Back to Journals » International Journal of Nanomedicine » Volume 17
How Do Extracellular Vesicles Play a Key Role in the Maintenance of Bone Homeostasis and Regeneration? A Comprehensive Review of Literature
Authors Ren J , Yu R, Xue J , Tang Y , Su S , Liao C, Guo Q, Guo W, Zheng J
Received 6 June 2022
Accepted for publication 31 October 2022
Published 17 November 2022 Volume 2022:17 Pages 5375—5389
DOI https://doi.org/10.2147/IJN.S377598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Farooq A. Shiekh
Junxian Ren,1,* Rongcheng Yu,1,* Jingyan Xue,1 Yiqi Tang,1 Sihui Su,1 Chenxi Liao,1 Quanyi Guo,2 Weimin Guo,3 Jinxuan Zheng1
1Hospital of Stomatology, Guangdong Provincial Key Laboratory of Stomatology, Sun Yat-sen University, Guangzhou, 510055, People’s Republic of China; 2Institute of Orthopedics, Chinese PLA General Hospital, Key Laboratory of Regenerative Medicine in Orthopedics, Key Laboratory of Musculoskeletal Trauma & War Injuries PLA, Beijing, 100853, People’s Republic of China; 3Department of Orthopedic Surgery, Guangdong Provincial Key Laboratory of Orthopedics and Traumatology, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 510055, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weimin Guo, Department of Orthopedic Surgery, Guangdong Provincial Key Laboratory of Orthopedics and Traumatology, First Affiliated Hospital, Sun Yat-sen University, 58 Zhongshan 2nd Road, Guangzhou, 510080, People’s Republic of China, Tel +86 20 87755766#6236, Email [email protected] Jinxuan Zheng, Hospital of Stomatology, Guangdong Provincial Key Laboratory of Stomatology, Sun Yat-sen University, 56 Lingyuan West Road, Guangzhou, 510055, People’s Republic of China, Tel +86 20 83802802, Email [email protected]
Abstract: The maintenance of bone homeostasis includes both bone resorption by osteoclasts and bone formation by osteoblasts. These two processes are in dynamic balance to maintain a constant amount of bone for accomplishing its critical functions in daily life. Multiple cell type communications are involved in these two complex and continuous processes. In recent decades, an increasing number of studies have shown that osteogenic and osteoclastic extracellular vesicles play crucial roles in regulating bone homeostasis through paracrine, autosecretory and endocrine signaling. Elucidating the functional roles of extracellular vesicles in the maintenance of bone homeostasis may contribute to the design of new strategies for bone regeneration. Hence, we review the recent understandings of the classification, production process, extraction methods, structure, contents, functions and applications of extracellular vesicles in bone homeostasis. We highlight the contents of various bone-derived extracellular vesicles and their interactions with different cells in the bone microenvironment during bone homeostasis. We also summarize the recent advances in EV-loaded biomaterial scaffolds for bone regeneration and repair.
Keywords: bone, homeostasis, metabolism, extracellular vesicles, regeneration
Introduction
Bone homeostasis includes not only osteogenesis by osteoblasts, osteocytes and bone marrow stem cells (BMSCs) but also the absorption of bone matrix by osteoclasts.1 Histological staining has shown that osteoclastic bone resorption is followed by osteoblastic bone formation,2 leading to the concept that bone resorption and bone formation are mechanistically “coupled” to the search for “coupling factors”.3 However, multiple factors are involved in the maintenance of bone homeostasis, and no single factor has been proven to link these coupled processes.3
Interruption of the balance between bone formation and bone absorption likely leads to osteoporosis or other bone metabolism-related diseases.4 A study published in LANCET indicated that more than 8.9 million fractures are caused by osteoporosis every year.5 Bone loss and repair are involved in unbalanced bone homeostasis.6 This complex and continuous process involves communication among multiple cell types.7 Approximately 50% of women and 20% of men older than 50 years of age may experience bone fracture in their remaining lifetime.8 Therefore, the design of new strategies for regulating bone homeostasis may be a potential method for curing bone defects.
Extracellular vesicles (EVs) are diversified nanoscale membrane-restricted vesicles released by prokaryotic and eukaryotic cells.9 Initial studies showed that the released EVs can be considered debris (“cell dust”), and the release of EVs constitutes part of the disposal mechanism of cells discarding unwanted materials.10–12 However, subsequent studies have shown that biofluids, such as semen, amniotic fluid, cerebrospinal fluid, bile, blood, multiple exudates, ascites, breast milk, saliva, CSF, and urine, can contain a large number of EVs.9,13–22 EVs then transport a variety of molecules from parental cells to target cells. Substances that can play the role of intercellular communication in EVs include proteins, nucleic acids and lipids.10,23–25 Therefore, the release of EVs is also an important medium for cellular communication,14,26–30 and EVs are involved in physiological and pathological processes.
A recent study revealed that EVs derived from mesenchymal stem cells (MSCs) have promising osteogenic potential.31 EVs secreted by BMSCs might play a role in osteoblast differentiation during bone healing.32 Osteocyte-derived EVs can accelerate bone degradation by promoting osteoclast formation.33 Moreover, EVs secreted by different cell types can interact with each other during bone homeostasis. Thus, EVs secreted by osteocytes, osteoblasts, osteoclasts, BMSCs and other cells related to bone homeostasis have a great potential role in bone remodeling. We first review the definition, classification, secretion processes, extraction methods and contents of EVs, discuss different origins of EVs, and then elucidate their biofunctional contents in bone homeostasis and regeneration.
Extracellular Vesicles
The structure of EVs released by parental cells is a lipid bilayer surrounded by vesicles and contains cellular components from parental cells.12,26 EVs can be separated into 3 types according to the mechanism of release and their size: exosomes (diameter smaller than 150 nm), microvesicles (diameter between 100 and 1000 nm), and apoptotic bodies (diameter greater than 1000 nm).9,11,15 When referring to EVs, exosomes, microvesicles and apoptotic bodies are mainly mentioned. This article mainly discusses exosomes and microvesicles. EV biogenesis and secretion can be divided into two different pathways. Microvesicles (ectosomes) are mainly produced by outward budding and division directly through the plasma membrane, and exosomes are produced by invasions of the endosomal membrane pathway.
