Back to Journals » Clinical Optometry » Volume 14
How Can We Best Measure the Performance of Scleral Lenses? Current Insights
Authors Macedo-de-Araújo RJ , Fadel D , Barnett M
Received 13 January 2022
Accepted for publication 24 March 2022
Published 7 April 2022 Volume 2022:14 Pages 47—65
DOI https://doi.org/10.2147/OPTO.S284632
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Mr Simon Berry
Rute J Macedo-de-Araújo,1 Daddi Fadel,2 Melissa Barnett3
1Clinical & Experimental Optometry Research Laboratory (CEORLab), Physics Centre of Minho and Porto Universities (CF-UM-UP), University of Minho, Braga, Portugal; 2Private Practice, Rieti, Italy; 3Davis Eye Center, University of California, Sacramento, CA, USA
Correspondence: Rute J Macedo-de-Araújo, Email [email protected]
Abstract: Scleral lenses (SLs) present several unique advantageous characteristics for patients. As these lenses are mainly fitted in severely diseased eyes, a thorough evaluation of the ocular surface before and after SL fitting and the on-eye SL fitting evaluation are essential and help minimize potential physiological complications. This review will explore the current and emerging techniques and instrumentation to best measure SL performance ensuring optimal lens fitting, visual quality, comfort and physiological responses, highlighting some potential complications and follow-up recommendations. A single physician could perform the great majority of evaluations. Still, the authors consider that the assessment of SL fitting should be a collaborative and multidisciplinary job, involving contact lens practitioners, ophthalmologists and the industry. This publication has reviewed the most up-to-date work and listed the most used techniques; however, the authors encourage the development of more evidence-based recommendations for SL clinical practice.
Keywords: scleral lens, performance, instrumentation
Introduction
Scleral lenses (SLs) present several advantageous characteristics for multiple patients. The general principle of SL fitting is that the lens rests on the conjunctival surface overlying the sclera, avoiding corneal and limbal touch, and is centered on the cornea with minimal movement.1 The post-lens fluid reservoir created by a SL – along with rigid gas permeable material – provides a smoother anterior ocular surface and neutralizes corneal irregularities while simultaneously keeping the anterior ocular surface moistened. This lack of contact between the SL and the densely innervated corneal surface promotes better comfort2–7 and enhanced optical quality8,9 compared to other contact lens modalities. Thus, SLs have a wide range of indications, from optical correction of irregular corneas and ocular surface protection to refractive error correction.10–13 Until recently, the instruments used to evaluate SL fitting were quite basic – practitioners relied on the use of slit-lamp, with and without using sodium fluorescein, to evaluate lens vault and haptic zone alignment, and keratometry or Placido-based topography to map corneal curvature. However, the continuous development and improvement of SL materials, advances in manufacturing, ophthalmic instrumentations, and ocular imaging technologies have helped the pronounced resurgence of SLs into the market.14,15 Currently available instrumentations include, and are not limited to, Placido ring corneal topography, anterior segment optical coherence tomography (AS-OCT), pachymetry, scleral topography/profilometry, scheimpflug tomography, specular microscopy, and confocal microscopy. Although these devices may not be required for SL fitting, many of them can provide additional and unique information to help practitioners achieve SL fit success. This review will explore the current and emerging techniques and instrumentation to best measure SL performance ensuring optimal lens fitting, visual quality, comfort and physiological responses, highlighting some potential complications and follow-up recommendations.
Measuring Scleral Lens Performance
A thorough evaluation of the eye prior to SL fitting, the on-eye SL fitting, the ocular surface after lens wear, lens care regimen and follow-up care are essential and crucial to maintaining pristine ocular health. Assessing the on-eye SL fitting can be challenging for both new and experienced practitioners. A SL should optimally align on the conjunctiva, without provoking conjunctival compression, impingement, or edge lift. At the same time, the lens should vault the entire corneal surface and limbus without allowing any mechanical interaction with those structures. In addition, to guarantee that the fit meets all of these requirements, practitioners should also ensure good vision at all distances and physical comfort during the time of lens wear while preserving ocular physiology.
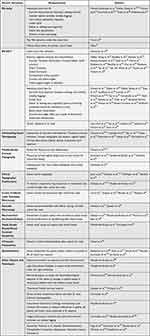
A prospective study that aimed to assess the learning curve of a novel practitioner with minor previous experience in SL fitting using a diagnostic lens set (without other advanced equipment) found that the practitioner’s experience will reduce the number of diagnostic lenses needed after the 60th fit.16 Fortunately, many devices to aid with initial SL fitting or measure on-eye SL performance have been developed and upgraded. Thus, practitioners must be up-to-date with the latest advancements in technology and be informed about current and specific techniques to measure SL performance. Table 1 highlights some of the devices and measurements that may be performed in the different phases of SL fitting, from the initial patient selection, fitting process, on-eye lens assessment, and follow-up care.
Instrumentation for Ocular Surface Evaluation and Initial Lens Selection
An accurate initial lens selection and evaluation will influence lens performance over time. Although studies have shown that SL fitting can be completed efficiently by using diagnostic trial lenses in most cases,7,16 molding/impression-based and scanning techniques17 proved to be very useful in patients with complex eye disease, unable to wear standard SL successfully.18 Although a SL lands exclusively on the conjunctiva over the sclera, some work has concluded that Placido-ring corneal topography could help with initial diagnostic lens sagittal height selection by considering corneal sagittal height measured over a specified chord length.19,20 Using an AS-OCT or a Scheimpflug tomography can provide several anterior segment measurements to aid the SL fitting. Additionally, several corneal topographers and scleral topographers include software-guided options to help SL fit.21 Harthan et al22 conducted a survey to evaluate SL fitting and assessment strategies and found some differences between new and experienced prescribers. For initial diagnostic lens selection, the authors found that new prescribers considered the base curve first (60%), while experienced prescribers considered sagittal depth first (63%). All experienced lens prescribers (100%) reported estimating tear reservoir thickness compared with the SL thickness using a slit-lamp beam at least some time. All experienced prescribers scheduled follow-up visits and assessed conjunctival compression and corneal and conjunctival staining after lens removal. The authors also found that new prescribers commonly use tomographic or topographic analysis (51%), corneal profile assessment (40%), and AS-OCT (9%) for initial lens selection. In contrast, experienced prescribers do not use corneal profile assessment (0%) but use topographic or tomographic analysis (80%) and AS-OCT (20%) for initial lens selection.
Initial evaluation of SL fitting can be done with a gross evaluation even outside the slit-lamp. Practitioners need to check for air bubbles, detect wettability issues, inspect the presence of blanching, assess central and limbal vault (initial fluorescein pattern), observe lens movement and confirm adequate settling. If the fitting is acceptable, a detailed lens fitting evaluation must proceed from an “in-out” approach, starting centrally and moving toward the periphery of the lens. More specifically, the following parameters must be assessed using a slit-lamp with white and/or cobalt blue light or AS-OCT:
- Central fluid reservoir thickness
- Mid-peripheral reservoir thickness
- Limbal reservoir thickness
- Conjunctival landing - in every quadrant
- Edge profile - in every quadrant
- Need for customization (notches, localized vaulting, etc.)
Modifications to the diagnostic lens can be made based on the findings and lens design. Although it could vary widely based on lens design, practitioners should remember that if one parameter is modified, it could impact the others. Consulting with the manufacturer (or using their online tools when available) will help obtain the most favorable outcome. Various Instrumentations and techniques to assess on-eye SL fitting will be reviewed.
