Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 11
How Common is Eosinophilia in Tuberculosis? Case Report
Authors Haftu H , Tadese K, Gebrehiwot T , Gebregziabher H
Received 31 December 2019
Accepted for publication 1 February 2020
Published 20 February 2020 Volume 2020:11 Pages 59—63
DOI https://doi.org/10.2147/PHMT.S244155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Hansa Haftu,1 Kibeten Tadese,2 Teklu Gebrehiwot,3 Hagos Gebregziabher4
1Mekelle University, College of Health Science, Pediatric and Child Health Department, Tigray, Ethiopia; 2Pediatric and Child Health Department, Tigray Regional Health Bureau, Tigray, Ethiopia; 3Mekelle University, College of Health Science, Clinical Pharmacist, Tigray, Ethiopia; 4Mekelle University, College of Health Science, Surgeon, Tigray, Ethiopia
Correspondence: Hansa Haftu Email [email protected]
Abstract: Though peripheral blood eosinophilia is common due to allergic diseases, drugs, parasitic infections, and malignancies, it is rarely reported due to tuberculosis (TB). The association between eosinophilia and TB is not well known. We have a case of the 9-year-old female present with abdominal pain in the right upper quadrant which is non-radiating associated with decreased appetite, weight loss, malaise and low-grade fever and vomiting of ingested of two weeks. On examination, she had severe wasting and hepatomegaly. On investigations, she had leukocytosis with 50% of eosinophilia, high ESR, multiple liver cysts (abdominal ultrasound and CT) and biopsy suggestive of TB. Finally, the patient started on anti-TB and her response was followed by clinical and laboratory parameters. After three weeks of treatment with anti-TB, she starts to gain weight, improve abdominal pain, appetite loss, and the investigation also normalized (leukocyte and the eosinophil become normalized, ESR corrected). The patient was to follow up for two years in the clinic and finally discharged. The coexistence of eosinophilia and TB in our patient is suggested because of the biopsy results in conjunction with the improvement of peripheral blood eosinophilia with anti-TB treatment. This example hopefully will encourage future investigations and researchers to look at the prevalence and a clear association of TB and peripheral eosinophilia.
Keywords: tuberculosis, eosinophilia, liver
Introduction
Tuberculosis (TB) remains one of the deadliest diseases. According to the World Health Organization (WHO) estimation, there are 8 million new cases per year.1,2 Patient manifestations mainly depend on the area where the infection occurs. Liver TB accounts for less than 1% of all TB infections. The clinical presentation of hepatic TB is usually insidious and often nonspecific symptoms, with the most frequently observed clinical finding being, high-grade fever, upper abdominal pain, weight loss, and hepatomegaly.3–5 Though the white blood cell count (WBC) may increase and it causes lymphocyte-predominant. Eosinophilia is not an uncommon finding in clinical practice, with a self-limiting in mild cases, but it is extremely rare to find in TB.5,6 Common causes of eosinophilia in children include helminthic parasite infections, allergic diseases, malignancies, and adverse drug reactions.1 Normal eosinophil count in the human blood varies from 0–350/mm3 of the blood.5 This amount accounts for about 1–3% of the differential leukocyte count.7 None of the English textbooks of medicine also describe eosinophilia in TB. Most of the reports of eosinophilia in TB describe the presence of local eosinophilia than getting peripheral eosinophilia.8 To the best of our knowledge, reported cases are so scarce, as a result we report on one of the rare sites for TB, Liver TB which had significant peripheral eosinophilia, and the treatment response.