Exosomes
Exosomes were discovered during the era of the rapid development of electron microscopy. The term “exosome” was first used by Dr. Rose Johnstone.13,34 Exosomes originate from the endosome system, whereas microbubbles originate from the plasma membrane. Exosomes are produced by invasions of the endosomal membrane pathway.26,35 First, the inward budding of the plasma membrane leads to the formation of endosomes.36 The endosomal network is a membranous chamber that classifies the various luminal vesicles (ILVs) and directs them to their appropriate destinations, including lysosomes and cell surface membranes.10,37 During this process, the endosome targets some proteins/lipids for lysosomal degradation, whereas others are targeted for recycling or exocytosis. The endosomes can be further subdivided into three different parts: early endosome, late endosome and circulating endosome.38 Early endosomes fuse with endocytic vesicles and integrate their contents into those destined for circulation, degradation, or exocytosis. The material to be recovered is classified as a recyclable inner body.39 The remaining early endosomes undergo a series of transformations and become late endosomes.40 During this transformation, contents destined for degradation or output are preferentially divided into 30–100-nm vesicles, which are formed in the lumen of the late endoplast by budding further inwards through the endosome-limiting membrane.41 Because these late endosomes are observed as multiple vesicles (vesicles are sometimes referred to as luminal vesicles or ILVs), they are also referred to as multivesicular bodies (MVBs).42,43 During this process, cytoplasmic contents and transmembrane and peripheral proteins are incorporated into the invaginated membrane.11 Late endosomes are targeted during lysosome or plasma membrane fusion.44 Fusion with lysosomes destroys the content of late endosomes.45 In contrast, fusion with the plasma membrane results in the secretion of 50–150-nm vesicles into the extracellular space. These excreted vesicles are exosomes labeled with CD9, CD63, TSG101, and RAB27A/B.38,46
Microvesicles
Although most microvesicles are larger (50–2000 nm) than exosomes (less than 150 nm), they still overlap with exosomes in size. Therefore, the difference between microvesicles and exosomes is not primarily in size but in the way they occur. Microvesicles were formerly known as ectosomes. They are mainly produced by outward budding and division directly through the plasma membrane.26 Microvesicles occur due to the dynamic interaction between phospholipid redistribution and cytoskeletal contraction.42 ARF-6 overexpression in tumor cells can induce the secretion level of microvesicles.47
As with exosomes, external factors (calcium influx-induced phospholipid redistribution and HIF-dependent expression of Rab22) have also been suggested to promote microvesicle release.13,48 The microvesicle content also appears to be a highly enriched subset of proteins.47 However, transferrin receptors are highly enriched in exosomes but noticeably absent in microvesicles.49 We summarized the biogenesis and secretion of exosomes and microvesicles in Figure 1 base on the literatures related.10,11,13,26,34–45
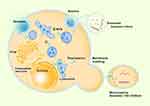 |
Figure 1 Biogenesis and secretion of exosomes and microvesicles. |
Apoptotic Bodies
Exosomes and microvesicles are secreted in normal cell processes. However, apoptotic bodies are only formed during the process of programmed cell death. After the nuclear chromatin condenses, the membrane blistering encapsulates the cell contents to form apoptotic bodies.50,51 Membrane blistering is partially mediated by actin-myosin interactions.52–54 Apoptotic bodies are usually larger in size55,56 and characterized by the presence of organelles in vesicles.51,52,55 Genetic information is transmitted not only by exosomes and microvesicles but also by apoptotic bodies.42
Structure, Content and Function of Extracellular Vesicles
EVs are a group of membrane-covered heterogeneous nanoparticles of different sizes and with different characteristics produced by prokaryotic and eukaryotic cells.9 EV membranes are formed by lipid bilayers and integrins to protect the EV contents from proteases and nucleases.26
The proteins in EVs are commonly associated with their biogenesis, such as proteins associated with endosomal pathways (ALIX and TSG101 in ESCRT). In addition, EVs often contain proteins related to the formation and release process, such as RAB27A, RAB11B, and ARF6, as well as tetraspanins (CD63, CD81, CD9), epidermal growth factor receptor (EGFR), and major histocompatibility complex (MHC). In general, proteins associated with the endoplasmic reticulum, Golgi apparatus, and nucleus are not present in EVs. However, some transcription factors, such as Notch and Wnt, which are usually found in the nucleus, have also been shown to exist in EVs.13
The lipids in EVs are generally related to the lipids in parental cells. The levels of phospholipcholine and diglycerol in EVs are lower than those in parental cells,54 whereas the level of phosphatidylserine in EVs is higher than that in parental cells.13 The amount of lipids in EVs is associated with the type of cargos and the transport of vesicles.44
The genetic material detected in EVs is diverse. Most of the genetic material is mainly small RNA, including ribosome 18S and 28S RNA, tRNA,13 mRNA57 and many miRNAs57 and noncoding RNAs.13 The length of RNA in EVs is approximately 200 nucleotides, indicating that they are probably RNA fragments. Only a small portion of RNA in EVs is complete mRNA and noncoding RNA. In some studies, EVs have been found to contain genomic and mitochondrial DNA.11,58
The function of EVs depends on their abilities to interact with receptor cells and deliver the contents to target cells.15 EVs make intercellular connections between cells through the horizontal transfer of proteins, nucleic acids and other biologically active molecules.58 Analyses of the contents of EVs can help reveal the functional status of parental cells. Studies have shown that EVs play crucial roles in the maturation of red blood cells, platelet adhesion, cytolytic apoptosis, cancer progression, neurodegenerative diseases, cardiovascular diseases, infectious diseases, and autoimmune diseases.15,58
EVs in the circulation can be used as biomarkers for many diseases and have good application prospects.59,60 For example, EVs extracted from cancer patients are utilized to evaluate tumor prognoses.11 In addition, the elimination of certain groups of EVs could prevent disease progression and may be a potential therapeutic strategy with broad research implications.15 The ability to pass through cell membranes makes EVs useful in translational medicine.11 Furthermore, stem cell-derived EVs induce tissue regeneration.61
Extraction of Extracellular Vesicles