Assessment of Central, Mid-Peripheral, and Limbal Reservoir Thickness
Post-lens fluid reservoir thickness is one of the most important on-eye lens fitting characteristics that practitioners should measure at every visit. The amount of vault should not be excessive as it may reduce oxygen transmissibility to the cornea inducing corneal edema in patients with reduced endothelial cell density.91,92,100 The post-lens fluid reservoir will decrease gradually over the day due to the pliable nature of the conjunctiva.45,48,101 Several authors have evaluated the amount of settling in the first eight hours of SL wear, and the mean results range from 62.8±38.4 µm to 113.7 µm45,48,52,101,102 depending on lens design and intrinsic patient attributes.101–103 Because of lens settling, a reduced post-lens fluid reservoir thickness immediately after lens application could lead to unintended corneal touch with potential mechanical insult over time.104 In this sense, some authors recommend dispensing a SL with an extra vault in cases of progressive ectatic disorders, such as keratoconus, to allow for corneal progression.105 Manufacturers tend to suggest that the ideal vault should be between 100 and 200 µm after lens stabilization; however, an optimal post-lens fluid reservoir thickness is one that ensures good vision and a comfortable wearing experience without physiological disturbances of the anterior ocular surface.14
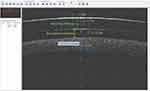
Initially, it is possible to perform a gross evaluation of the post-lens fluid reservoir (centrally, mid-peripherally and at limbal area) by adding sodium fluorescein prior to lens application and evaluating with diffuse cobalt blue light and a Wratten #12 filter to check for dark areas, which represent areas of a shallow vault or corneal touch. It should be evaluated in primary gaze position but also at all positions of gaze, always bearing in mind that scleral lenses can slightly decenter in the different gaze positions. Although using fluorescein and filters may be considered for a general overview, it may be misleading. Some areas, apparently without fluorescein, can be wrongly assumed as zones of touch, which may not be the case. This is especially true when evaluating the limbal area since the human eye can only visualize 15–20 µm.106 Apart from this general evaluation, it is crucial to quantify, even subjectively, the post-lens fluid reservoir thickness at every visit. Different instrumentations – such as optic section with a slit-lamp (either with or without fluorescein instillation)2,6,23,102 and AS-OCT23,45,47,107,108 - could be used to accurately measure the space between the anterior corneal surface or limbus and the posterior lens surface in the central corneal zone or different quadrants (Figure 1). Other authors have also described various methods to measure the post-lens fluid reservoir thickness using an optic biometer and image processing software.88
Although AS-OCT can provide the most accurate post-lens fluid reservoir measurements, not all practitioners have this technology. The most used technique is a slit-lamp. Observation with the slit-lamp should be viewed with low to medium magnification (10–16X), using an optic section with the slit beam rotated by approximately 45 degrees with white light.105 Visualizing tear reservoir thickness with low magnifications is essential to evaluate differences in the superior, central and inferior zones. As SLs tend to decenter infero-temporally,48,74,109–111 it is common to have narrower post-lens fluid reservoir thicknesses nasally and superior-nasally.108 So, it is important to scan the entire cornea (superior, inferior, nasal, and temporal) with an optic section, including the limbal area. Higher magnifications can be used to quantify tear reservoir thickness at a specific area. It is a good practice to evaluate the lens on the eye after 30 minutes to predict the final lens fit after settling, knowing that 50% to 80% of the lens settling has already occurred.45,47 To quantify this thickness, practitioners usually compare the corneal thickness or lens thickness to the distance between the anterior corneal surface to the posterior SL surface (post-lens fluid reservoir or vault), which may or may not be colored with fluorescein. As manufacturers can quickly provide the lens thickness on request, the corneal thickness is a less reliable basis for comparison in cases of corneal ectasia (variable and sometimes more thin corneal thickness). However, corneal thickness measurement could be difficult to perform, as it can only be measured with the lens on-eye with anterior OCT. Other devices such as Scheimpflug or ultrasound pachymetry require lens removal, with the later one needing anesthetic instillation to perform the measurement. Tear reservoir thickness measurement could be quite difficult for novice practitioners, especially if no fluorescein is available. Fortunately, a study has suggested that subjective measurements of the post-lens fluid reservoir thickness with slit-lamp will increasingly agree with objective methods as the practitioner gains more experience.88 Figure 2 shows a diagram and an image of an optic section using a slit-lamp, either with or without fluorescein. As the estimation of post-lens fluid reservoir thickness is relatively easier with fluorescein, sometimes novice practitioners rely on this dye to evaluate this thickness straightforwardly. While it is not a problem during initial lens selection and fitting, it could be problematic during follow-up evaluations where patients come to the clinic wearing the lenses for several hours without fluorescein. However, a SL should not be removed and refiled with fluorescein for this measurement because, after removal and reapplication, the lens will not return to its settled position.105 In fact, some pilot studies (unpublished data) demonstrated that removing and reapplying the SL filled with fresh preservative-free saline solution and fluorescein will increase the post-lens fluid reservoir thickness by 50 to 70 µm in both healthy corneas and corneas with keratoconus.112,113 Therefore, practitioners must develop the skill of visualizing and quantifying post-lens fluid reservoir thickness without using fluorescein.105
Assessment of Scleral Lens Position, Movement, Conjunctival Landing, Edge Profile, and Need for Customization
SLs should exhibit minimal movement on the eye. Various factors may cause unwanted lens movement, but generally, it is second to a thick post-lens fluid reservoir or non-optimal alignment on the conjunctiva. Lowering the fluid reservoir thickness and optimizing the alignment habitually fix this issue. Lens position/decentration should be measured in primary position of gaze horizontally and vertically using slit-lamp rulers or via topography over lenses. Due to gravity (lens thickness and weight) and scleral elevation (higher nasally, lower temporally), SL decentration occurs most of the time in the infero-temporal quadrant, inducing a thinning of the fluid reservoir in the supero-nasal quadrant.48,74,109–111 Decentration generates a misalignment of the visual and the optical axis, which can cause a prismatic effect. The unexpected prism may affect vision and disturb binocular vision, especially in patients with fragile binocularity and when a SL is worn in only one eye. If the decentration is high, or a better-centered lens is needed (wavefront-guided custom lenses), refinement of the peripheral curves and haptic zone is needed (toric or quadrant specific modifications). Sabesan et al83 measured horizontal and vertical lens decentration through images of the eye’s pupil and customized software in MATLAB for image analysis. Marsack et al84 used a custom-built Modular Ophthalmic Measurement Systems (MOMS) to assess on-eye translation and rotation of a SL. Ticak et al114 quantified on-eye rotation and translation of 3 SLs with the same method. Vincent et al described another method using AS-OCT imaging the horizontal and vertical meridians.48 However, all these techniques require the use of customized software. Thus, Vincent et al66 described a method using over-topography captured with a Placido-ring videokeratoscope and determined that SL decentration can be accurately quantified using tangential power maps with a standard scale without the need for customized instrumentation or image analysis software.66
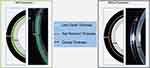
SLs should align evenly with the conjunctiva overlying the sclera. Suboptimal scleral alignment usually presents with conjunctival compression and impingement, which may occur simultaneously, or edge lift. SL alignment should be assessed in primary position of gaze but also at all positions of gaze, considering the possibility for slight decentering of the lens with eye movements. The resurgence of SLs into the market has renewed interest in the corneo-scleral junction and scleral shape. Different instruments have been used and developed to measure the anterior ocular surface shape and sagittal height (Figure 3), including Placido-based topography,19,66–68 Scheimpflug-based tomography,60–63 AS-OCT20,33,34,38–42,73 and scleral topography/ profilometry.69,70,72,73,115
Numerous publications that have analyzed scleral shape concluded that the sclera is asymmetrical in shape,70,107,116 with the nasal portion being flatter than other areas of the eye. Only 5.7% (Group 1) of 140 eyes evaluated with a scleral topographer (sMap3D) had a primarily spherical sclera.72 In the same study, 28.6% (Group 2) were primarily regularly toric, 40.7% (Group 3) had asymmetric depressions or asymmetric elevations, and 26% (Group 4) had a recognizable toric pattern with elevations and depressions that were irregularly spaced or did not have the customary 180° periodicity. When adding groups 3 and 4, the total is 65.7%; these eyes have non-regular scleral shapes different from commonly designed spherical or toric haptic designs. In this sense, scleral asymmetry assessment is a potential predictor for SL compression.74 Also, the limbal and scleral shape will influence SL design.117 There is significant asymmetry in the corneoscleral junction angles typically along the horizontal meridian, contributing to an excessive limbal vault or lens lifting off temporally. The alignment of the lens with the bulbar conjunctiva overlying the sclera can be assessed with slit-lamp and AS-OCT. Scleral topography/profilometry and corneal topography could also be used to monitor conjunctival ring impression or indentation, although this is an uncommon assessment in clinical practice.76 Like the post-lens fluid reservoir evaluation, the on-eye evaluation of peripheral lens alignment is usually done with a slit-lamp. In this case, practitioners should opt to use diffuse white light with low magnification. A SL should be evaluated in primary position of gaze and also at all positions of gaze. The alignment quality could be evaluated with clinical grading scales used in the context of previous work (good alignment: 0.00; edge lift from +0.5 to +2.0; blanching from −0.5 to −2.0).2 Practitioners should check for lens compression that is “Blanching of the conjunctival vessels that occurs because of excessive bearing/pressure of the SL peripheral curves,” impingement when “The edge of the lens focally pinches into the conjunctival tissue” or edge lift when “The edge of the lens is lifted off the ocular surface creating tear pooling, or in extreme cases, an air meniscus”.105 The landing zone should be carefully analyzed in all meridians as suboptimal SL alignment could appear in particular areas or quadrants (Figure 4).
