantly to HS pathogenesis; diabetes, dyslipidemia, the metabolic syndrome, and polycystic ovarian syndrome are among the most common comorbidities.12 The model based on female subjects which was established in this study confirmed this hypothesis by indicating a high probability of subjects having a history of pregnancy and hormone influence in the LCmo and LCse classes.
The LC analysis is a powerful method for classifying patients with similar patterns. LC analysis allowed for the determination of 3 clusters using Bayesian information and assigned each cluster of probabilities to the category of each variable.18
Data were collected prospectively from consecutive patients with a standardized CRF. Three classes of disease severity were defined for each gender: mild, moderate, and severe HS. Internal and external validation, with age and Hurley stage distribution, confirmed the reliability and the added value of the models. From a clinical point of view, it was necessary to take many factors into consideration when assessing the severity of the disease. In this study, modalities selected to describe latent classes were treated together, and it is the association of these different characteristics that provide the disease severity level. These results show the coherence and the added value of the models created.
The main limitation of the latent class method is that this process requires a balance between the number of variables to be used, their associated modalities, and the fineness of the classification. Multiple factors were considered during variable aggregation. There was also a possible bias in the number of previous treatments. Patients included in this study were predominantly outpatients; therefore, they had received fewer treatments than those patients who had attended follow-up in a hospital setting. Finally, it was not possible a posteriori, from the CRF, to re-classify patients according to the redefined Hurley. Similarly, we did not have the means to compare our results with the IHS4 score.
The lack of attention to HS research in the past means that there is still considerable room for improvement in this area of health care, and recent findings have indicated that there is phenotypic heterogeneity within the disease spectrum.19 Numerous scales and severity scores exist (Hurley, IHS4, HS-PGA, Hi-SCR, etc.) but they do not allow to assess the overall burden and severity of HS. The therapeutic arsenal is bound to expand considerably in this pathology. Defining the severity of the disease (in terms of therapeutic pathways, comorbidities, severity) will have a definite impact on therapeutic decisions and highlighted the role of the Excel tool developed here. The HS classifications determined in this study should have many implications and may help establish homogenous subgroups of HS patient populations that could be used for treatment selection and clinical randomized trials. This tool may contribute to current practice by helping with the classification of HS patients.
Conclusion
The classification method used in this study has shown different prognostic factors depending on patient gender. Three distinct levels of HS disease severity in male and female populations have been established. These classes are clearly defined and identified from mild to severe. This study developed a convenient classification tool, which is useful for facilitating decision support in routine practice. This tool could potentially assist with clinical testing by defining clinical subgroups in a study population. It could also identify certain candidate classes for future specific treatments.
Acknowledgments
The authors thank Qualees, who performed statistical analyses and helped in the manuscript writing process. The authors thank the ResoRecherche association for providing financial support. The authors thank the ResoVerneuil group for its contribution: Abramowitz L, Allal S, Barrachin JP, Bécherel PA, Begon E, Beraud V, Biard B, Sultan Bichat N, Bonnefoy Th, Brams A, Cambazard F, Fite C, Favreau Weltzer C, Fouilloux B, Gand-Gavanou, Huther M, Labeille B, Kupfer-Bessaguet I, Laurent Montelimard, Leclercq A, Masson I, Michel JL, Nicol I, Pavlovic-Ganascia M, Levigne V, Pouaha J, Raspado O, Quiles N, Roussel A, Salavert M, Servoz J, Beaulieu T, Tiffet O, Skowron F, Solyga B, Tisserand E, Vergote S, Vermersch A, Vuering F, Zukervar P, Biard B, Gregoire M, Parier J.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8):760–764. doi:10.1001/jamadermatol.2017.0201
2. Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318(20):2019–2032. doi:10.1001/jama.2017.16691
3. Sartorius K, Killasli H, Heilborn J, Jemec GBE, Lapins J, Emtestam L. Interobserver variability of clinical scores in hidradenitis suppurativa is low. Br J Dermatol. 2010;162(6):1261–1268. doi:10.1111/j.1365-2133.2010.09715.x
4. Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol. 2016;175(2):263–272. doi:10.1111/bjd.14475
5. Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa. Cochrane Database Syst Rev. 2015;(10):CD010081. doi:10.1002/14651858.CD010081.pub2
6. Nazzaro G, Passoni E, Muratori S, et al. Comparison of clinical and sonographic scores in hidradenitis suppurativa and proposal of a novel ultrasound scoring system. Ital J Dermatol Venerol. 2021;156(2):235–239. doi:10.23736/S2784-8671.18.06196-5
7. Wortsman X, Moreno C, Soto R, Arellano J, Pezo C, Wortsman J. Ultrasound in-depth characterization and staging of hidradenitis suppurativa. Dermatol Surg. 2013;39(12):1835–1842. doi:10.1111/dsu.12329
8. Canoui-Poitrine F, Le Thuaut A, Revuz JE, et al. Identification of three hidradenitis suppurativa phenotypes: latent class analysis of a cross-sectional study. J Invest Dermatol. 2013;133(6):1506–1511. doi:10.1038/jid.2012.472
9. Varly P, Abdallah A Utilisation des modèles à classes latentes pour la classification des élèves en niveaux scolaires; 2015.
10. Sartorius K, Emtestam L, Jemec GBE, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161(4):831–839. doi:10.1111/j.1365-2133.2009.09198.x
11. Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol. 2015;73(5 Suppl 1):S8–11. doi:10.1016/j.jaad.2015.07.045
12. Karagiannidis I, Nikolakis G, Zouboulis CC. Endocrinologic aspects of hidradenitis suppurativa. Dermatol Clin. 2016;34(1):45–49. doi:10.1016/j.det.2015.08.005
13. Kromann CB, Deckers IE, Esmann S, Boer J, Prens EP, Jemec GBE. Risk factors, clinical course and long‐term prognosis in hidradenitis suppurativa: a cross‐sectional study. Br J Dermatol. 2014;171(4):819–824. doi:10.1111/bjd.13090
14. Happle R. Korean gender differences in hidradenitis suppurativa: nature or nurture? J Eur Acad Dermatol Venereol. 2019;33(7). doi:10.1111/jdv.15507
15. Shibuya Y, Morioka D, Nomura M, Zhang Z, Utsunomiya H. Earwax of patients with hidradenitis suppurativa: a retrospective study. Arch Plast Surg. 2019;46(6):566–571. doi:10.5999/aps.2019.00290
16. Yüksel M, Basım P. Demographic and clinical features of hidradenitis suppurativa in Turkey. J Cutan Med Surg. 2020;24(1):55–59. doi:10.1177/1203475419887732
17. Choi E, Cook AR, Chandran NS. Hidradenitis suppurativa: an asian perspective from a Singaporean institute. Skin Appendage Disord. 2018;4(4):281–285. doi:10.1159/000481836
18. Magidson J, Vermunt JK. Latent class models for clustering: a comparison with K-means. Can J Mark Res. 2002;20:8.
19. Ingram JR, Piguet V. Phenotypic heterogeneity in hidradenitis suppurativa (acne inversa): classification is an essential step toward personalized therapy. J Invest Dermatol. 2013;133(6):1453–1456. doi:10.1038/jid.2012.476
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.