Different extraction methods are suitable for EVs for different purposes. The maximum number of EVs is extracted without maintaining the intact structure of EVs for diagnosis purposes. However, if EVs are used for therapy, such as drug carriers, their intact membrane should be maintained to carry contents specifically to the target cell.9 The most common methods of EV isolation are differential super centrifugation, density gradient centrifugation and filter lamps. These methods are based on the physical properties of EVs, such as their size and density. Recent studies have shown that EVs can be separated by size differentiation-based microfluidic filter systems, ultrasonic-based contact-free sorting, immunoaffinity isolation, precipitation and field-flow fractionation.9,11,16 After separation, EVs need to be identified before use. Optimized flow cytometry, which is based on Rosetta Calibration beads and the application of a fluorescent trigger threshold, can be utilized to identify EVs. This new approach improves the analysis power and counting capacity.62 Stoner et al developed a highly sensitive flow cytometry instrument called vesicle flow cytometry.63 In addition to flow cytometry, sequencing techniques, micronuclear magnetic response, nanoplasmonic exosome (nPLEX) sensors, integrated magnetic-electrochemical exosome (iMEX) sensors, and ExoScreen are other effective methods for the analysis of EVs.11,64
Extracellular Vesicles and Bone Remodeling
Osteoblasts are derived from mesenchymal precursor cells. These cells mainly secrete the extracellular matrix, including type I collagen and hydroxyapatite, in several ways (such as EVs).65 Osteoclasts originate from monocyte fusion and have approximately 2 to 12 nuclei per cell. The structure of osteoclasts is related to the degradation of the extracellular matrix in resorption lacuna, which allows the acidified extracellular environment to hydrolyze hydroxyapatite, and metalloproteases and cathepsin K will then degrade collagen fibrils through the exposition of collagen fibrils. The interdependence of osteoblasts and osteoclasts allows us to view them as bone remodeling units histologically and functionally. In addition to osteoblasts and osteoclasts, osteocytes also regulate bone homeostasis. In fact, the majority of cells in the bone are osteocytes. Osteocytes are derived from mature osteoblasts embedded in the bone matrix. Osteocytes are known to play a significant role in sensing mechanical loading and regulating calcium and phosphate homeostasis instead of being static bystanders during bone homeostasis. The activity of these cells is spatiotemporally coordinated through many molecules.66 BMSCs, osteoblasts, osteoclasts and osteocytes can release EVs.67–69 One of the significant mechanisms involved in intercellular communication is through EVs. miRNAs carried by EVs can regulate significant pathways during bone homeostasis, including PI3K/AKT, TGFβ, Ras/ERK and Wnt.7,61 Consequently, EVs and their contents can be used as biomarkers for some bone-related diseases.68 Osteogenic EVs and their bioactive substances orchestrate bone remodeling through paracrine, autosecretory, and endocrine mechanisms. Moreover, osteogenic EVs can modulate the differentiation of osteoclasts, osteoblasts and other cells.7,70 However, the effects of osteogenic EVs on bone homeostasis and regeneration are related to the amount of EVs, the age of the parent cells and the cell passage of ex vivo cell culture.61
Extracellular Vesicles and Osteocytes
EVs derived from osteocytes (OC-EVs) regulate mineral deposition through communication with osteoblasts or osteoclasts. EVs secreted by osteocytes are rich in RANKL.33 OC-EVs are able to reduce the cytoplasmic volume of osteoblasts and promote osteoclast formation.71,72 The number of OC-EVs increases after parathyroid hormone treatment, which induces osteoclast differentiation. The mechanical stimulation of osteocytes enhances EV production to transmit bone regulatory proteins. This process is associated with Ca2+-dependent contraction of osteocytes.73 Osteocytes adjacent to blood vessels and/or at the edge of the bone marrow space produce EVs that are present in the circulation and may transport their contents to remote recipient cells through the blood circulation.74,75 EVs located in the bone matrix are named bone matrix-derived EVs, which have been proven to originate from osteocytes. Recently, Wang et al found that aged bone matrix-derived EVs can target BMSCs in the bone marrow cavity, leading to adipogenesis and targeting vascular smooth muscle cells (VSMCs) in vascular tissue to augment the osteogenic transdifferentiation of VSMCs.76
Extracellular Vesicles and Osteoblasts
Osteoblast-derived EVs (OB-EVs) have the characteristics of exosomes: diameter between 30 and 100 nm, round shape with cup-like concavity, and expression of transmembrane markers TSG101 and FLOT1.70,77 Osteoblasts secrete EVs during the whole maturation stage.78 OB-EVs contain proteins and nucleic acids that play crucial roles in intercellular communication in bone.79 A total of 1536 proteins are involved in this process. Most of these proteins are located in the plasma membrane or cytoplasm and are involved in protein localization and intracellular signal transduction. These proteins are also engaged in the EIF2, integrin, BMP, Wnt and TGF-β signaling pathways, which are involved in bone formation by inducing osteoblast differentiation and promoting angiogenesis.80,81 The RAB13 protein is involved in vesicle transport, including vesicle production, cargo sorting and fusion with target cell membranes. RAB13 has the largest content in EV-mRNA. EVs containing RAB13 can regulate the proliferation and differentiation of potential target cells (stromal cells, chondrocytes, blood cells, and tumor cells), which interact physiologically with osteoblasts in the bone microenvironment.82 OB-EVs contain RANKL, which interacts with RANK on the precursors of osteoclasts to promote osteoclast formation.76,83,84 Thus, inhibiting OB-EV secretion may be a novel approach to prevent bone loss. In addition to RANKL, galectin-3 is also an important component of EVs. Weilner et al85 demonstrated a positive correlation between galectin-3 and osteogenic potential in vitro. Studies on the content of mouse OB-EVs have shown that many highly enriched proteins in EVs are similarly enriched in osteoblast-related pathways, including the mTOR pathway.80
Moreover, osteoblast-derived EVs contain 254 types of mRNAs specifically related to osteoblasts and EV-mediated functions, such as bone metabolism and osteoblast function.70 Most of the mRNAs are small RNA fragments with lengths of 25–1000 nucleotides82 that contain certain 3’-UTR fragments to protect the mRNA of recipient cells from miRNA degradation.86 Some OB-EVs contain medium-size full-length mRNAs.82 These results indicate that there are two different methods of information transmission of nucleic acids through OB-EVs: mRNA message translates into protein directly or 3’-UTR fragments completely bind miRNA to weaken its inhibition of translation.
