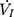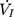Back to Journals » Nature and Science of Sleep » Volume 12
How Does Chronic Intermittent Hypoxia Influence Upper Airway Stability in Rats?
Authors Meng Y, Li W , Zou Y, Yao Y, Huang H, Sun J, Li X, Guo S , Zhang X, Wang W
Received 15 February 2020
Accepted for publication 14 September 2020
Published 15 October 2020 Volume 2020:12 Pages 749—758
DOI https://doi.org/10.2147/NSS.S249948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven A Shea
Yanling Meng,1 Wenyang Li,1 Ying Zou,1 Ye Yao,1 Hong Huang,1 Jianjun Sun,1 Xiaomeng Li,1 Shu Guo,2 Xilong Zhang,3 Wei Wang1
1Institute of Respiratory Disease, The First Hospital of China Medical University, Shenyang, People’s Republic of China; 2Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang, People’s Republic of China; 3Department of Respiratory and Critical Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China
C orrespondence: Wei Wang Email [email protected]
Background: Obstructive sleep apnea (OSA) is characterized by repetitive episodes of upper airway collapse during sleep. The contraction of upper airway dilator muscles plays a crucial role in maintaining UA patency. Chronic intermittent hypoxia (CIH) is the most important pathophysiological process of OSA. Exposure to CIH induced not only the damage of dilator muscles but also the plasticity of the muscles. This study aimed to dynamically assess the influence of CIH on the upper airway.
Methods: The experiments were performed on 44 rats. They were randomly divided into a normoxia (NO) group (n=22) and CIH group (n=22). In each group (n=6, respectively), EMG, transcranial magnetic stimulation (TMS) response, and critical pressure (Pcrit) value were recorded on day 0 (the day before exposure), and the 7th, 14th, 21st, and 28th day of air/CIH exposure. For each group, 16 rats were used for transmission electron microscopy observations on day 0, and the 7th, 14th and 28th day of air/CIH exposure (n=4 for every time point).
Results: Compared to the NO group at the same point, the CIH group showed a damaged ultrastructure of genioglossus, increased activity of genioglossus corticomotor area, and increased Pcrit of the upper airway from the 7th to the 28th day of CIH. Increased EMG activity occurred at the 14th day of CIH and lasted for 2 weeks.
Conclusion: The elevated genioglossus corticomotor excitability in response to the CIH could not counterbalance the damage effect of CIH on upper airway dilator muscles, which ultimately increased the collapsibility of the upper airway.
Keywords: chronic intermittent hypoxia, ultrastructure, transcranial magnetic stimulation, upper airway critical pressure, electromyogram
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent partial or complete collapse of the upper airway during sleep, but it does not occur during wakefulness. The patency of the upper airway mainly depends on the balance between the negative airway pressure and the contraction of upper airway dilator muscles such as genioglossus, sternohyoid muscle. As a typical upper airway dilator muscle, genioglossus contracts synergistically with other dilator muscles to keep the upper airway open. Katz and White1 found that genioglossus activity decreased during sleep and compensatively increased during wakefulness in OSA patients. McSharry et al2 reported that, when compared to control subjects, OSA patients showed significantly decreased muscle fiber conduction velocity of genioglossus which meant increased fatigue. These suggest the dysfunction of genioglossus plays an important role in the development of OSA.
Chronic intermittent hypoxia (CIH) is the dominant pathophysiological feature of OSA. Exposure to CIH during neonatal development caused sternohyoid muscle weakness in male and female rats, and re-exposure to CIH in adulthood could still cause sternohyoid muscle weakness.3,4 However, it is known that hypoxia could also induce long-term facilitation of respiratory muscles. A recent study showed that CIH could bring a beneficial outcome to mitigate OSA by initiating respiratory plasticity.5 Our previous experiments also observed increased genioglossus corticomotor activity in CIH rats assessed by single-pulse transcranial magnetic stimulation (TMS).6,7 Furthermore, previous investigators suggested that dynamic response of the upper airway depended on the coordinated action of numerous rather than any single dilator muscle. In terms of the above changes of dilator muscles, what could happen to upper airway stability during CIH? It has not been systematically evaluated yet.