Case Presentation
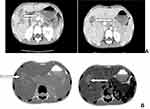
A 9-year-old a female patient from Adigudem was admitted to Mekelle hospital's pediatric ward complaining of right upper quadrant abdominal pain for the previous two weeks, with associated decrease in appetite and vomiting. She also had significant weight loss, malaise, and low-grade intermittent fever. For this, she visited a local health center where she was given unspecified oral medication for 7 days, but saw no improvement. There was no history of diarrhea, yellowish discoloration of the eyes or contact with yellowish discoloration of the eye, bleeding from any site. No history of stool or urine color change, use of herbal medication or tattooing, blood transfusion, river water contact, or trauma or surgery, There was no history of cough, shortness of breath or chest pain, contact with patients having chronic cough or a TB-diagnosed person. On physical examination the patient was conscious. The vital sign was stable and in the normal range with a maximum temperature of 37.8 c. Anthropometry findings were; Weight – 25 kg, Height – 136 cm, MUAC – 14.5 cm (moderate wasting), BMI – 13.5 kg/M2 (underweight). The other indexes were normal. The pertinent finding was in an abdominal examination: The patient had palpable liver 3 cm below the right costal margin with a total liver span of ~12 cm which was a non-tender and sharp edge. No splenomegaly or fluid collection. On Initial investigation, leukocytosis with severe Eosinophilia (WBC − 15,500 with Eos – 50%, Neut – 25%, Lym – 20%, Hematocrit − 39.13%, Platelet – 501,700. An organ function test (both liver and kidney), chest x-ray and stool exam were normal. HIV test, HBsAg and HCV results were negative, Abdominal U/S hepatomegally with abnormal echo pattern with abnormal cystic changes (Table S1). With the diagnosis of pyogenic hepatic abscesses to R/O hydatid cyst; she started on Albendazole (15mg/kg/day) and IV antibiotics (Ceftriaxone 100 mg/kg/day and metronidazole 30 mg/kg/day). Computed Tomography (CT) and it shows; multiple cystic and small daughter cysts like a communicating hypoechoic mass-like lesion (Figure 1). After a CT scan, the Hydatid cyst was considered and the patient continued with Albendazole and IV antibiotics were discontinued. After the patient taken to the operation theater (OR), the intra-operative finding was 3×3cm, 2×2cm, small 1×1cm (about 5 sites) on the anterolateral aspect of the liver. Upon entering the cysts, there was greenish pus with some calcifications. The surgeon opened the two large cavities and drained them and the smaller ones on the edge of the liver were excised for histopathology. Because of the intraoperative findings, post-op Dx of pyogenic liver abscess reconsider and she restarted the IV antibiotics (Metronidazole, Ceftriaxone, Gentamyecine) with Albendazole. After the biopsy result, which shows partly capsulated lobules of hepatocytes with numerous epithelioid granulomas, giant cells, necrosis, and microabscesses and with chronic inflammatory cells, mainly lymphocytes and eosinophils with a pathologic diagnosis of Liver TB (Figure 2). After the biopsy result, all antibiotics and Albendazole were discontinued. She started the anti-TB medication (2ERHZ/4RH) daily. The effectiveness of the treatment was assessed by clinical resolution of symptoms, serial (abdominal ultrasound, WBC with differentials, and ESR). After 1 week of anti-TB treatment, she regained her appetite and the abdominal pain decreased and she was discharged with an appointment two weeks later. After three weeks of anti-TB, all symptoms resolved (vomiting, abdominal pain, and appetite loss) with significant weight gain (Figure 3) and decrement in peripheral eosinophilia. During follow up after anti-TB medication, the serial WBC and eosinophilia decrease significantly and normalized (Figure S1). The patient was followed up with serial abdominal U/S and it showed improvement and the final U/S only showed a healed scar. The patient took anti-TB for six months and at the end of treatment, our patient was symptom-free, with significant weight gain (she gained a total of 5 kg) and all laboratory results were normal (Figure 3). The patient was also followed for adherence and any adverse drug reaction clinically (vomiting, bleeding, jaundice, rash) and serial laboratory measurements (Table S1). She took anti-TB for the first two months of direct observational therapy (DOT) in the hospital and the nearby health center. For a further 4 months, she continued visiting nearby health centers to assess for adherence and any adverse effects. She was an adherent and no adverse effects were reported or found during the investigations. Currently, the patient is on followed up with normal laboratory findings and free from clinical signs and symptoms (with normal anthropometry) for the last two years.
 |
Figure 2 Liver biopsy shows numerous epithelioid granulomas, giant cells, necrosis, and microabscesses and with chronic inflammatory cells, mainly lymphocytes and eosinophils. |
 |
Figure 3 Serial weight measurement during a hospital stay and on follow up after anti-TB treatment began. |
Discussion and Conclusion