Peripheral alignment should also be evaluated after several hours of lens wear to let the lens completely settle into the conjunctiva. When clinical signs are observed (such as conjunctival blanching, lens edge lifting off, and rebound hyperemia after lens removal), the lens parameters should be changed accordingly. Conjunctival prolapse – elevation of peripheral conjunctival tissue (Figure 4A) - could also be and indicative of peripheral misalignment. The action of compression and suction forces and the extent of limbal settling were associated with this phenomenon.1,118 Back surface toricity or customized peripheral designs can reduce several aspects such as localized blanching, conjunctival prolapse, debris, formation of air bubbles, lens flexure and decentration. Impingement may be challenging to address with a slit-lamp in cases without associated compression; it occurs because of a steep lens edge. It is easy to observe arcuate conjunctival staining related to lens impingement after lens removal. To properly evaluate edge lift with a slit-lamp, fluorescein or lissamine green could be applied to the front surface of the lens or bulbar conjunctiva. After a few blinks, practitioners can look for the dye uptake or exchange underneath the lens. This technique will allow to easily find suboptimal alignment patterns with slit-lamp since edge lift will be highlighted with the dye. AS-OCT has been previously used to quantify alterations in the thickness profile of tissues located adjacent to the haptic zones following SL wear. As described, macroscopic alterations such as an indentation ring and fluorescein staining can be observed with slit-lamp imaging. However, superficial thinning of the bulbar conjunctiva and sclera was also observed with AS-OCT imaging. Short-term changes in the bulbar conjunctiva and scleral tissue following SL wear have been identified with scleral topographers.35,75,76,119 Gimenez-Sanchis et al54 evaluated the peripheral SL fitting with AS-OCT angiography, which imaged limbal and conjunctival blood flow, and concluded that SL fitting could be optimized with the use of this technology.
Assessment of Visual Performance
When fitting SLs in primary or secondary corneal ectasias, the main goal is to achieve the best optical quality possible. The optical quality can be assessed with different objective and subjective methods such as high and low contrast visual acuity,8,87,110,120 visual contrast sensitivity function,81,87,120 residual high order aberrations,8,81,120 and visual and quality of life questionnaires.24,87 Some authors have calculated visual metrics to evaluate the optical performance of a SL.8,120 Macedo-de-Araújo et al8 used the reconstruction of the wavefront measured with and without SL to compute the point-spread function (PSF) with an image chart template to simulate the retinal image quality for different patients. In the same work, the authors evaluated the optical behavior under dim light conditions of SL wearers with corneal irregularities and healthy corneas using the Light Disturbance Analyzer (LDA) device. Hastings et al120 used visual image quality metrics such as the logarithm of the visual Strehl ratio (logVSX) to compare the optical performance of conventional SL with individualized wavefront-guided SL corrections. These publications point to a statistically significant increase in the optical quality with SL even under challenging conditions. However, many factors could degrade the visual performance during SL wear that should be assessed appropriately – residual astigmatism, lens flexure, lens rotation, midday fogging, poor wettability, and deposits on the lens surface. Therefore, practitioners need to identify these problems to improve the SL fit and reduce visual-related complaints.
Residual Astigmatism and Lens Flexure
If the lens has front surface toricity to correct residual astigmatism, the rotation of the lens should be evaluated at every visit. Manufacturers mark the lenses to identify the correct orientation, such as 6:00 or 12:00 or at the flat (or steep) axis of the lens back surface toricity. Practitioners should always evaluate the orientation of the markings by orientating the slit-lamp beam with the markings. A misalignment of the lens markings indicates lens rotation that may decrease SL optical performance. Increasing peripheral curves or incorporating customized peripheral curves will help to minimize lens rotation.
Whenever residual astigmatism is found by over-refraction, it is necessary to determine whether this reduced vision is due to true astigmatism, lens flexure, or warpage. Flexure may occur because of the haptic zone misalignment with the scleral region, reduced lens thickness, lens diameter, or modulus of the material.25,121 Therefore, solutions include switching to toric, quadrant specific, or free form peripheral curves, increasing central lens thickness, changing the lens diameter or material modulus.25 However, as central lens thickness could be linked to increased corneal edema,45,91,122 increasing the central thickness should not be done in an eye with reduced endothelial cell density. Keratometry or topography over the SL may be used to evaluate the flexure of rigid gas permeable contact lens materials. If over-keratometry over a spherical SL reveals toric or atypical readings, it indicates that the lens is flexing or warping. On the other hand, if over-keratometry readings remain spherical, it indicates that the residual astigmatism is internal, and a front-surface toric lens should be fitted. Vincent et al25 used a Placido-based videokeratoscope to examine the influence of center thickness in SL flexure and concluded that reduced SL center thickness and SL toricity were related to increased lens flexure.
Midday Fogging
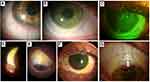
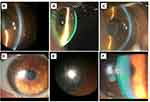
Midday fogging is a condition characterized by the accumulation of debris (lipids, proteins, pro-inflammatory molecules)123,124 in the post-lens fluid reservoir beneath the SL after a few hours of lens wear, creating a blurred image similar to a fog sensation (Figure 5A–C). Incapacitating fogging characterized by a significant reduction in visual quality is estimated to occur in approximately 20% to 33%125,126 SL wearers. The hallmark symptom of midday fogging is hazy or blurry vision, often described as looking through a “fog”.1 Fogging can be easily observed with the slit-lamp using an optic section with white light. Analysis of AS-OCT images with ImageJ software50 and Scheimpflug-based64,65 devices have been used to observe and quantify the turbidity of the fluid underneath the SL. When midday fogging occurs, patients may remove and reapply the lens filled with a fresh-saline solution to recover visual quality. However, this may represent a challenge for many patients and expose them to serious hygienic consequences deriving from removing the lenses during the day in sanitary conditions that are not always optimal.
Although it seems that midday fogging is a patient-specific phenomenon, practitioners can reduce the severity by carefully evaluating lens fitting to adjust the lens fit and manage the ocular surface disease. Reducing overall sagittal depth, flattening the curve over the limbus, or creating an asymmetric peripheral landing zone for a better alignment have been recommended to minimize midday fogging.127 Allergies and dry eye disease, particularly Meibomian gland dysfunction, should be treated if present. Some patients may benefit from an eyewash in the morning prior to SL application.127 Patients may wish to change the application solution from saline to a high-viscosity, preservative-free artificial tear to help reduce debris. A recent study investigated the benefits of using a solution that closely mimics the composition of the tears and reported significant improvements in comfort and subjective visual quality compared to other filling solutions. However, there was no significant change in visual acuity and the presence of particulate in the fluid reservoir.128 Further studies are needed to investigate the etiology of midday fogging and its management.
Assessment of the Scleral Lens Surface
Lens fogging may be due to the anterior lens surface caused by the presence of deposits and poor wettability. If deposits are present, practitioners should properly question and revise products used by the patient (hand soap, make-up, lotion) and the frequency of the problem and related activities when it occurs. The deposition of proteins, lipids, and mucin from the tear film will also have a negative impact on the overall wettability of the lens.129 Clinicians should also evaluate the lens to check for scratches or any defects and replace it when they are present.
The reflection of the light on the surface should exhibit a nice sharp reflex. If there is a wetting defect, inconsistency of the reflex will be seen (Figure 5D–F), and the best-corrected visual acuity will be impaired. Lens surface wettability refers to the ability of a liquid to spread onto a contact lens surface and is a fundamental factor in determining the compatibility between the lens material and the eye.129 Wettability is commonly determined by measuring the contact angle formed between a drop of liquid and the lens surface. A low contact angle indicates good wettability, which will lead to a more stable tear film distribution over the lens surface.130 Poor wettability causes a greasy lens surface and multiple issues such as discomfort, reduced wearing time, and blurry or hazy vision.131 Patients with blepharitis, Sjögren’s disease, ocular rosacea, or graft versus host disease are more predisposed to develop issues with lens surface wettability127 (Figure 5D–F). It is also important to diagnose and manage the presence of ocular conditions such as giant papillary conjunctivitis or Meibomian gland dysfunction. To minimize poor wettability, it is crucial to treat these conditions before or when starting the SL fitting process.
When poor wettability is present at SL delivery, the cause is likely laboratory-related issues. Substances can be transferred during the manufacturing process or by manipulation that will affect the overall quality of the lens surface.132 It is crucial to inspect the lens once received before the dispensing visit. The SL should be cleaned with an appropriate gas permeable extra strength disinfectant and cleanser and then stored in a multipurpose solution before its application on the patient’s eye. If the lens wettability was adequate upon dispensing and the patient returns with wettability issues, an investigation into the care regimen, plunger hygiene, hand soap, cosmetics, and lotions should be carefully done. Avoiding handwashing soaps containing lanolin, moisturizers, or oil and switch to products indicated for SL or acne treatment may be helpful to improve lens surface wettability. Also, applying the SL before applying make-up and facial products, removing the SL before make-up and facial products removal, and avoiding the application of cosmetics inside the eyelid margin on the waterline of the eyelid may be beneficial.
Adding a surface coating such as plasma or Tangible Hydra-Peg™ (Tangible Science LLC, Menlo Park, CA, USA) may improve lens wettability, comfort and reduce surface deposits.133 A study that evaluated polyethylene glycol surface-treated SLs in participants with dry eye determined that those SLs provided improved comfort, reduced dry eye symptoms, and decreased ocular surface compromise compared with untreated SLs.134 It is also important to choose a material with a balanced relationship between oxygen permeability (Dk) and contact angle.131 An ideal lens material has a high Dk and a low contact angle to optimize wettability and oxygen supply to the cornea.1 Thus, selecting the appropriate material, solutions and reinforcing education on lens handling and care will resolve lens wettability issues.