Critical pressure (Pcrit) has been considered to directly reflect the dynamics of the upper airway because it is an integrated measure of many combined factors. By using negative pressure, it can show us how the contraction of upper airway dilator muscles influence the patency of the upper airway. Cao et al8 reported that Pcrit was correlated with the susceptibility of upper airway collapse. The upper airway of a normal human usually presents a negative Pcrit, instead, a positive Pcrit is frequently observed in OSA patients.9,10 Sforza et al11 reported that there was a positive correlation between the elevated Pcrit and the severity of OSA. These suggested that Pcrit could be used to evaluate the stability of the upper airway.
Based on the above considerations, we hypothesized that CIH could cause different degrees of damage and compensation of upper airway dilator muscles which depended on the time of exposure. These changes might dynamically affectthe upper airway patency. Therefore, we aimed to assess the influence of CIH on the upper airway in the rats at different stages of CIH. Given that genioglossus was an important dilator muscle, we also studied the structure of genioglossus and its neuromuscular response at the same time.
Materials and Methods
Animals
Adult male Wistar rats were provided by Liaoning Changsheng Biotechnology Company (Benxi City, China). The weight ranged from 280–320 g. All rats had free access to water and food, and were housed under controlled conditions (temperature 24±2°C, relative air humidity 40%) with a 12–12 hour light–dark cycle (lights on at 8:00 am and lights off at 8:00 pm). All procedures were performed in accordance with the National Institution of Health Guide for Care and Use of Laboratory Animals, and approved by the animals Ethics and Use Committee of China Medical University.
CIH
The establishment of CIH was based on the principal of N2 dilution. During the exposure of CIH, the rats were housed in a polypropylene animal chamber (A-15274-P-EVAC, BioSpherix), and the capacity of the chamber was 15”W×20”D×20”H. All rats were randomly divided into two groups: the normoxia group (NO group) and the CIH group. The rats in the CIH group were subjected to an oxycycler (Oxycycler model A84XOV, BioSpherix, NY, USA). In this apparatus, hypoxia (10% O2 in N2 lasted for 45 seconds) and normoxia (21% O2 in N2 lasted for 72 seconds) were alternated every 180-second cycle (20 per hour) for 8 hours per day (from 8:00 am to 16:00 pm). The O2 concentration was continuously measured by the O2 analyzer of the oxycycler and changed by a computer-controlled gas outlet. CIH exposure lasted for 4 weeks. The rats in the NO group were subjected to alternating cycles of air under identical experimental conditions in parallel.
Electromyogram (EMG)
Each rat was appropriately anesthetized with isoflurane induction at first, then intraperitoneal injection of sodium pentobarbital was used to maintain anesthesia. Effective anesthesia was judged by abolition of the pedal withdrawal and corneal blink reflexes. Then the rat was positioned on a wooden board with its head, body, and limbs restrained. A concentric needle electrode (NM-131T, Nihon Kohden, Japan) was inserted into the genioglossus. The EMG signal of genioglossus was amplified and filtered (JB-904BK, Nihon Kohden, filter frequency was 300–10,000 Hz, time constant: 200 ms). The integrated signal was digitized and rectified with an electric stimulator (Neuropack Manager, Nihon Kohden). After the EMG recording, the electrode was removed gently and the submental region was cleaned with iodophor disinfectant solution.
TMS
The rats were prepared as EMG measurement by inserting a centric needle electrode (NM-131 T, NIHON Kohden, Japan) into the genioglossus. The TMS response of the genioglossus corticomotor area was recorded from the electrode and described by the latency and the amplitude of the corresponding motor evoked potential (MEP). A computer software package (AxoScopesoftware9.0, Axon Instruments, Inc., USA) was used to collect the signals. Single-pulse TMS was carried out by a Magstim 200 stimulator (Magstim, Whiteland, Dyfed, UK) with a 70 mm figure-eight coil. The coil was hold against the rat’s head, and the stimulation site was 3.0–5.0 mm rostral to bregma and 2.0–4.0 mm lateral from the midline according to the previous studies.6,7 The coil position with the best response to TMS (the highest MEP amplitude and the shortest MEP latency) was defined as the optimal position and marked with the special pen. The coil was kept constantly on the optimal position by a high-precision multi-positional support. For each rat, five stimuli were applied at least 30 second intervals and averaged for mean TMS response. MEP signals were amplified (with filters at 10 Hz to 5 kHz), digitized and recorded by a computer software package (AxoScopesoftware9.0, Axon Instruments, Inc., USA).