miRNAs are another portion of the OB-EV content. Cui et al found that multiple miRNAs in OB-EVs, including miR-667-3p, miR-6769b-5p, miR-7044-5p, miR7668-3p and miR-874-3p, increase during bone mineralization. These miRNAs target axin, a negative regulator of the Wnt signaling pathway, to increase the expression of nuclear-located β-catenin and activate Wnt signaling. Furthermore, miR-140-3p contained in OB-EVs and BMSC-derived exosomes promote osteoblast differentiation.87–89 In the microenvironment of bone remodeling, EVs also deliver specific proteins, miRNAs (such as miR214-3p, miR-183-5p, miR-196a) and growth factors (such as TGF-β) to osteoblasts to promote bone formation.68 In contrast, osteoblast differentiation is inhibited by adipocyte-derived EVs.90,91 Weilner et al found that senescent endothelial cells are miR-31 producers and deliver miR-31 in EVs. When miR-31 is captured by mesenchymal stem cells, it inhibits osteogenic differentiation by antagonizing its target gene frizzered-3.92
miRNAs of osteoblast lineage cell-derived EVs, such as those belonging to the let-7 miRNA family, can regulate the activity of osteoblasts through autosecretion.70 Exosomes from preosteoblast cell lines (such as MC3T3-E1) promote the osteogenic differentiation of BMSC cell lines (such as ST2) characterized by upregulated expression of osteoblast markers and enhanced matrix mineralization.89 miR-143-3p suppresses osteoblast differentiation by targeting Cbfb mRNA. The miR-218 content in osteoblast-derived EVs inactivates Wnt inhibitors and stimulates Wnt/β-catenin signaling to promote osteoblast differentiation of BMSCs.93 EVs derived from MC3T3-E1 osteoblast-like cells have been demonstrated to affect mineralization of the BMSC line ST2. These EVs contain multiple miRNAs, including miR-30d-5p, miR-133b, and miR-140-3p. A previous study showed that miR-30d-5p and miR-133b target RUNX2 to inhibit osteoblast differentiation.66,89 miR-181a promotes osteoblast differentiation in C2C12 and MC3T3 cells by inhibiting TGF-β signaling molecules by targeting the TGF-β-induced osteoblast differentiation negative regulatory factors TGF-βI and TβRI/Alk5.94 In conclusion, osteoblast-cell-line-derived EVs targeting osteoblasts may promote osteoclast differentiation and osteoclastogenesis.95
At the first stage of mineralization, EVs secreted by osteoblasts are mainly matrix vesicles (MVs).65 Calcium (Ca) and inorganic phosphate (Pi) are absorbed by MVs to form hydroxyapatite crystals. These processes may have multiple impacts on collagen fibril synthesis and extracellular matrix mineralization. Active vitamin D is necessary for intestinal calcium absorption, and fibroblast growth factor 23 (FGF23) is a key regulator of phosphate metabolism. A lack of calcium or phosphate could impair bone mineralization. Overaction of FGF23 leads to renal phosphate consumption and impairs the activation of vitamin D. Extracellular phosphate regulates FGF23 expression through FGFR signaling, which suggests that phosphate metabolism is closely related to the FGFR pathway.96
Extracellular Vesicles and Osteoclasts
Mature osteoclasts and their precursors secrete EVs that are similar in size and morphology and express highly specific surface markers, such as EpCAM and tetraspanin CD63.70 EVs derived from osteoclast precursors enhance 1,25-dihydroxyvitamin D3-dependent osteoclast formation in mouse marrow cultures. In contrast, EVs from differentiated osteoclasts inhibit osteoclast formation.75,83 The underlying mechanism may be that EVs from mature osteoclasts contain RANK, which can compete with RANK on the surface of osteoclasts to bind to limited RANKL. However, a lower level of RANK is detected in EVs produced by osteoclast precursors.70,81 Removing EVs that contain RANK secreted by osteoclasts rescues their inhibitory effect on osteoclast formation.97 Therefore, EVs from mature osteoclasts can act as a RANK inhibitor.83
EVs derived from osteoclasts are engaged in modulating bone mineral metabolism through their proteins and nucleic acids. RANK-RANKL interactions can establish contact between osteoclasts and osteoblasts. RANK-rich EVs target the RANKL-rich membrane surface to deliver proteins, mRNAs, miRNAs and noncoding RNAs to regulate osteoblasts.83 EVs secreted by osteoclasts interact with EphA2 on osteoblasts through ephrinA2 carried by EVs and thus specifically identify osteoblasts. miR-214-3p carried by osteoclast-derived EVs inhibits osteoblast differentiation by directly targeting ATF4.87 miR-214 may be a target for the diagnosis, treatment and prognosis of osteoporosis. These studies have shown that osteoclasts can communicate with osteoblasts through EVs to coordinately inhibit osteogenesis. Sun et al showed that miR-148, miR-21, miR-214 and miR-183A-5p are significantly upregulated in EVs secreted by osteoclasts.87 Among these miRNAs, only miR-21 and miR-148a are considered to promote osteoclast formation.68,70,98,99 Cui et al found that lncRNA MALAT-1 enrichment in HEK-293T cell-derived EVs inhibits miR-124 and enhances MMP9, CSK, CP5, and CAR2 expression, which results in increasing osteoclastogenesis and bone resorption.100
Extracellular Vesicles and Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are multipotent stromal cells originating from bone marrow, fat and other mesenchymal tissue sources or derived from induced pluripotent stem cells (IPS-MSCs). EVs are one of the main components secreted by MSCs.101 EVs derived from a variety of MSC types have successfully induced bone formation in preclinical models.61 hBMSC-derived EVs induce angiogenesis and increase osteocalcin expression in rat skull defects.102 A study confirmed that exosomes are required for bone repair by evaluating the effects of exosomes isolated from MSC-conditioned medium on bone fracture healing in CD9−/− mice. As we know, CD9−/− mice produce very low levels of exosomes. The delivery of EVs secreted by MSCs to bone fracture sites rescues the phenotype of delayed fracture healing. However, when conditioned medium is injected with exosomes, bone fractures could not heal as well as in the extracted exosome groups, suggesting that a high concentration of exosomes is needed for tissue repair.101
The contents of EVs isolated from the same type of cell may differ.61 BMSC-derived EVs have tetraspanin markers on their surface, including CD9, CD63 and CD81, and other markers, including HSP90, HSP70 and Flot-1.70 EVs of BMSCs may play a role in osteoblast differentiation, bone healing, bone protection and regeneration,75 among which EVs may act as mediators. Martin et al found that hBMSCs grown under osteogenic conditions release EVs with osteogenic induction potential by upregulating BMP2, SP7, SPP1, BGLAP/IBSP and ALP expression in undifferentiated BMSCs.103 There are differences in the therapeutic ability of BMSC-derived EVs, which may be due to differences in cell origin, cell age and other factors.61
The key elements of EV cargo are miRNAs and small noncoding RNAs that play crucial roles in bone-related gene expression and thus affect bone healing by regulating cell proliferation, differentiation, and apoptosis.87 EVs lose their osteogenic potential after prolonged incubation with RNases, which emphasizes the role of miRNAs in bone formation.61 A molecular analysis of MSC-derived exosomes in bone defect models revealed the presence of several highly expressed miRNAs,101 including miR-29b-3p, which significantly increase the callus volume and density.98 Different miRNAs are involved in the regulation of the osteogenic differentiation of BMSCs,104 and these include miR-27a, miR-21, miR-217, miR-26a, miR-148a, miR-200b, miR-335-3p, miR-92a, miR-9, miR199b-5p, miR885-5p, miR-199b and miR-196a.32,87,105 A small number of miRNAs and pathways in BMSC-derived EVs, such as miR-21, miR-4532, miR125b-5p and miR-338-3p, are believed to play an important role in bone regeneration, and among these, miR-21 is an anti-apoptotic miRNA that can promote the osteogenic differentiation of MSCs.