If patients experience poor wettability during the day, the lens may be removed, cleaned adequately, and reapplied. Also, the lens may be conditioned while on the eye using a cotton swab or removal plunger, which should be moistened with a gas permeable multipurpose solution.
Assessment of Ocular Surface Physiology/ Safety
Monitoring corneal physiology from the baseline findings is crucial for the success of SL fit, whether fitting an irregular or diseased cornea or a healthy one.123 An improperly fitted SL can disrupt the normal epithelial physiology and cause a wide range of complications, from increased swelling or redness to infiltrates or neovascularization. In this sense, practitioners should evaluate and document slit-lamp findings at baseline to compare with the findings at follow-up visits. Additional measurements such as corneal thickness, endothelial cell density, meibography, and intra-ocular pressure should also be performed whenever possible.
Anterior Segment Photography
Photography of the anterior segment can be extremely beneficial and accessible with modern technology. Anything from a built-in camera on a biomicroscope to a mobile phone held up to an ocular can be used to document the anterior segment from the baseline findings (before lens fitting) to all follow-up visits. It helps to monitor and document anterior ocular surface findings and on-eye lens fitting through time. Capturing images becomes particularly important to monitor a transient ocular surface condition such as a persistent epithelial defect,135 filamentary keratitis,136 or neurotrophic keratopathy.137,138 These conditions all have transient defects and varying severity of ocular surface features such as abrasions, neovascularization and pannus, scarring, and conjunctivalization. However, practitioners relying on this technique need to account for patient privacy.
Ocular Surface Assessment
Slit-lamp is an essential tool for overall contact lens fitting in optometric practice. Using a slit-lamp, fluorescein, and proper filters, practitioners will be able to evaluate several aspects related to conjunctival and corneal physiology.2 In the specific case of a SL fit, practitioners should assess the potential corneal epithelial staining defects commonly associated with SLs. For instance, epithelial “Bogging” is a condition that refers to the uneven spread of sodium fluorescein over the cornea after SL removal. This appears to be a post-lens fluid film phenomenon123 thought to be caused by the prolonged soaking of the cornea in saline with an absence of tear exchange and electrolytes to nourish the cornea.1 There are no known negative effects of epithelial bogging, but a practitioner should be able to detect and monitor the condition by removing the SL and staining with fluorescein at follow-up visits. Another possible condition is punctate epithelial keratopathy, which may occur when wearing SLs and is caused by toxic material trapped in the fluid reservoir.139 Removing the lens during the day, rinsing, and reapplying it may help minimize this phenomenon. Slit-lamp could also help to assess clinical findings related to hypoxic stress of the cornea and evaluate the conjunctiva and sclera after lens removal. Checking for hyperemia, conjunctival staining (conjunctival arcuate staining), or an impression ring (SL imprint into the conjunctival tissue) will help practitioners change the lens parameters accordingly and enhance ocular surface physiology.
The tear film should also be assessed with SL wearers as, similarly to other types of contact lenses,140 it will be disrupted. Serramito et al26 studied the influence of SL wear on the ocular surface and anterior SL surface wettability. The authors evaluated tear film surface quality (TFSQ) with Medmont E300 topographer, Schirmer test, and tear break-up time before and after one month of SL wear and concluded that the surface of a SL keeps its wettability after one month of lens wear, but the ocular surface wettability was reduced after SL wear.
Although it is not crucial for SL fitting, practitioners can monitor potential morphologic changes of meibomian glands with topography or retinography with specific software and other more sophisticated devices that can help image Meibomian glands under infrared lighting.141 This type of testing is becoming a standard of care when assessing and managing patients with dry eye syndrome. It may provide some clues about SL management on patients complaining about eye dryness or those developing chronic lipid deposition on the SL surface.
Hypoxic Stress of the Cornea
The theoretical studies on corneal hypoxia have shown that corneal edema may be avoided by fitting the SL with a minimum lens thickness of 250 µm and a maximum fluid reservoir thickness of 200 µm.92,100 Also, a lens with material a Dk ≥125 is advised to prevent corneal edema.142 However, many current lenses have a thickness of 300 µm or greater with a fluid reservoir thickness greater than 200 µm. Clinical evidence showed minimal clinically significant swelling with SLs.143–145 Nevertheless, corneal swelling in diseased eyes with SL was reported and attributed to hypoxia and SL-cornea interactions during lens overwear.146 The edema can appear as corneal haze, bullae, and anterior surface mottling;146 it is confirmed using pachymetry on an instrument such as an AS-OCT or Scheimpflug tomography or ultrasound pachymetry devices (corneal center).45,49,55,122,147 However, only anterior OCT allows to measure corneal thickness with the lens on eye. Scheimpflug and ultrasound pachymetry require lens removal, with the later one needing anesthetic instillation to perform the measurement. Special attention should be given to post-penetrating keratoplasty cornea as this condition is a risk factor for developing corneal edema with SL wear.136,146 Hypoxic stress may be reduced by increasing oxygen permeability of the lens material, reducing lens and post-lens fluid reservoir thicknesses, adjusting the peripheral curves to allow more tear exchange, incorporating channels and fenestrations, and limiting hours of SL wear.148 Hypoxic stress is not the only factor to consider in the equation.149 SLs used to treat ocular surface disorders provide larger benefits than the limited risk of hypoxic stress over time. Practitioners should not refrain from fitting SLs whenever the patient’s condition dictates that it is the best option available. However, regardless of the condition or lens design used, SL fitting should always limit potential negative impacts.
Specular Microscopy
It may be important to assess the endothelium avoiding risks of complications with SL wear. Specular microscopy can be done prior to SL fitting to estimate endothelial function and during the follow-up visits to monitor endothelium integrity. Some practitioners recommend using a limit of 500 cells/mm2 or 800 cells/mm2 when fitting SLs; those with less than these cut-offs are poor SL candidates. Individuals with endothelial diseases such as Fuch’s endothelial dystrophy or post corneal transplantation are at greatest risk of endothelial compromise that could affect corneal homeostasis with SL wear (ie, edema). Aging can also affect the endothelium, although more than twice the cell density needed for the normal function of the tissue remains at age 65.150 The endothelium of patients with keratoconus or other ectasias are not typically functionally affected by endothelial dysfunction unless the cornea develops hydrops.151 Ultimately, specular microscopy can be an excellent screening tool in predicting corneal edema and endothelial-related complications with SL wear, but it is not essential for SL practice and management.
Intraocular Pressure
Intraocular pressure (IOP) rise is a controversial potential complication of SL wear.152 Modern studies have reported small increases in IOP after SL removal98,99,153,154 but with the expectation that any spike would quickly return to normal after a SL is removed.155 Thus, considering that SL wear may be associated with a slight increase in IOP during lens wear in some patients, it is a good practice to measure IOP in all patients wearing SLs immediately after lens removal.98,152 Patients with glaucoma or ocular hypertension should be monitored closely, especially when first beginning SL wear, because IOP may not be controlled with a SL on the eye.156,157 Portable tonometer system (ie, Icare, Tonopen) can help accommodate the quick measurement when available. Instruments such as a pneumotonometer or Diaton tonometer may be used to measure IOP with the SL on the eye. For irregular cornea patients, IOP measurement may not be accurate using the gold-standard applanation tonometer. Other tools based on the biomechanical factors of the cornea (such as Corvis ST or Ocular Response Analyzer) may be preferable.158 Furthermore, care should be taken to educate patients about careful application and removal to reduce unnecessary force against the conjunctiva and sclera.
The variation of IOP pressure may not be evident at lens removal159 since the outflow is automatically restored. Whenever an increased IOP during SL wear is suspected, careful observation of the optic nerve, with optic nerve imaging, ocular coherence tomography, and visual fields, is mandatory and must be performed periodically. Analysis of the optic nerve with posterior segment OCT scans may be required when glaucoma is suspected or in patients already under glaucoma treatment but recently fitted with SLs.
Assessment of Lens Comfort
It is important to assess patient comfort immediately after lens application. Significant discomfort may be generated by SL that is too flat or too steep on the ocular surface, or lens touching the cornea. A new lens, with other parameters, must then be selected. A lens with excessive sagittal depth may cause a tight fitting and be uncomfortable or create a trapped air bubble under the lens, causing discomfort after 20–30 minutes of wear. Removing and reapplying the lens with an appropriate volume of fluid is mandatory. Different questionnaires have been used to assess patients’ physical comfort,2,26,94,160,161 visual comfort,8,26,160 and quality of life,86,162–166 with SL wear.