Pcrit
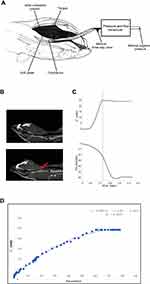
Each rat was appropriately anesthetized with isoflurane induction at first, then intraperitoneal injection of sodium pentobarbital was used to maintain anesthesia. Airway secretion after anesthesia was gently cleaned by a slender cotton swab. The preparation of the upper airway for Pcrit measurement is illustrated in Figure 1A. In order to noninvasively and dynamically investigate the alteration of the mechanical properties of the UA, a double-deck 30.0 mm-long PE cannula with a side hole at the tip was intraorally inserted. The tip of the inner cannula protruded 3.7 mm out of the outer one. The OD of the inner and outer cannula was 2.1 and 3.7 mm separately. The tip of the inner tube was positioned at the boundary of the soft palate and oropharynx. The outer cannula was connected with fresh air. The upper airway Pcrit was determined as previously described.12 To enable the measurement of the negative driving pressure (Pd) and instantaneous airflow (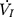 ) across the velopharynx simultaneously, the downstream end of the inner cannula was connected to a pressure and airflow transducer, while the other end of the transducer was further connected to a medical negative pressure suction device. The gradually increased negative pressure were given until the
) across the velopharynx simultaneously, the downstream end of the inner cannula was connected to a pressure and airflow transducer, while the other end of the transducer was further connected to a medical negative pressure suction device. The gradually increased negative pressure were given until the 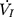 plateaued or decreased. When the negative pressure was administered through the cannula, the lips and snout of rats were well-sealed with acrylate adhesive. Maximum expiratory flow (
plateaued or decreased. When the negative pressure was administered through the cannula, the lips and snout of rats were well-sealed with acrylate adhesive. Maximum expiratory flow (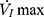 ) was determined by rapidly lowering the upper airway pressure (Pd). With the increment of the absolute value of negative driving pressure, the upper airway CT 3D reconstruction technology was performed to verify the site of collapse (Figure 1B). Pd and
) was determined by rapidly lowering the upper airway pressure (Pd). With the increment of the absolute value of negative driving pressure, the upper airway CT 3D reconstruction technology was performed to verify the site of collapse (Figure 1B). Pd and 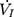 were amplified and digitized for real-time display, and analyzed by the PowerLab software (AD Instruments, Colorado Springs, CO, USA). The negative pressure through the intra-oral cannula were considered to induce flow limitation when instantaneous flow (
were amplified and digitized for real-time display, and analyzed by the PowerLab software (AD Instruments, Colorado Springs, CO, USA). The negative pressure through the intra-oral cannula were considered to induce flow limitation when instantaneous flow (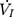 ) plateaued or decreased despite a persistent increase in negative driving pressure (Pd) (Figure 1C). The UA dynamic response of flow-limited twitches was modeled by characterizing the Pd vs
) plateaued or decreased despite a persistent increase in negative driving pressure (Pd) (Figure 1C). The UA dynamic response of flow-limited twitches was modeled by characterizing the Pd vs 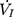 relationship (including a flow value ranging from 0 to
relationship (including a flow value ranging from 0 to 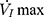 ) with a second-degree polynomial regression model (
) with a second-degree polynomial regression model (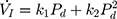 ). The flow-pressure relationships fitted well with this polynomial regression model (r2≥0.90, P<0.001, Figure 1D). Solving this equation (
). The flow-pressure relationships fitted well with this polynomial regression model (r2≥0.90, P<0.001, Figure 1D). Solving this equation (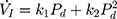 ) for
) for 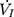 =0 and Pd≠0 provided a theoretical value of Pcrit.