Moreover, EVs derived from BMSCs participate in bone remodeling by regulating the proliferation and activity of osteoblasts and osteoclasts.81 MSC-derived EVs with miR31A-5P enhance adipogenesis, activate osteoclasts and decrease osteogenesis in aging rats, showing dual functions of osteogenic inhibition and osteoclastic generation.106 Qin et al found that miR-196a, miR-27a, and miR207 are upregulated in BMSC-derived EVs, and among these, miR-196a is the most osteogenic inducible factor.32 Luo et al found that the upregulation of many miRNAs, particularly miR-26a, in BMSC-derived EVs promotes osteogenesis. The silencing of miR-26a completely eliminates the osteogenic potential of BMSC-derived EVs in vitro.107 Ji-feng Xu et al found that BMSC-derived EVs upregulate let-7a, miR-199b, miR218, miR-148a, miR-135b, miR203, miR299-5p and miR-302b expression in osteoblastic differentiation and significantly decrease the levels of miR-221, miR-155, miR-885-5p, miR-181a and miR-320.104,105 The overexpression of miR-885-5p by delivered hBMSCs-EVs impair wnt5a and runx2 expression, which ultimately dampens the osteogenic potential of hBMSCs.66
Extracellular Vesicles in Multiple Cell Interactions
With the communication between different types of cells, bioinformation interflows among them. There are different ways of intercellular communication: direct contact between cells, communication by secreted chemicals and gap junctions. EV secretion is an important method for the transfer of cargoes to target cells. Thus, intercellular communication through EVs is a type of communication via secreted chemicals. The effects of this type of cell communication depend on cargos carried by EVs to target cells. As reviewed above, multiple cell types, such as osteocytes, osteoblasts, osteoclasts, MSCs and BMSCs, produce EVs targeting neighboring or remote cells or bone matrix. The contents of EVs participating in bone homeostasis are organized into a table for reference (Table 1). The schematic image of these cell communication processes was shown in Figure 2 and the specific references can be found in Table 1.
 |
Table 1 Content of EVs Contributing to Bone Homeostasis |
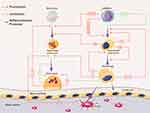 |
Figure 2 Interaction of EVs and multiple cells during bone homeostasis. Note: Parts of the figure were created using images from Servier Medical Art by Servier, licensed under a Creative Commons Atribution 3.0 Unported License (https://smart.servier.com/). |
BMSC-derived EVs could be effectively internalized by targeted osteoblasts and thus upregulate the expression of osteoblast-related genes and promote osteoblast differentiation.32 MSC-derived exosomes can firmly bind extracellular matrix proteins, such as type I collagen and fibronectin, to the bone surface.108 Conversely, the content of osteoblast-derived exosomes, such as EIF2, can induce osteoblastic differentiation of MSCs.80 The RANK-RANKL interaction makes osteoclast-osteoblast communication by EVs possible. Deng et al showed that RANKL-rich exosomes released by osteoblasts stimulate osteoclast formation.76 The paracrine release of RANK-rich exosomes from osteoclasts targets osteoblasts to inhibit their activity.70,109 Moreover, ephrinA2 carried by osteoclast-derived EVs interacts with EphA2 on osteoblasts. EVs secreted by osteoclasts containing miR-214-3p can target osteoblasts and inhibit osteogenesis.110 miR-214 directly targets ATF4 to inhibit the activity of osteoblasts, whereas reverse signaling through ephrinA2 into osteoclasts enhances osteoclast formation.109 EVs derived from osteoblasts carrying miR-125b can be transferred to the bone matrix and then inhibit the differentiation of osteoclast precursors, thereby increasing the bone mass in mice.111
Extracellular Vesicless with Biomaterial Scaffolds in Bone Regeneration
Taking advantage of EVs in cell communication, several studies have combined EVs and biomaterials to construct functionalized implants for use in bone repair (Tables 2 and 3).112 When applied with biomaterials, the source of EVs is not restricted to bone-related cells. Cell-free exosome-coated 3D-printed titanium alloy scaffolds show efficiency for bone regeneration.113 The combination of hiPSC-MSC-exosomes and β-TCP scaffolds can promote osteogenesis and angiogenesis and thus improves bone regeneration.114,115 Li et al used polydopamine (pDA) to modify a poly(lactic-co-glycolic acid) (PLGA) scaffold and then loaded the scaffold with exosomes derived from human adipose tissue-derived stem cells (hASCs). The results indicated that the resulting scaffold can release exosomes slowly and constantly in vitro and enhance bone regeneration in vivo.116 Wei et al utilized BMP2-treated macrophage-derived exosomes to modify titanium nanotube implants, and this incorporation increases alveolar bone osteogenesis around implants.117 Mineral-doped PLA-based porous scaffolds combined with MSC-derived exosomes exert promising osteogenic results and bone healing efficiency.118 Swanson et al encapsulated exosomes derived from hDPSCs in PLGA and poly(ethylene glycol) (PEG) triblock copolymer microspheres and then incorporated them into nanofibrous PLAA scaffolds. These scaffolds can promote BMSC differentiation and mineralization and thus increase osteogenesis.119 Wang et al synthesized a type of coralline hydroxyapatite (CHA)/silk fibroin (SF)/glycol chitosan (GCS)/difunctionalized polyethylene glycol (DF-PEG) self-healing hydrogel, which can be used as a scaffold to combine human umbilical cord mesenchymal stem cell (hUCMSC)-derived exosomes for promoting bone defect healing.120 Yang et al developed an injectable hydrogel system that embeds hydroxyapatite (HAP) in in situ cross-linked hyaluronic acid-alginate (HA-ALG). The combination of hUCMSC-derived exosomes and the hydrogel system significantly promotes bone regeneration.121 Fan et al combined BMSC-derived exosomes and tannic acid-modified sulfonated polyether ether ketone (TA-SPEEK) and developed BMSC-derived exosome-functionalized implants, which play a significant role in osteoimmunomodulation and thus promote osteogenesis both in vitro and in vivo.122 Liu et al delivered lyophilized BMSC-derived exosomes to a mesoporous bioactive glass (MBG) scaffold. The implant enhances the bone reparative cavity in a rat cranial defect model.123 Huang et al showed that when MSC-EVs are encapsulated in 3D hydrogels, they can prolong delivery while also maintaining the EV structural integrity and osteoinductive functionality in vitro and enhancing bone regeneration in vivo. These hydrogels may possibly be formulated as an injective material or a bulk hydrogel that is suitable for regenerative medicine applications.124
 |
Table 2 General Characteristics of EV-Functionalized Biomaterials |
 |
Table 3 Application of EV-Loaded Biomaterials |
Conclusion and Prospective
Bone homeostasis is modulated by relevant cell communication through EVs. Osteoclast-derived EVs regulate bone matrix mineralization and the differentiation of osteoblasts and osteoclasts. OB-derived matrix vesicles are involved in matrix mineralization. OB-EVs and their contents (nucleic acids, proteins) mediate intercellular communication in bone. Premature osteoblast-derived EVs promote osteogenic differentiation and thus promote bone formation, whereas mature osteoblast-derived EVs deliver RANKL to promote osteoclast formation. Premature osteoclast-derived EVs enhance osteoclast formation. However, mature osteoclast-derived EVs inhibit osteoclast formation. BMSC-EVs or MSC-EVs are crucial in bone remodeling, bone healing, bone protection and regeneration. In addition, different types of cells interact with each other and orchestrate bone homeostasis and regeneration.