Follow-Up/Aftercare
The follow-up schedule for SL wearers varies depending on the patient and the severity of the underlying condition. Once a SL has been dispensed, the patient should build up a wearing time to allow gradual adaptation of ocular metabolism to the lens system, and the patient can return for a follow-up 1–3 weeks later. It is important to note that follow-up testing is essentially useless if there is no baseline data to compare it to; hence, baseline data collection is critical for SL management. At an initial follow-up, the patient should come in wearing their SLs for as many hours as possible (minimum of 4 hours in the authors’ opinion), so that maximum settling can be observed46,63 and complications such as midday fogging and corneal edema can be detected. SL fitting assessment (post-lens fluid reservoir thickness, mid-periphery, and lens landing), comfort, optical quality, and proper evaluation of ocular surface physiology after lens removal with the aforementioned techniques should be performed at every follow-up visit. Evaluation of the lens fit in white light prior to removal will allow assessment of the lens after settling. Visual acuity should be assessed in each eye monocularly at distance. In multifocal or monovision contact lens wear, binocular distance and near visual acuity should be evaluated. A spherical and then sphero-cylindrical over-refraction should be performed over each eye. Lens care, handling, and solutions should be reviewed at each follow-up. New wearers should be questioned about handling at each follow-up visit since SL handling is the primary reason for SL dropout.1,167
Most patients will require visits every 6 months. Individuals with more advanced and unstable conditions will require more frequent follow-ups. An example is those who wear SLs after a corneal transplant with reduced endothelial cell density. Severe ocular surface disease patients may require more frequent visits than six months to monitor the ocular surface in combination with other treatments such as meibomian gland expression, punctal occlusion, immunomodulators, or autologous serum drops in conjunction with SL wear.
Conclusions
SL wear is generally safe but may have unique physiological responses due to their distinct characteristics, demonstrating the practitioner’s need to be constantly aware. Practitioners should know how to properly assess on-eye SL fitting and assess the common physiological responses for better and fast eye care. A single practitioner could perform most evaluations; however, the authors consider that the evaluation of SL fitting (including corneal physiology) should be a collaborative and multidisciplinary job, involving the contact lens practitioners, ophthalmologists and the industry. This publication has reviewed the most up-to-date work, and the more used techniques were listed. Still, the authors encourage the development of more evidence-based recommendations for clinical practice, especially regarding IOP and corneal metabolism and hypoxia in non-healthy eyes.
Disclosure
Dr Melissa Barnett reports Speaker/Advisory Board from ABB, Acculens, Bausch + Lomb, Contamac, Gas Permeable Lens Institute, Scleral Lens Education Society, Synergeyes, Tangible Science, Global Ambassador/Speaker from BCLA, Advisory Board/Podcast Sponsor from CooperVision, Advisory Board from Mojo Vision, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Barnett M, Courey C, Fadel D, et al. BCLA CLEAR - Scleral lenses. Contact Lens Anterior Eye. 2021;44(2):270–288. doi:10.1016/j.clae.2021.02.001
2. Macedo-de-araújo RJ, Amorim-de-sousa A, van der Worp E, González-Méijome JM. Clinical findings and ocular symptoms over 1 year in a sample of scleral lens wearers. Eye Contact Lens Sci Clin Pract. 2020;46(6):e40–e55. doi:10.1097/ICL.0000000000000672
3. Yan P, Kapasi M, Conlon R, et al. Patient comfort and visual outcomes of mini-scleral contact lenses. Can J Ophthalmol. 2017;52(1):69–73. doi:10.1016/j.jcjo.2016.07.008
4. Bergmanson JPG, Walker MK, Johnson LA. Assessing scleral contact lens satisfaction in a keratoconus population. Optom Vis Sci. 2016;93(8):855–860. doi:10.1097/OPX.0000000000000882
5. Visser E-S, Visser R, van Lier HJJ, Otten HM. Modern scleral lenses part II: patient satisfaction. Eye Contact Lens. 2007;33(1):21–25. doi:10.1097/01.icl.0000228964.74647.25
6. Schornack MM, Patel SV. Scleral lenses in the management of keratoconus. Eye Contact Lens. 2010;36:39–44. doi:10.1097/ICL.0b013e3181c786a6
7. Schornack MM, Pyle J, Patel SV. Scleral lenses in the management of ocular surface disease. Ophthalmology. 2014;121(7):1398–1405. doi:10.1016/j.ophtha.2014.01.028
8. Macedo-de-araújo RJ, Faria-Ribeiro M, Mcalinden C, van der Worp E, González-Méijome JM. Optical quality and visual performance for one year in a sample of scleral lens wearers. Optom Vis Sci. 2020;97(9):775–789. doi:10.1097/OPX.0000000000001570
9. Schornack M, Nau C, Nau A, Harthan J, Fogt J, Shorter E. Visual and physiological outcomes of scleral lens wear. Contact Lens Anterior Eye. 2019;42(1):3–8. doi:10.1016/j.clae.2018.07.007
10. Visser ES, Visser R, Van Lier HJJ, Otten HM. Modern scleral lenses part I: clinical features. Eye Contact Lens. 2007;33(1):13–20. doi:10.1097/01.icl.0000233217.68379.d5
11. Pullum KW, Buckley RJ. A study of 530 patients referred for rigid gas permeable scleral contact lens assessment. Cornea. 1997;16(6):612–622. doi:10.1097/00003226-199711000-00003
12. Rosenthal P, Croteau A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005;31(3):130–134. doi:10.1097/01.ICL.0000152492.98553.8D
13. Barnett M, Ross J, Durbin-Johnson B. Preliminary clinical exploration of scleral lens performance on normal eyes. J Contact Lens Res Sci. 2018;2(2):e14–e21. doi:10.22374/jclrs.v2i2.32
14. Vincent SJ, Alonso‐caneiro D, Collins MJ. Optical coherence tomography and scleral contact lenses: clinical and Research applications. Clin Exp Optom. 2019;102(3):224–241. doi:10.1111/cxo.12814
15. Vincent SJ. The rigid lens renaissance: a surge in sclerals. Contact Lens Anterior Eye. 2018;41(2):139–143. doi:10.1016/j.clae.2018.01.003
16. Macedo-de-araújo RJ, van der Worp E, González-Méijome JM. Practitioner learning curve in fitting mini-scleral contact lenses in irregular and regular corneas using a fitting trial. Biomed Res Int. 2019;2019(Article ID 5737124):11. doi:10.1155/2019/5737124
17. Nguyen MTB, Thakrar V, Chan CC. EyePrintPRO therapeutic scleral contact lens: indications and outcomes. Can J Ophthalmol. 2018;53(1):66–70. doi:10.1016/j.jcjo.2017.07.026
18. Nau A, Shorter ES, Harthan JS, Fogt JS, Nau CB, Schornack M. Multicenter review of impression-based scleral devices. Contact Lens Anterior Eye. 2021;44(5):101380. doi:10.1016/j.clae.2020.10.010
19. Macedo-de-araújo RJ, Amorim-de-sousa A, Queirós A, van der Worp E, González-Méijome JM. Relationship of placido corneal topography data with scleral lens fitting parameters. Contact Lens Anterior Eye. 2019;42:20–27. doi:10.1016/j.clae.2018.07.005
20. Kojima R, Caroline PJ, Graff T, Copilevitz L, Achng-Coan R, van der Worp E. Eye shape and scleral lenses. Contact Lens Spectr. 2013;28:38–43.
21. Jedlicka J. Initial lens selection. In: Contemporary Scleral Lenses: Theory and Application. Sharjah, UAE: Bentham Science Publishers; 2017:183–200.
22. Harthan J, Shorter E, Nau C, et al. Scleral lens fitting and assessment strategies. Contact Lens Anterior Eye. 2019;42(1):9–14. doi:10.1016/j.clae.2018.10.020
23. Yeung D, Sorbara L. Scleral lens clearance assessment with biomicroscopy and anterior segment optical coherence tomography. Optom Vis Sci. 2018;95(1):13–20. doi:10.1097/OPX.0000000000001164
24. Yeung D, Murphy PJ, Sorbara L. Objective and subjective evaluation of clinical performance of scleral lens with varying limbal clearance in keratoconus. Optom Vis Sci. 2020;97(9):703–710. doi:10.1097/OPX.0000000000001561
25. Vincent SJ, Kowalski LP, Alonso-Caneiro D, Kricancic H, Collins MJ. The influence of centre thickness on miniscleral lens flexure. Cont Lens Anterior Eye. 2018;41:S74–S75. doi:10.1016/j.clae.2018.07.003
26. Serramito M, Privado-Aroco A, Batres L, Carracedo GG. Corneal surface wettability and tear film stability before and after scleral lens wear. Contact Lens Anterior Eye. 2019;42(5):520–525. doi:10.1016/j.clae.2019.04.001
27. Serramito M, Carpena-Torres C, Carballo J, Piñero D, Lipson M, Carracedo G. Posterior cornea and thickness changes after scleral lens wear in keratoconus patients. Contact Lens Anterior Eye. 2019;42(1):85–91. doi:10.1016/j.clae.2018.04.200
28. Fuller DG, Chan N, Smith B. Neophyte Skill Judging Corneoscleral Lens Clearance. Optom Vis Sci. 2016;93(3):300–304. doi:10.1097/OPX.0000000000000800
29. Skidmore K V., Walker MK, Marsack JD, Bergmanson JPG, Miller WL. A measure of tear inflow in habitual scleral lens wearers with and without midday fogging. Contact Lens Anterior Eye. 2019;42(1):36–42. doi:10.1016/j.clae.2018.10.009
30. Tse V, Tan B, Kim YH, Zhou Y, Lin MC. Tear dynamics under scleral lenses. Contact Lens Anterior Eye. 2019;42(1):43–48. doi:10.1016/j.clae.2018.11.016
31. Meier D. Das cornea-scleral-profil – ein kriterium individueller kontactlinsenanpassung. Die Kontaktlinse. 1992;10:4–10.