=0 and Pd≠0 provided a theoretical value of Pcrit.
Transmission Electron Microscopy
The rats were anesthetized with isoflurane induction at first and then intraperitoneal injection of sodium pentobarbital to maintain anesthesia until the absence of the pedal withdrawal and corneal blink reflexes. According to the research of McClung and Goldberg,13 we removed the genioglossus rapidly after the rats were euthanized. The genioglossus was sliced into several tissue pieces with a volume of about 1.0 mm3 for each. They were fixed in 2.5% glutaraldehyde phosphate buffer at 4°C to process for paraffin embedded tissue sections. Subsequently, the sections were fixed into 1% osmium tetroxide at 4°C for 30 minutes, flushed with 0.1 mmol/L of PBS three times, dehydrated in graded alcohol, and embedded in paraffin by standard methods. The samples were examined under a transmission electron microscope (JEM-101, Jeol electron Inc., Japan) by three independent investigators with the same and standardized criteria. Mitochondrial analysis was determined by blinded quantification of mitochondria number and area per section using a computerized image analysis system (Image Pro Plus, ver. 6.0 Media Cybernetics, Silver Spring, MD), as previously reported.14,15
Experimental Protocol
The experiments were performed on 44 rats. They were randomly divided into a NO group (n=22) and CIH group (n=22). In each group (n=6, respectively), EMG, TMS response, and Pcrit were performed in one single animal in this order on day 0 (the day before exposure), the 7th, 14th, 21st and 28th day of air/CIH exposure. Each procedure took about 5 minutes and an additional 5 minutes was used for recovery. For each group, 16 rats were used for transmission electron microscopy observations on day 0, and the 7th, 14th and 28th day of air/CIH exposure (n=4 for every time point). The experimental protocol is shown in Supplementary Figure 1.
Statistical Analyses
The results are reported as means±SD. Unpaired t-test was used in two independent series of specimen data. Two-way repeated measures analysis of variance (ANOVA) was performed for the analysis of genioglossus EMG, TMS, and Pcrit between two groups. For the analysis of mitochondria, we used a two-way ANOVA method. The grouping factors of the two-way ANOVA were time (day) and stimulus (chronic intermittent hypoxia). All analyses were performed with SPSS 17.0. P<0.05 was considered as statistically significant.
Results
Genioglossus Ultrastructure of Different Groups
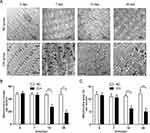
For the NO group and day 0 of the CIH group, genioglossus ultrastructure showed regular myofibrils composing of clear discernible parazones (containing regularly arranged Z lines) and a dark band (containing evident H bands and M lines). The shape of the mitochondria was regular with abundant cristae and an intact inner. Compared with the corresponding NO group, the CIH group presented a damaged ultrastructure characterized by disordered arrangement of partial myofibrils, little vacuolar degeneration, slight edema, and disruption of cristae occurred in some mitochondria on the 7th day of CIH. On the 14th and 28th day, the CIH group showed more obvious ultrastructural changes and presented by disordered structure of myofibrils with blurred parazones and dark band, such as massive lysis and vacuolar degeneration, aggregated, and edematous mitochondria with lysis of mitochondria cristae (Figure 2A). The quantification of mitochondria number results are as follows: Two-way analysis of variance revealed that a significant main effect between two groups (F=98.743, P<0.01, partial η2=80.4%). The main effect of hypoxia time was also significant (F=40.382, P<0.01, partial η2=83.5%), and there was a significant interaction effect (F=37.271, P<0.01, partial η2=82.3%). The quantification of mitochondria area results were as follows: Two-way analysis of variance revealed a significant main effect between two groups (F=69.741, P<0.01, partial η2=74.4%). The main effect of hypoxia time was also significant (F=16.015, P<0.01, partial η2=66.7%), and there was a significant interaction effect (F=20.048, P<0.01, partial η2=71.5%). The quantification of mitochondria number and area decreased from day 14 to day 28 of CIH when compared with those of the NO group (Figure 2B and C).