Even though bone cell-derived EVs display potential functions, the application of EVs only in regeneration medicine is a difficult challenge. Thus, strategies that combine EVs with modified biomaterials have emerged for the functionalization of implants in bone repair and have shown remarkable progress. However, there is still some way to go before bone-cell-derived EVs can be applied in the clinic. The sources of EVs, the selected cargos in EVs, the optimized biomaterials and the preservation of biological agents need further in-depth research.
Overall, EVs play indispensable and multidimensional roles in bone homeostasis, particularly in cell communication, cell differentiation and extracellular matrix deposition. Elucidating the role of EVs in the maintenance of bone homeostasis indicate a new direction for bone regeneration in the future and identify potential biomarkers for bone metabolism-related diseases.
Abbreviations
EVs, extracellular vesicles; ILVs, luminal vesicles; MVB, multivesicular bodies; BMSC, bone marrow stem cell; MVs, matrix vesicles; MSC, marrow stem cell; hBMSC, human bone marrow stem cell; pDA, polydopamine; PLGA, poly(lactic-co-glycolic acid); PEG, poly(ethylene glycol); CHA, coralline hydroxyapatite; SF, silk fibroin; GCS, glycol chitosan; DF-PEG, functionalized polyethylene glycol; HAP, hydroxyapatite; HA-ALG, hyaluronic acid-alginate; MBG, mesoporous bioactive glass; TA-SPEEK, tannic acid-modified sulfonated polyether ketone.
Acknowledgments
We apologize to everyone in the EV field whose work could not be included due to space constraints.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81900958, 82102552) and the Natural Science Foundation of Guangdong Province (2020A1515010059).
Disclosure
The authors report no conflicts of interest associated with this work.
References
1. Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9(1):66. doi:10.1186/1741-7015-9-66
2. Frost HM. Intermediary organization of the skeleton; 1986.
3. Rodan GA. Bone homeostasis. Proc Natl Acad Sci U S A. 1998;95(23):13361–13362.
4. Reeve J, Hesp R, Williams D, et al. Anabolic effect of low doses of a fragment of human parathyroid hormone on the skeleton in postmenopausal osteoporosis. Lancet. 1976;1(7968):1035–1038.
5. Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367(9527):2010–2018.
6. Park-Min KH. Metabolic reprogramming in osteoclasts. Semin Immunopathol. 2019;41(5):565–572.
7. Huang X, Xiong X, Liu J, Zhao Z, Cen X. MicroRNAs-containing extracellular vesicles in bone remodeling: an emerging frontier. Life Sci. 2020;254:117809.
8. Bone health and osteoporosis: a report of the surgeon general. Reports of the Surgeon General. 2004.
9. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347.
10. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
11. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118(4):1917–1950. doi:10.1021/acs.chemrev.7b00534
12. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi:10.1038/s41556-018-0250-9
13. Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–312. doi:10.1007/s10571-016-0366-z
14. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi:10.1083/jcb.201211138
15. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:4.
16. Lyu H, Xiao Y, Guo Q, Huang Y, Luo X. The role of bone-derived exosomes in regulating skeletal metabolism and extraosseous diseases. Front Cell Dev Biol. 2020;8:89. doi:10.3389/fcell.2020.00089
17. Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8(19):4083–4099. doi:10.1002/pmic.200800109
18. Properzi F, Logozzi M, Fais S. Exosomes: the future of biomarkers in medicine. Biomark Med. 2013;7(5):769–778. doi:10.2217/bmm.13.63
19. Wu M, Ouyang Y, Wang Z, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114(40):10584–10589. doi:10.1073/pnas.1709210114
20. Street JM, Koritzinsky EH, Glispie DM, Yuen PST. Urine exosome isolation and characterization. Methods Mol Biol. 2017;1641:413–423.
21. Manek R, Moghieb A, Yang Z, et al. Protein biomarkers and neuroproteomics characterization of microvesicles/exosomes from human cerebrospinal fluid following traumatic brain injury. Mol Neurobiol. 2018;55(7):6112–6128. doi:10.1007/s12035-017-0821-y
22. Leiferman A, Shu J, Upadhyaya B, Cui J, Zempleni J. Storage of extracellular vesicles in human milk, and MicroRNA profiles in human milk exosomes and infant formulas. J Pediatr Gastroenterol Nutr. 2019;69(2):235–238. doi:10.1097/MPG.0000000000002363
23. Li Y, Yin P, Guo Z, et al. Bone-derived extracellular vesicles: novel players of interorgan crosstalk. Front Endocrinol (Lausanne). 2019;10:846. doi:10.3389/fendo.2019.00846
24. Tao SC, Guo SC, Zhang CQ. Modularized extracellular vesicles: the dawn of prospective personalized and precision medicine. Adv Sci. 2018;5(2):1700449. doi:10.1002/advs.201700449
25. Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–1059. doi:10.3727/096368913X667709
26. Cocozza F, Grisard E, Martin-Jaular L, Mathieu M, Thery C. SnapShot: extracellular Vesicles. Cell. 2020;182(1):262–262 e1. doi:10.1016/j.cell.2020.04.054
27. Holme PA, Solum NO, Brosstad F, Roger M, Abdelnoor M. Demonstration of platelet-derived microvesicles in blood from patients with activated coagulation and fibrinolysis using a filtration technique and Western blotting. Thromb Haemost. 1994;72(5):666–671. doi:10.1055/s-0038-1648939
28. Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999;163(8):4564–4573.
29. Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi:10.1016/j.tcb.2008.11.003
30. Gyorgy B, Modos K, Pallinger E, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117(4):e39–48.
31. Gugliandolo A, Fonticoli L, Trubiani O, et al. Oral bone tissue regeneration: mesenchymal stem cells, secretome, and biomaterials. Int J Mol Sci. 2021;22:10.
32. Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961.
33. Holliday LS, Patel SS, Rody WJ
34. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420.
35. Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125.
36. Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51.
37. Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15.
38. Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608.
39. Morelli AE, Larregina AT, Shufesky WJ, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–3266.
40. Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL. Late endosomes derive from early endosomes by maturation. Cell. 1991;65(3):417–427.
41. Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44(1):11–15.
42. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1–11.
43. Laulagnier K, Grand D, Dujardin A, et al. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004;572(1–3):11–14.
44. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208.
45. Luzio JP, Gray SR, Bright NA. Endosome-lysosome fusion. Biochem Soc Trans. 2010;38(6):1413–1416.
46. Juan T, Furthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018;74:66–77.
47. Muralidharan-Chari V, Clancy J, Plou C, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875–1885.
48. Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology. 2005;20:22–27.
49. Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10):1603–1611.
50. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257.
51. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516.
52. Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9(3):231–241.
53. Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3(4):339–345.
54. Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3(4):346–352.
55. Gonda DD, Akers JC, Kim R, et al. Neuro-oncologic applications of exosomes, microvesicles, and other nano-sized extracellular particles. Neurosurgery. 2013;72(4):501–510.
56. Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104(9):2761–2766.
57. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659.
58. Iraci N, Leonardi T, Gessler F, Vega B, Pluchino S. Focus on extracellular vesicles: physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci. 2016;17(2):171.
59. Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015;9(3–4):358–367.
60. Junker K, Heinzelmann J, Beckham C, Ochiya T, Jenster G. Extracellular vesicles and their role in urologic malignancies. Eur Urol. 2016;70(2):323–331.
61. Murali VP, Holmes CA. Mesenchymal stromal cell-derived extracellular vesicles for bone regeneration therapy. Bone Rep. 2021;14:101093.
62. Simeone P, Celia C, Bologna G, et al. Diameters and fluorescence calibration for extracellular vesicle analyses by flow cytometry. Int J Mol Sci. 2020;21:21.
63. Stoner SA, Duggan E, Condello D, et al. High sensitivity flow cytometry of membrane vesicles. Cytometry A. 2016;89(2):196–206.
64. Silvestro S, Chiricosta L, Gugliandolo A, et al. Extracellular vesicles derived from human gingival mesenchymal stem cells: a transcriptomic analysis. Genes. 2020;11:2.
65. Rilla K, Mustonen AM, Arasu UT, Harkonen K, Matilainen J, Nieminen P. Extracellular vesicles are integral and functional components of the extracellular matrix. Matrix Biol. 2019;75-76:201–219.
66. Muraca M, Cappariello A. The role of Extracellular Vesicles (EVs) in the epigenetic regulation of bone metabolism and osteoporosis. Int J Mol Sci. 2020;21:22.
67. Murphy C, Withrow J, Hunter M, et al. Emerging role of extracellular vesicles in musculoskeletal diseases. Mol Aspects Med. 2018;60:123–128.
68. Liu M, Sun Y, Zhang Q. Emerging role of extracellular vesicles in bone remodeling. J Dent Res. 2018;97(8):859–868.
69. Liu J, Li D, Wu X, Dang L, Lu A, Zhang G. Bone-derived exosomes. Curr Opin Pharmacol. 2017;34:64–69.
70. Patil KC, Soekmadji C. Extracellular vesicle-mediated bone remodeling and bone metastasis: implications in prostate cancer. Subcell Biochem. 2021;97:297–361.
71. Webster DJ, Schneider P, Dallas SL, Muller R. Studying osteocytes within their environment. Bone. 2013;54(2):285–295.
72. Paic F, Igwe JC, Nori R, et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45(4):682–692.
73. Morrell AE, Brown GN, Robinson ST, et al. Mechanically induced Ca(2+) oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Res. 2018;6:6.
74. Sato M, Suzuki T, Kawano M, Tamura M. Circulating osteocyte-derived exosomes contain miRNAs which are enriched in exosomes from MLO-Y4 cells. Biomed Rep. 2017;6(2):223–231.
75. Qin W, Dallas SL. Exosomes and extracellular RNA in muscle and bone aging and crosstalk. Curr Osteoporos Rep. 2019;17(6):548–559.
76. Wang ZX, Luo ZW, Li FX, et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat Commun. 2022;13(1):1453.
77. Cappariello A, Loftus A, Muraca M, Maurizi A, Rucci N, Teti A. Osteoblast-derived extracellular vesicles are biological tools for the delivery of active molecules to bone. J Bone Miner Res. 2018;33(3):517–533.
78. Morhayim J, van de Peppel J, Demmers JA, et al. Proteomic signatures of extracellular vesicles secreted by nonmineralizing and mineralizing human osteoblasts and stimulation of tumor cell growth. FASEB J. 2015;29(1):274–285.
79. Yin P, Lv H, Li Y, Deng Y, Zhang L, Tang P. Exosome-mediated genetic information transfer, a missing piece of osteoblast-osteoclast communication puzzle. Front Endocrinol. 2017;8:336.
80. Ge M, Ke R, Cai T, Yang J, Mu X. Identification and proteomic analysis of osteoblast-derived exosomes. Biochem Biophys Res Commun. 2015;467(1):27–32.
81. Tao SC, Guo SC. Extracellular vesicles in bone: “dogrobbers” in the “eternal battle field”. Cell Commun Signal. 2019;17(1):6.
82. Morhayim J, van de Peppel J, Dudakovic A, Chiba H, van Wijnen AJ, Van Leeuwen JP. Molecular characterization of human osteoblast-derived extracellular vesicle mRNA using next-generation sequencing. Biochim Biophys Acta Mol Cell Res. 2017;1864(7):1133–1141.
83. Huynh N, VonMoss L, Smith D, et al. Characterization of regulatory extracellular vesicles from osteoclasts. J Dent Res. 2016;95(6):673–679.
84. Deng L, Wang Y, Peng Y, et al. Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;79:37–42.
85. Weilner S, Keider V, Winter M, et al. Vesicular Galectin-3 levels decrease with donor age and contribute to the reduced osteo-inductive potential of human plasma derived extracellular vesicles. Aging. 2016;8(1):16–33.
86. Batagov AO, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3’-untranslated regions. Biol Direct. 2013;8:12.
87. Hadjiargyrou M, Komatsu DE. The therapeutic potential of MicroRNAs as orthobiologics for skeletal fractures. J Bone Miner Res. 2019;34(5):797–809.
88. Wang N, Liu X, Tang Z, et al. Increased BMSC exosomal miR-140-3p alleviates bone degradation and promotes bone restoration by targeting Plxnb1 in diabetic rats. J Nanobiotechnology. 2022;20(1):97.
89. Cui Y, Luan J, Li H, Zhou X, Han J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590(1):185–192.
90. Zhang Y, Yu M, Dai M, et al. miR-450a-5p within rat adipose tissue exosome-like vesicles promotes adipogenic differentiation by targeting WISP2. J Cell Sci. 2017;130(6):1158–1168.