32. Gemoules G. A novel method of fitting scleral lenses using high resolution optical coherence tomography. Eye Contact Lens. 2008. doi:10.1097/ICL.0b013e318166394d
33. Bandlitz S, Bäumer J, Conrad U, Wolffsohn J. Scleral topography analysed by optical coherence tomography. Contact Lens Anterior Eye. 2017;40(4):242–247. doi:10.1016/j.clae.2017.04.006
34. Ebneter A, Häner NU, Zinkernagel MS. Metrics of the normal anterior sclera: imaging with optical coherence tomography. Graefe’s Arch Clin Exp Ophthalmol. 2015;253(9):1575–1580. doi:10.1007/s00417-015-3072-5
35. Alonso-Caneiro D, Vincent SJ, Collins MJ. Morphological changes in the conjunctiva, episclera and sclera following short-term miniscleral contact lens wear in rigid lens neophytes. Contact Lens Anterior Eye. 2016;39(1):53–61. doi:10.1016/j.clae.2015.06.008
36. Read SA, Alonso-Caneiro D, Vincent SJ, et al. Anterior eye tissue morphology: Scleral and conjunctival thickness in children and young adults. Sci Rep. 2016;6(1):33796. doi:10.1038/srep33796
37. Hall LA, Hunt C, Young G, Wolffsohn J. Factors Affecting Corneoscleral Topography. Investig Opthalmology Vis Sci. 2013;54(5):3691. doi:10.1167/iovs.13-11657
38. Kasahara M, Shoji N, Morita T, Shimizu K. Comparative optical coherence tomography study of differences in scleral shape between the superonasal and superotemporal quadrants. Jpn J Ophthalmol. 2014;58(5):396–401. doi:10.1007/s10384-014-0329-1
39. Choi HJ, Lee S-M, Lee JY, Lee SY, Kim MK, Wee WR. Measurement of anterior scleral curvature using anterior segment OCT. Optom Vis Sci. 2014;91(7):793–802. doi:10.1097/OPX.0000000000000298
40. Ritzmann M, Caroline PJ, Börret R, Korszen E. An analysis of anterior scleral shape and its role in the design and fitting of scleral contact lenses. Contact Lens Anterior Eye. 2017;41(2):205–213. doi:10.1016/j.clae.2017.10.010
41. Tan B, Graham AD, Tsechpenakis G, Lin MC. A novel analytical method using OCT to describe the corneoscleral junction. Optom Vis Sci. 2014;91(6):650–657. doi:10.1097/OPX.0000000000000267
42. van der Worp E, Graft T, Caroline P. Exploring beyond the corneal borders. Contact Lens Spectr. 2010;4:28–32.
43. Vincent SJ, Alonso-Caneiro D, Kricancic H, Collins MJ. Scleral contact lens thickness profiles: The relationship between average and centre lens thickness. Contact Lens Anterior Eye. 2019;42(1):55–62. doi:10.1016/j.clae.2018.03.002
44. Valdes G, Romaguera M, Serramito M, Cerviño A, Carracedo G. OCT Applications in contact lens fitting. Contact Lens Anterior Eye.
45. Esen F, Toker E. Influence of apical clearance on mini-scleral lens settling, clinical performance, and corneal thickness changes. Eye Contact Lens. 2017;43:230–235. doi:10.1097/ICL.0000000000000266
46. Kauffman MJ, Gilmartin CA, Bennett ES, Bassi CJ. A Comparison of the short-term settling of three scleral lens designs. Optom Vis Sci. 2014;91(12):1462–1466. doi:10.1097/OPX.0000000000000409
47. Courey C, Michaud L. Variation of clearance considering viscosity of the solution used in the reservoir and following scleral lens wear over time. Cont Lens Anterior Eye. 2017;40(4):260–266. doi:10.1016/j.clae.2017.03.003
48. Vincent SJ, Alonso-Caneiro D, Collins MJ. The temporal dynamics of miniscleral contact lenses: central corneal clearance and centration. Cont Lens Anterior Eye. 2018;41(2):162–168. doi:10.1016/j.clae.2017.07.002
49. Tan B, Zhou Y, Yuen TL, Lin K, Michaud L, Lin MC. Effects of scleral-lens tear clearance on corneal edema and post-lens tear dynamics: a Pilot Study. Optom Vis Sci. 2018;95(6):481–490. doi:10.1097/OPX.0000000000001220
50. Carracedo G, Serramito-Blanco M, Martin-Gil A, Wang Z, Rodriguez-Pomar C, Pintor J. Post-lens tear turbidity and visual quality after scleral lens wear. Clin Exp Optom. 2017;100(6):577–582. doi:10.1111/cxo.12512
51. Carracedo G, Pastrana C, Serramito M, Rodriguez‐Pomar C. Evaluation of tear meniscus by optical coherence tomography after different sodium hyaluronate eyedrops instillation. Acta Ophthalmol. 2019;97(2). doi:10.1111/aos.13887
52. Rathi VM, Mandathara PS, Dumpati S, Sangwan VS. Change in vault during scleral lens trials assessed with anterior segment optical coherence tomography. Contact Lens Anterior Eye. 2017;40(3):157–161. doi:10.1016/j.clae.2017.03.008
53. Sonsino J, Mathe DS. Central vault in dry eye patients successfully wearing scleral lens. Optom Vis Sci. 2013;90(9):
54. Gimenez-Sanchis I, Palacios-Carmen B, García-Garrigós A, Cantó-Vañó J, Pérez-Ortega AJ, Piñero DP. Anterior segment optical coherence tomography angiography to evaluate the peripheral fitting of scleral contact lenses. Clin Optom. 2018;10:103–108. doi:10.2147/OPTO.S164454
55. Kim YH, Tan B, Lin MC, Radke CJ. Central corneal edema with scleral-lens wear. Curr Eye Res. 2018;43(11):1305–1315. doi:10.1080/02713683.2018.1500610
56. Haque S, Simpson T, Jones L. Corneal and epithelial thickness in keratoconus: a comparison of ultrasonic pachymetry, orbscan II, and optical coherence tomography. J Refract Surg. 2006;22(5):486–493. doi:10.3928/1081-597X-20060501-11
57. Isozaki VL, Chiu GB. Transient corneal epithelial bullae associated with large diameter scleral lens wear: a case series. Contact Lens Anterior Eye. 2018;41(5):463–468. doi:10.1016/j.clae.2018.05.002
58. Vincent SJ, Alonso-Caneiro D, Collins MJ. The time course and nature of corneal oedema during sealed miniscleral contact lens wear. Contact Lens Anterior Eye. 2018;55:6421–6429. doi:10.1016/j.clae.2018.03.001
59. Jesus J, Dias L, Almeida I, Costa T, Chibante-Pedro J. Analysis of conjunctival vascular density in scleral contact lens wearers using optical coherence tomography angiography. Contact Lens Anterior Eye. 2021:101403. doi:10.1016/j.clae.2020.12.066
60. Vincent SJ, Alonso-Caneiro D, Collins MJ. Corneal changes following short-term miniscleral contact lens wear. Cont Lens Anterior Eye. 2014;37(6):461–468. doi:10.1016/j.clae.2014.08.002
61. Vincent SJ, Alonso-Caneiro D, Collins MJ. Miniscleral lens wear influences corneal curvature and optics. Ophthalmic Physiol Opt. 2016;36(2):100–111. doi:10.1111/opo.12270
62. Consejo A, Alonso‐Caneiro D, Wojtkowski M, Vincent SJ. Corneal tissue properties following scleral lens wear using Scheimpflug imaging. Ophthalmic Physiol Opt. 2020;40(5):595–606. doi:10.1111/opo.12710
63. Nau CB, Schornack MM. Region-specific changes in postlens fluid reservoir depth beneath small-diameter scleral lenses over 2 hours. Eye Contact Lens Sci Clin Pract. 2018;44(1):S210–S215. doi:10.1097/ICL.0000000000000382
64. Akkaya Turhan S, Dizdar Yigit D, Toker E. Impact of changes in the optical density of postlens fluid on the clinical performance of miniscleral lenses. Eye Contact Lens Sci Clin Pract. 2020;46(6):353–358. doi:10.1097/ICL.0000000000000674
65. Schornack MM, Nau CB. Changes in optical density of postlens fluid reservoir during 2 hours of scleral lens wear. Eye Contact Lens. 2018;44(Suppl 2):S344–S349. doi:10.1097/ICL.0000000000000500
66. Vincent SJ, Collins MJ. A topographical method to quantify scleral contact lens decentration. Contact Lens Anterior Eye. 2019;42(4):462–466. doi:10.1016/j.clae.2019.04.005
67. Schornack MM, Patel SV. Relationship between corneal topographic indices and scleral lens base curve. Eye Contact Lens. 2010;36(6):330–333. doi:10.1097/ICL.0b013e3181eb8418