Genioglossus EMG Activity of Two Groups
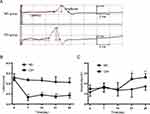
Figure 3A showed typical EMG activity of genioglossus in the NO and CIH groups on day 28 of exposure. Two-way repeated measures analysis of variance revealed a significant main effect between two groups (F=32.908, P<0.01, partial η2=86.8%). The main effect of hypoxia time was also significant (F=32.181, P<0.01, partial η2=86.6%), and there was a significant interaction effect (F=13.669, P<0.01, partial η2=73.2%). The comparison of genioglossus EMG activity between two groups at the same time point is shown in Figure 3B. When compared with the NO group, the CIH group showed a significantly increased genioglossus EMG activity from the 14th day of CIH which lasted for 2 weeks.
TMS Responses of Genioglossus Corticomotor Areas in CIH and NO Groups
The typical TMS responses of genioglossus corticomotor area are shown in Figure 4A. Motor evoked potentials (MEP) latency results are as follows: Two-way repeated measures analysis of variance revealed a significant main effect between two groups (F=224.310, P<0.01, partial η2=97.8%). The main effect of hypoxia time was also significant (F=24.099, P<0.01, partial η2=82.8%), and there was a significant interaction effect (F=11.135, P<0.01, partial η2=69.0%). MEP amplitude results were as follows: Two-way repeated measures analysis of variance revealed a significant main effect between two groups (F=19.741, P<0.01, partial η2=79.8%). The main effect of hypoxia time was also significant (F=3.807, P=0.019, partial η2=43.2%), and there was a significant interaction effect (F=3.652, P=0.022, partial η2=42.2%). Compared with the NO group, the CIH group showed shorter motor evoked potentials (MEP) latency from the 7th day to the 28th day and higher MEP amplitude on the 21st and 28th day of exposure (Figure 4B and C).
Pcrit Measurements for CIH and NO Groups
Two-way repeated measures analysis of variance revealed a significant main effect between two groups (F=31.611, P<0.01, partial η2=86.3%). There was no statistical difference in the main effect of hypoxia time and interaction effect as well. When compared to the NO group, the CIH group showed increased Pcrit on the 7th day of CIH, and this increase lasted to the 28th day of hypoxia exposure (Table 1).
 |
Table 1 The Comparison of Pharyngeal Critical Pressure (Pcrit) Between NO and CIH Groups |
Discussion
Previous studies have observed abnormal structure and function of genioglossus in OSA patients. Carrera et al16 reported that OSA patients showed greater fatigue and a higher percentage of type II fibers in genioglossus than control subjects. Jordan et al17 found that genioglossus activity significantly reduced during REM stage, which might be responsible for the higher severity of OSA at that stage. Hence, does CIH induced by OSA contribute to the structural and functional abnormality of genioglossus and increase upper airway collapsibility? Up to now, there has been no explicit conclusion. Huang et al18 found that CIH could lead to the impairment of genioglossus ultrastructure after 5 weeks of CIH, suggesting that recurrent hypoxia-reoxygenation may be a major driving force for the dysfunction of the upper airway. The present study further dynamically observed the change of genioglossus structure at different stages of CIH, but also explored genioglossus activity and its central control at the same time. The ultrastructure damage of genioglossus occurred on the 7th day of CIH and gradually aggravated with the extension of CIH. Meanwhile, CIH could induce the central compensation first and then elicited an elevation of the genioglossal activity. However, combined with the results of Pcrit, we confirmed that, during CIH, although genioglossus and its central control showed compensation to increase the activity, the collapsibility of the upper airway still increased because of ultrastructure damage of upper airway dilator muscles. This was the first study to systematically investigate the effect of CIH on the upper airway from the perspective of muscle, central regulation to upper airway dynamics.