91. Martin PJ, Haren N, Ghali O, et al. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol. 2015;16:10.
92. Weilner S, Schraml E, Wieser M, et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell. 2016;15(4):744–754.
93. Qin Y, Peng Y, Zhao W, et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J Biol Chem. 2017;292(26):11021–11033.
94. Bhushan R, Grunhagen J, Becker J, Robinson PN, Ott CE, Knaus P. miR-181a promotes osteoblastic differentiation through repression of TGF-beta signaling molecules. Int J Biochem Cell Biol. 2013;45(3):696–705.
95. Uenaka M, Yamashita E, Kikuta J, et al. Osteoblast-derived vesicles induce a switch from bone-formation to bone-resorption in vivo. Nat Commun. 2022;13(1):1066.
96. Michigami T. Skeletal mineralization: mechanisms and diseases. Ann Pediatr Endocrinol Metab. 2019;24(4):213–219.
97. Xie Y, Chen Y, Zhang L, Ge W, Tang P. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J Cell Mol Med. 2017;21(5):1033–1041.
98. Sugatani T, Vacher J, Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood. 2011;117(13):3648–3657.
99. Cheng P, Chen C, He HB, et al. miR-148a regulates osteoclastogenesis by targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. J Bone Miner Res. 2013;28(5):1180–1190.
100. Cui Y, Fu S, Sun D, Xing J, Hou T, Wu X. EPC-derived exosomes promote osteoclastogenesis through LncRNA-MALAT1. J Cell Mol Med. 2019;23(6):3843–3854. doi:10.1111/jcmm.14228
101. Furuta T, Miyaki S, Ishitobi H, et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl Med. 2016;5(12):1620–1630. doi:10.5966/sctm.2015-0285
102. Takeuchi R, Katagiri W, Endo S, Kobayashi T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. PLoS One. 2019;14(11):e0225472. doi:10.1371/journal.pone.0225472
103. Martins M, Ribeiro D, Martins A, Reis RL, Neves NM. Extracellular vesicles derived from osteogenically induced human bone marrow mesenchymal stem cells can modulate lineage commitment. Stem Cell Rep. 2016;6(3):284–291. doi:10.1016/j.stemcr.2016.01.001
104. Hao ZC, Lu J, Wang SZ, Wu H, Zhang YT, Xu SG. Stem cell-derived exosomes: a promising strategy for fracture healing. Cell Prolif. 2017;50(5):5. doi:10.1111/cpr.12359
105. Xu JF, Yang GH, Pan XH, et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9(12):e114627. doi:10.1371/journal.pone.0114627
106. Xu R, Shen X, Si Y, et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell. 2018;17(4):e12794. doi:10.1111/acel.12794
107. Luo ZW, Li FX, Liu YW, et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11(43):20884–20892. doi:10.1039/C9NR02791B
108. Narayanan R, Huang CC, Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016;2016:3808674. doi:10.1155/2016/3808674
109. Sun W, Zhao C, Li Y, et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016;2(1):16015. doi:10.1038/celldisc.2016.15
110. Li D, Liu J, Guo B, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7(1):10872. doi:10.1038/ncomms10872
111. Yoshiko Y, Minamizaki T. Emerging roles of microRNAs as extracellular vesicle cargo secreted from osteoblasts. J Oral Biosci. 2020;62(3):228–234. doi:10.1016/j.job.2020.05.006
112. Al-Sowayan B, Alammari F, Alshareeda A. Preparing the bone tissue regeneration ground by exosomes: from diagnosis to therapy. Molecules. 2020;25(18):18. doi:10.3390/molecules25184205
113. Zhai M, Zhu Y, Yang M, Mao C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv Sci. 2020;7(19):2001334. doi:10.1002/advs.202001334
114. Qi X, Zhang J, Yuan H, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12(7):836–849. doi:10.7150/ijbs.14809
115. Zhang J, Liu X, Li H, et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7(1):136. doi:10.1186/s13287-016-0391-3
116. Li W, Liu Y, Zhang P, et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces. 2018;10(6):5240–5254. doi:10.1021/acsami.7b17620
117. Wei F, Li M, Crawford R, Zhou Y, Xiao Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019;86:480–492. doi:10.1016/j.actbio.2019.01.006
118. Gandolfi MG, Gardin C, Zamparini F, et al. Mineral-doped Poly(L-lactide) acid scaffolds enriched with exosomes improve osteogenic commitment of human adipose-derived mesenchymal stem cells. Nanomaterials. 2020;10(3):3. doi:10.3390/nano10030432
119. Swanson WB, Zhang Z, Xiu K, et al. Scaffolds with controlled release of pro-mineralization exosomes to promote craniofacial bone healing without cell transplantation. Acta Biomater. 2020;118:215–232. doi:10.1016/j.actbio.2020.09.052
120. Wang L, Wang J, Zhou X, et al. A new self-healing hydrogel containing hucMSC-derived exosomes promotes bone regeneration. Front Bioeng Biotechnol. 2020;8:564731. doi:10.3389/fbioe.2020.564731
121. Yang S, Zhu B, Yin P, et al. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. ACS Biomater Sci Eng. 2020;6(3):1590–1602. doi:10.1021/acsbiomaterials.9b01363
122. Fan L, Guan P, Xiao C, et al. Exosome-functionalized polyetheretherketone-based implant with immunomodulatory property for enhancing osseointegration. Bioact Mater. 2021;6(9):2754–2766. doi:10.1016/j.bioactmat.2021.02.005
123. Liu A, Lin D, Zhao H, et al. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272:120718. doi:10.1016/j.biomaterials.2021.120718
124. Huang CC, Kang M, Shirazi S, et al. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. 2021;126:199–210. doi:10.1016/j.actbio.2021.03.030
125. Fushimi S, Nohno T, Nagatsuka H, Katsuyama H. Involvement of miR-140-3p in Wnt3a and TGFbeta3 signaling pathways during osteoblast differentiation in MC3T3-E1 cells. Genes Cells. 2018;23(7):517–527. doi:10.1111/gtc.12591
126. Zhong LN, Zhang YZ, Li H, Fu HL, Lv CX, Jia XJ. Overexpressed miR-196a accelerates osteogenic differentiation in osteoporotic mice via GNAS-dependent Hedgehog signaling pathway. J Cell Biochem. 2019;120(12):19422–19431. doi:10.1002/jcb.29166
127. Sun X, Li X, Qi H, et al. MiR-21 nanocapsules promote early bone repair of osteoporotic fractures by stimulating the osteogenic differentiation of bone marrow mesenchymal stem cells. J Orthop Translat. 2020;24:76–87. doi:10.1016/j.jot.2020.04.007
128. Hassan MQ, Maeda Y, Taipaleenmaki H, et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem. 2012;287(50):42084–42092. doi:10.1074/jbc.M112.377515
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