68. Caroline P, André MP. Contact lens case reports: calculating scleral lens sag. Contact Lens Spectr. 2014;29:56.
69. Jesus DA, Kedzia R, Iskander DR. Precise measurement of scleral radius using anterior eye profilometry. Cont Lens Anterior Eye. 2017;40(1):47–52. doi:10.1016/j.clae.2016.11.003
70. Consejo A, Rozema JJ. Scleral shape and its correlations with corneal astigmatism. Cornea. 2018;37(8):1047–1052. doi:10.1097/ICO.0000000000001565
71. Consejo A, Llorens-Quintana C, Radhakrishnan H, Iskander DR. Mean shape of the human limbus. J Cataract Refract Surg. 2017;43(5):667–672. doi:10.1016/j.jcrs.2017.02.027
72. Denaeyer G, Sanders DR, Van Der Worp E, Jedlicka J, Michaud L, Morrison S. Qualitative assessment of scleral shape patterns using a new wide field ocular surface elevation topographer: the SSSG Study. J Contact Lens Res Sci. 2017;1(Group4):12–22. doi:10.22374/jclrs.v1i1.11
73. Bandlitz S, Esper P, Stein M, Dautzenberg T, Wolffsohn JS. Corneoscleral topography measured with fourier-based profilometry and scheimpflug imaging. Optom Vis Sci. 2020;97(9):766–774. doi:10.1097/OPX.0000000000001572
74. Consejo A, Behaegel J, Van Hoey M, Iskander DR, Rozema JJ. Scleral asymmetry as a potential predictor for scleral lens compression. Ophthalmic Physiol Opt. 2018;38(6):609–616. doi:10.1111/opo.12587
75. Consejo A, Behaegel J, Van Hoey M, Wolffsohn JS, Rozema JJ, Iskander DR. Anterior eye surface changes following miniscleral contact lens wear. Contact Lens Anterior Eye. 2018. doi:10.1016/j.clae.2018.06.005
76. Macedo-de-araújo RJ, van der Worp E, González-Méijome JM. In vivo assessment of the anterior scleral contour assisted by automatic profilometry and changes in conjunctival shape after miniscleral contact lens fitting. J Optom. 2018;12:131–140. doi:10.1016/j.optom.2018.10.002
77. Tse V, Zhou Y, Truong T, Lin K, Tan B, Lin MC. Corneal health during three months of scleral lens wear. Optom Vis Sci. 2020;97(9):676–682. doi:10.1097/OPX.0000000000001566
78. Alipour F, Soleimanzade M, Latifi G, Aghaie SH, Kasiri M, Dehghani S. Effects of soft toric, rigid gas-permeable, and mini-scleral lenses on corneal microstructure using confocal microscopy. Eye Contact Lens Sci Clin Pract. 2020;46(2):74–81. doi:10.1097/ICL.0000000000000612
79. Bonnet C, Lee A, Shibayama VP, Tseng C-H, Deng SX. Clinical outcomes and complications of fluid-filled scleral lens devices for the management of limbal stem cell deficiency. Contact Lens Anterior Eye. 2021:101528. doi:10.1016/j.clae.2021.101528
80. Wang Y, Kornberg DL, St Clair RM, et al. Corneal nerve structure and function after long-term wear of fluid-filled scleral lens. Cornea. 2015;34(4):427–432. doi:10.1097/ICO.0000000000000381
81. Montalt JC, Porcar E, España-Gregori E, Peris-Martínez C. Visual quality with corneo-scleral contact lenses for keratoconus management. Contact Lens Anterior Eye. 2018;41(4):351–356. doi:10.1016/j.clae.2018.01.002
82. Giasson CJ, Rancourt J, Robillard J, Melillo M, Michaud L. Corneal endothelial blebs induced in scleral lens wearers. Optom Vis Sci. 2019;96(11):810–817. doi:10.1097/OPX.0000000000001438
83. Sabesan R, Johns L, Tomashevskaya O, Jacobs DS, Rosenthal P, Yoon G. Wavefront-guided scleral lens prosthetic device for keratoconus. Optom Vis Sci. 2013;90(4):314–323. doi:10.1097/OPX.0b013e318288d19c
84. Marsack JD, Ravikumar A, Nguyen C, et al. Wavefront-guided scleral lens correction in keratoconus. Optom Vis Sci. 2014;91(10):1221–1230. doi:10.1097/OPX.0000000000000275
85. Rijal S, Hastings GD, Nguyen LC, Kauffman MJ, Applegate RA, Marsack JD. The Impact of misaligned wavefront-guided correction in a scleral lens for the highly aberrated eye. Optom Vis Sci. 2020;97(9):732–740. doi:10.1097/OPX.0000000000001577
86. Bhattacharya P, Mahadevan R. Quality of life and handling experience with the PROSE device: an Indian scenario. Clin Exp Optom. 2017;100(6):710–717. doi:10.1111/cxo.12519
87. Ozek D, Kemer OE, Altiaylik P. Visual performance of scleral lenses and their impact on quality of life in patients with irregular corneas. Arq Bras Oftalmol. 2018;81(6). doi:10.5935/0004-2749.20180089
88. Macedo-de-araújo RJ, Amorim-de-sousa A, Queirós A, van der Worp E, González-Méijome JM. Determination of central corneal clearance in scleral lenses with an optical biometer and agreement with subjective evaluation. Contact Lens Anterior Eye. 2018;42(1):28–35. doi:10.1016/j.clae.2018.11.013
89. Moschos MM, Chatziralli IP, Koutsandrea C, Siasou G, Droutsas D. Assessment of the macula in keratoconus: an optical coherence tomography and multifocal electroretinography study. Ophthalmologica. 2013;229(4):203–207. doi:10.1159/000350801
90. Amorim-de-Sousa A, Macedo-de-Araújo R, Fernandes P, Queirós A, González-Méijome JM. Multifocal electroretinogram in keratoconus patients without and with scleral lenses. Curr Eye Res. 2021;46(11):1732–1741. doi:10.1080/02713683.2021.1912781
91. Compañ V, Oliveira C, Aguilella-Arzo M, Molla S, Peixoto-de-matos SC, González-Méijome JM. Oxygen diffusion and edema with modern scleral rigid gas permeable contact lenses. Investig Ophthalmol Vis Sci. 2014;55(10):6421–6429. doi:10.1167/iovs.14-14038
92. Michaud L, van der Worp E, Brazeau D, Warde R, Giasson CJ. Predicting estimates of oxygen transmissibility for scleral lenses. Contact Lens Anterior Eye. 2012;35(6):266–271. doi:10.1016/j.clae.2012.07.004
93. Carracedo G, Wang Z, Serramito-Blanco M, Martin-Gil A, Carballo-Alvarez J, Pintor J. Ocular surface temperature during scleral lens wearing in patients with keratoconus. Eye Contact Lens Sci Clin Pract. 2016:1. doi:10.1097/ICL.0000000000000273
94. Macedo-de-araújo RJ, Serramito-Blanco M, van der Worp E, Carracedo G, González-Méijome JM. Differences between inferior and superior bulbar conjunctiva goblet cells in scleral lens wearers: a Pilot Study. Optom Vis Sci. 2020;97(9):726–731. doi:10.1097/OPX.0000000000001575
95. Fogt JS, Nau CB, Schornack M, Shorter E, Nau A, Harthan JS. Comparison of pneumatonometry and transpalpebral tonometry measurements of intraocular pressure during scleral lens wear. Optom Vis Sci. 2020;97(9):711–719. doi:10.1097/OPX.0000000000001574
96. Formisano M, Franzone F, Alisi L, Pistella S, Spadea L. Effects of scleral contact lenses for keratoconus management on visual quality and intraocular pressure. Ther Clin Risk Manag. 2021;17:79–85. doi:10.2147/TCRM.S293425
97. Obinwanne CJ, Echendu DC, Agbonlahor O, Dike S. Changes in scleral tonometry and anterior chamber angle after short-term scleral lens wear. Optom Vis Sci. 2020;97(9):720–725. doi:10.1097/OPX.0000000000001568
98. Michaud L, Samaha D, Giasson CJ. Intra-ocular pressure variation associated with the wear of scleral lenses of different diameters. Contact Lens Anterior Eye. 2019;42(1):104–110. doi:10.1016/j.clae.2018.07.004
99. Aitsebaomo AP, Wong-Powell J, Miller W, Amir F. Influence of scleral lens on intraocular pressure. J Contact Lens Res Sci. 2019;3(1):e1–e9. doi:10.22374/jclrs.v3i1.34
100. Jaynes JM, Edrington TB, Weissman BA. Predicting scleral GP lens entrapped tear layer oxygen tensions. Contact Lens Anterior Eye. 2015;38(1):44–47. doi:10.1016/j.clae.2014.09.008
101. Bray C, Britton S, Yeung D, Haines L, Sorbara L. Change in over-refraction after scleral lens settling on average corneas. Ophthalmic Physiol Opt. 2017;37(4):467–472. doi:10.1111/opo.12380
102. Kauffman MJ, Gilmartin CA, Bennett ES, Bassi CJ. A Comparison of the short-term settling of three scleral lens designs. Optom Vis Sci. 2014;91(12):1462–1466. doi:10.1097/OPX.0000000000000409
103. Otchere H, Jones LW, Sorbara L. Effect of time on scleral lens settling and change in corneal clearance. Optom Vis Sci. 2017;94(9):908–913. doi:10.1097/OPX.0000000000001111
104. Edrington TB, Szczotka LB, Barr JT, et al. Rigid contact lens fitting relationships in keratoconus. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Optom Vis Sci. 1999;76(10):692–699.