Hepatic TB is rare, accounts for less than 1% of all TB infection. As with our patient, patients present with different symptoms which can mimic a variety of other conditions. The imaging appearances of hepatic TB are nonspecific and may appear identical to a pyogenic abscess, metastases, and primary liver tumors such as hepatocellular carcinoma. Multiple micro-nodular lesions may coalesce into a macro-nodular lesion presenting as a multi-loculated cystic mass.5 This multiloculated cystic mass may be due to different disease entities which may be difficult to differentiate clinically and through the imaging. In our patient, Ultrasound and CT scans were unable to differentiate among pyogenic, amebic abscess, hydatid cyst, and TB. Imaging alone may be insufficient in reaching a conclusive diagnosis and tissue sampling is needed in most cases.1,5 This was true in our patient, with non-specific imaging findings and the need for a biopsy for definitive diagnosis. The most important issue for our patient was the presence of eosinophilia in both the peripheral and infection site, which is extremely rare in TB, especially peripheral eosinophilia. however, In certain disease states, eosinophils can selectively accumulate in the peripheral blood or any tissue in the body.9 It has been shown that eosinophilia may participate in the inflammatory response initiated by Mycobacterium TB. In animal models, the rapid accumulation of eosinophils in infection sites of M. TB was documented. These studies showed that mycobacteria induce the attraction of eosinophils to local inflammatory sites and can phagocytize these bacilli.10,11 Though there are reports of local site eosinophilia after TB infection, it is extremely rare to find peripheral eosinophilia. In the medical literature and standard medical references, there is no clear documentation of TB as a well-known cause of eosinophilia. There have been very few case reports of TB associated with eosinophilia in the literature; and to the best of our knowledge, this has not been reported on for many years. Ray et al Suggested the possibility of early hypersensitivity reaction to the Mycobacterium antigen triggering local pulmonary eosinophilia state in susceptible patients. IL-5 is the most important cytokine providing the expansion of peripheral eosinophilia in patients with pulmonary TB.12
Gill et al reported a case of peripheral eosinophilia in abdominal TB. He diagnosed the patients in a case of abdominal TB based on histopathological examination of peritoneal tissue, which helps to confirm the diagnosis of TB as a cause of peripheral eosinophilia.13 Similarly, Flores et al reported a case of peripheral blood eosinophilia with TB. The patient presented with weakness, fatigue, weight loss, and lymphadenopathy. A biopsy of the lymph node revealed a granulomatous lesion.14 Hsu et al reported mild peripheral blood and peritoneal fluid eosinophilia in a patient undergoing peritoneal dialysis. Eosinophilia persisted despite stopping the dialysis, but it disappeared after starting anti-TB treatment.10 The image finding in our patient was non-specific and mimics other common disease entities, and a biopsy was helpful to reach a diagnosis. Besides, the clinical response shown by the patient and normalization of an eosinophilic count after starting anti-TB treatment with 2ERHZ/4RH proves the association of TB with peripheral eosinophilia. So, it is good to consider TB as a differential diagnosis in those patients with peripheral eosinophilia, especially after the most common causes are already excluded.
Consent for Publication
Written informed consent was obtained from the patient’s legal guardian (her mother) for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgment
I thank pediatric residents, seniors, surgeon and other staffs in Mekelle hospitals contributed to the hospital management of our patients. I am also thankful for the patient and her mother for their willingness for the publication and Dr. Kibeten, Dr. Hagos and Mr. Teklu for their support in writing this case report.
Availability of Data and Material
Please contact the correspondence author for data requests.
Author Contributions
All authors contributed to data analysis, drafting or revising the article gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Florence R, Peter F. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol. 2010;126(1):39–43. doi:10.1016/j.jaci.2010.04.011
2. American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi:10.1164/ajrccm.161.4.16141
3. Uma D, Vasudevan R. Abdominal tuberculosis of the gastrointestinal tract. World J Gastroenterol. 2014;20(40):14831–14840. doi:10.3748/wjg.v20.i40.14831
4. Wu Z, Wang W-L, Zhu Y, et al. Diagnosis and treatment of hepatic tuberculosis, report of five cases and review of literature. Int J Clin Exp Med. 2013;6(9):845–850.
5. Vincent R, Maria M, Mejia S, et al. The many faces of hepatic tuberculosis, cross-sectional imaging manifestations. TB Corner. 2015;1(2):1–6.
6. Gunjan G, Atul G. Persistent marked peripheral eosinophilia due to tuberculosis: a case report. Iran J Med Sci. 2017;42(1):102–105.
7. Shrestha S, Dongol S, Shrestha N, et al. Clinical and laboratory profile of children with eosinophilia. Kathmandu Uni Med J. 2012;11(2):58–61.
8. Morton G. Eosinophilia in tuberculosis. Br Med J. 1940;2:220–221.
9. Marc E. Mechanisms of disease eosinophilia. N Engl J Med Rev Art. 1998;338(22):1592. doi:10.1056/NEJM199805283382206
10. Hsin-Hui W, Ling-Yu Y, Chang J-W, Hung Y-T, Lee T-Y, Tang R-B. Eosinophilic peritonitis: an unusual manifestation of tuberculous peritonitis in a peritoneal dialysis patient. J Chin Med Assoc. 2011;74:322–324. doi:10.1016/j.jcma.2011.05.009
11. Joanna K, Zamsyari Z, Kathy M, et al. Role of eosinophils in the pathogenesis of Mycobacterium Bovis, BCG infection in gamma interferon receptor-deficient mice. Infect Immun. 2000;68(5):2976–2978. doi:10.1128/IAI.68.5.2976-2978.2000
12. Ray D, Abel R. Hypereosinophilia in association with pulmonary tuberculosis in a rural population in south India. Indian J Med Res. 1994;100:219–222.
13. Gill A. Eosinophilia in tuberculosis. Br Med J. 1940;2:220–221. doi:10.1136/bmj.2.4154.220
14. Flores M, Merino-Angulo J, Tanago J, et al. Late generalized tuberculosis, and eosinophilia. Arch Intern Med. 1983;143:182. doi:10.1001/archinte.1983.00350010194044
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.