Ding and Liu19 found that CIH could decrease the fatigue resistance of genioglossus at the end of 5-week’s specific hypoxia (a 2-minute cycle, 1 minute on, 1 minute off with a nadir O2 at 6–8%, 8 hours per day for successive 5 weeks). These could be explained by the observation of Huang et al18 that the genioglossus ultrastructure of the rats was obviously damaged after 5-week’s CIH (a 2-minute cycle, 1 minute on, 1 minute off with a nadir O2 at 5–6%, 8 hours per day for successive 5 weeks), mainly manifested as structural impairment and dysfunction of the mitochondria. Our results confirmed this damage and further found that this damage occurred at the first week of CIH and gradually aggravated with the time of CIH exposure. Genistein treatment could reverse the above fatigability by down-regulating the oxidative stress level and up-regulating antioxidant enzymatic activity through the ERK1/2 signaling pathway.19 The good part of intermittent hypoxia was that it could initiate respiratory plasticity. In a previous study, CIH induced the longterm facilitation of genioglossus in rats.20 In our study, 1 week of CIH was long enough to induce the central compensation of genioglossus in rats, but the increased genioglossus corticomotor excitability and EMG activity could not compensate for the damage of genioglossus ultrastructure resulting from CIH. These ultimately increased the collapsibility of the upper airway. This suggested that the damage was related to the oxidant–antioxidant imbalance to some extent.
Few studies have been done to directly evaluate the effect of CIH on upper airway dynamics. This was the first study to dynamically observe the change of upper airway stability during different stages of CIH. We found that CIH could induce structural damage and central compensation of upper airway dilator muscle, which might eventually increase the collapsibility of the upper airway. In the 4th week of CIH, the stability of the upper airway still decreased even if EMG activity of the genioglossus increased. Ray et al21 also revealed that exposure to CIH in lean Zuker rats increased the upper airway collapsibility, but they only observed the effect of CIH in the 12th week of CIH. These suggested that CIH might contribute to the development of OSA.
Several limitations of this study should be considered. First, in order to keep the integrity of the upper airway, we did not isolate the upper airway to measure Pcrit. With reference to the method of Series and Ethier,12 we applied a second-degree polynomial regression model to calculate Pcrit and tested the occlusion of the upper airway by 3D CT. It had good stability and repeatability. Furthermore, the Pcrit values in the NO group of our study were similar to those reported by the previous study.22 Second, this study was conducted only in male rats and did not explore the effect of sex. The incidence of OSA shows a sex difference. It is higher in males than in females before demonstration. This indicates that estrogen might play a role in keeping upper airway patency. Li et al23 suggested that estrogen might protect OSA patients by affecting genioglossus muscle tension and upper airway collapsibility. Therefore, we only chose male rats for this study to avoid the influence of sex. Besides intermittent hypoxia, hypercapnia is another important pathophysiological change in obstructive sleep apnea patients. It also has an important effect on the activity of upper airway dilator muscles. The combined effect of CO2 and CIH on the upper airway dilator muscles deserves further study. However, this study mainly aimed to observe the influence of CIH itself. We will conduct further studies to assess the effect of CO2 on the upper airway.
In conclusion, the present study revealed that the CIH could result in the ultrastructural damage and the central compensation of genioglossus. However, the elevated genioglossus corticomotor excitability in response to the CIH could not counterbalance its damage effect on upper airway dilator muscles, which ultimately increased the collapsibility of the upper airway. It will be interesting to continuously explore whether the above changes could be reversed by reoxygenation.
Ethics Statement
The procedures and experiment protocols were performed in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals, and were approved by the animal Ethics and Use Committee of the First Hospital of China Medical University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
National Natural Science Foundation of China: 81670085.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest and report no conflicts of interest for this work.
References
1. Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(5):553–560. doi:10.1164/rccm.200403-262OC
2. McSharry D, O’Connor C, McNicholas T, et al. Genioglossus fatigue in obstructive sleep apnea. Respir Physiol Neurobiol. 2012;183(2):59–66. doi:10.1016/j.resp.2012.05.024
3. McDonald FB, Williams R, Sheehan D, O’Halloran KD. Early life exposure to chronic intermittent hypoxia causes upper airway dilator muscle weakness, which persists into young adulthood. Exp Physiol. 2015;100(8):947–966. doi:10.1113/EP085003
4. McDonald FB, Dempsey EM, O’Halloran KD. Early life exposure to chronic intermittent hypoxia primes increased susceptibility to hypoxia-induced weakness in rat sternohyoid muscle during adulthood. Front Physiol. 2016;7:69. doi:10.3389/fphys.2016.00069
5. Mateika JH, Komnenov D. Intermittent hypoxia initiated plasticity in humans: a multipronged therapeutic approach to treat sleep apnea and overlapping co-morbidities. Exp Neurol. 2017;287(Pt 2):113–129. doi:10.1016/j.expneurol.2016.05.011
6. Li T, Wang W, Kong DL, Su J, Kang J. Effects of intermittent hypoxia on the responses of genioglossus motor cortex to transcranial magnetic stimulation in rats. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35(4):283–285.
7. Su J, Wang W, Sun L, Li T, Kong D, Kang J. Raphe serotonergic neurons modulate genioglossus corticomotor activity in intermittent hypoxic rats. Respir Res. 2014;15(1):76. doi:10.1186/1465-9921-15-76
8. Cao Y, McGuire M, Liu C, Malhotra A, Ling L. Phasic respiratory modulation of pharyngeal collapsibility via neuromuscular mechanisms in rats. J Appl Physiol (1985). 2012;112(5):695–703. doi:10.1152/japplphysiol.00136.2011
9. Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol (1985). 1988;64(2):535–542. doi:10.1152/jappl.1988.64.2.535
10. Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol (1985). 1988;64(2):789–795. doi:10.1152/jappl.1988.64.2.789
11. Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med. 1999;159(1):149–157. doi:10.1164/ajrccm.159.1.9804140
12. Series F, Ethier G. Site of phrenic nerve stimulation-induced upper airway collapse: influence of expiratory time. J Appl Physiol (1985). 2002;92(2):665–671. doi:10.1152/japplphysiol.00582.2001
13. McClung JR, Goldberg SJ. Functional anatomy of the hypoglossal innervated muscles of the rat tongue: a model for elongation and protrusion of the mammalian tongue. Anat Rec. 2000;260(4):378–386. doi:10.1002/1097-0185(20001201)260:4<378::AID-AR70>3.0.CO;2-A
14. Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292(3):R1271–1278. doi:10.1152/ajpregu.00472.2006
15. Huang H, Jiang X, Dong Y, et al. Adiponectin alleviates genioglossal mitochondrial dysfunction in rats exposed to intermittent hypoxia. PLoS One. 2014;9(10):e109284. doi:10.1371/journal.pone.0109284
16. Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Agusti AG. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med. 1999;159(6):1960–1966. doi:10.1164/ajrccm.159.6.9809052
17. Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32(3):361–368. doi:10.1093/sleep/32.3.361
18. Huang H, Zhang X, Ding N, Li Q, Min Y, Zhang X. Effects of chronic intermittent hypoxia on genioglossus in rats. Sleep Breath. 2012;16(2):505–510. doi:10.1007/s11325-011-0532-y
19. Ding W, Liu Y. Genistein attenuates genioglossus muscle fatigue under chronic intermittent hypoxia by down-regulation of oxidative stress level and up-regulation of antioxidant enzyme activity through ERK1/2 signaling pathway. Oral Dis. 2011;17(7):677–684. doi:10.1111/j.1601-0825.2011.01822.x
20. Zou Y, Wang W, Nie X, Kang J. Chronic intermittent hypoxia induces the long-term facilitation of genioglossus corticomotor activity. Can Respir J. 2018;2018:5941429. doi:10.1155/2018/5941429
21. Ray AD, Magalang UJ, Michlin CP, et al. Intermittent hypoxia reduces upper airway stability in lean but not obese zucker rats. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R372–378. doi:10.1152/ajpregu.00038.2007
22. Ogasa T, Ray AD, Michlin CP, Farkas GA, Grant BJ, Magalang UJ. Systemic administration of serotonin 2A/2C agonist improves upper airway stability in zucker rats. Am J Respir Crit Care Med. 2004;170(7):804–810. doi:10.1164/rccm.200312-1674OC
23. Li QY, Wan HY, Huang SG. Protective effect of estrogen on patients with obstructive sleep apnea hypopnea syndrome. Zhonghua Jie He He Hu Xi Za Zhi. 2007;30(12):941–943.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.