105. Johns LK, McMahon J, Barnett M. Scleral lens evaluation. In: Contemporary Scleral Lenses: Theory and Application. Vol. 4. Bentham Science Publishers Ltd.; 2017:201–240.
106. Guillon M, Sammons W. Contact lens design. In: Contact Lens Practice. London: Chapman & Hall; 1994:87–112.
107. Sorbara L, Maram J, Mueller K. Use of the VisanteTM OCT to measure the sagittal depth and scleral shape of keratoconus compared to normal corneae: pilot study. J Optom. 2013;6(3):141–146. doi:10.1016/j.optom.2013.02.002
108. Vincent SJ, Alonso-Caneiro D, Collins MJ. Regional variations in postlens tear layer thickness during scleral lens wear. Eye Contact Lens Sci Clin Pract. 2020;46(6):368–374. doi:10.1097/ICL.0000000000000676
109. van der Worp E. A Guide to Scleral Lens Fitting Version 2.0. Forest Grove, OR: Pacific University; 2015.
110. Visser E-S, Van der Linden BJJJ, Otten HM, Van der Lelij A, Visser R. Medical applications and outcomes of bitangential scleral lenses. Optom Vis Sci. 2013;90(10):1078–1085. doi:10.1097/OPX.0000000000000018
111. Kowalski LP, Collins MJ, Vincent SJ. Scleral lens centration: the influence of Centre thickness, scleral topography, and apical clearance. Contact Lens Anterior Eye. 2020;43(6):562–567. doi:10.1016/j.clae.2019.11.013
112. López-Alcón D. Variación del reservorio lacrimal tras quitar y volver a poner una lente escleral [Tear reservoir variation after scleral lens re-insertion].
113. Macedo-de-araújo RJ, Albert T, Amorim-de-sousa A, González-méijome JM. Espessura do reservatório lacrimal durante o uso de lentes esclerais (Tear reservoir thickness during Scleral Lens wear).
114. Ticak A, Marsack JD, Koenig DE, et al. A comparison of three methods to increase scleral contact lens on-eye stability. Eye Contact Lens Sci Clin Pract. 2015:1. doi:10.1097/ICL.0000000000000145
115. Consejo A, Llorens-Quintana C, Radhakrishnan H, Iskander DR. Mean shape of the human limbus. J Cataract Refract Surg. 2017;43(5):667–672. doi:10.1016/j.jcrs.2017.02.027
116. van der Worp E, Denaeyer G, Caroline PJ. Understanding anterior ocular surface shape. In: Barnett M, Johns LK, editors. Contemporany Scleral Lenses: Theory and Applications.
117. Fadel D. The influence of limbal and scleral shape on scleral lens design. Contact Lens Anterior Eye. 2018;41(4):321–328. doi:10.1016/j.clae.2018.02.003
118. Fisher D, Collins MJ, Vincent SJ. Conjunctival prolapse during open eye scleral lens wear. Contact Lens Anterior Eye. 2021;44(1):115–119. doi:10.1016/j.clae.2020.09.001
119. Courey C, Courey G, Michaud L. Conjunctival inlapse: nasal and temporal conjuctival shape variations associated with scleral lens wear. J Contact Lens Res Sci. 2018;2(1). doi:10.22374/jclrs.v2i1.19
120. Hastings GD, Applegate RA, Nguyen LC, Kauffman MJ, Hemmati RT, Marsack JD. Comparison of wavefront-guided and best conventional scleral lenses after habituation in eyes with corneal ectasia. Optom Vis Sci. 2019;96(4):238–247. doi:10.1097/OPX.0000000000001365
121. Sorbara L, Fonn D, MacNEILL K. Effect of rigid gas permeable lens flexure on vision. Optom Vis Sci. 1992;69(12):953–958. doi:10.1097/00006324-199212000-00008
122. Vincent SJ, Alonso-Caneiro D, Collins MJ, et al. Hypoxic corneal changes following eight hours of scleral contact lens wear. Optom Vis Sci. 2016;93(3):293–299. doi:10.1097/OPX.0000000000000803
123. Walker MK, Bergmanson JP, Miller WL, Marsack JD, Johnson LA. Complications and fitting challenges associated with scleral contact lenses: a review. Contact Lens Anterior Eye. 2016;39(2):88–96. doi:10.1016/j.clae.2015.08.003
124. Postnikoff C, Pucker A, Laurent J, Huisingh C, McGwin G, Nichols J. Identification of leukocytes associated with midday fogging in the post-lens tear film of scleral contact lens wearers. Invest Ophthalmol Vis Sci. 2019;60(1):226–233. doi:10.1167/iovs.18-24664
125. Caroline PJ. Cloudy vision with sclerals. Contact Lens Spectr. 2014;45:e23.
126. McKinney A, Miller W, Leach N, Polizzi C, Worp E, Bergmanson J. The cause of midday visual fogging in scleral gas permeable lens wearers. Child Abuse Neglect. 2013. doi:10.1016/j.chiabu.2004.11.003
127. Fadel D. Chapter 3. Scleral lens issues & complications. Their recognition, etiology, and management. In: Scleral Lens Issues & Complications. Their Recognition, Etiology, and Management. Ontario Canada: Dougmar Publishing Group; 2020:41–48.
128. Fogt JS, Karres M, Barr JT. Changes in symptoms of midday fogging with a novel scleral contact lens filling solution. Optom Vis Sci. 2020;97(9):690–696. doi:10.1097/OPX.0000000000001559
129. Tonge S, Jones L, Goodall S, Tighe B. The ex vivo wettability of soft contact lenses. Curr Eye Res. 2001;23(1):51–59. doi:10.1076/ceyr.23.1.51.5418
130. Lin MC, Svitova TF. Contact lenses wettability in vitro: effect of surface-active ingredients. Optom Vis Sci. 2010;87(6):440–447. doi:10.1097/OPX.0b013e3181dc9a1a
131. Fadel D. Chapter 6 - Scleral lens issues & complications. Their recognition, etiology, and management. In: Scleral Lens Issues & Complications. Their Recognition, Etiology, and Management. Ontario Canada: Dougmar Publishing Group; 2020:238–337.
132. Fadel D. Chapter 3 - Scleral lens issues & complications. Their recognition, etiology, and management. In: Scleral Lens Issues & Complications. Their Recognition, Etiology, and Management. Ontario Canada: Dougmar Publishing Group; 2020:94–98.
133. Harthan JS, Shorter E. Therapeutic uses of scleral contact lenses for ocular surface disease: patient selection and special considerations. Clin Optom. 2018;10:65–74. doi:10.2147/OPTO.S144357
134. Mickles CV, Harthan JS, Barnett M. Assessment of a novel lens surface treatment for scleral lens wearers with dry eye. Eye Contact Lens Sci Clin Pract. 2021;47(5):308–313. doi:10.1097/ICL.0000000000000754
135. Lim P, Ridges R, Jacobs DS, Rosenthal P. Treatment of persistent corneal epithelial defect with overnight wear of a prosthetic device for the ocular surface. Am J Ophthalmol. 2013;156(6):1095–1101. doi:10.1016/j.ajo.2013.06.006
136. Bhattacharya P, Mahadevan R. Case report: post-keratoplasty filamentary keratitis managed with scleral lens. Optom Vis Sci. 2018;95(8):682–686. doi:10.1097/OPX.0000000000001252
137. Grey F, Carley F, Biswas S, Tromans C. Scleral contact lens management of bilateral exposure and neurotrophic keratopathy. Contact Lens Anterior Eye. 2012;35(6):288–291. doi:10.1016/j.clae.2012.07.009
138. Gould HL. Treatment of neurotrophic keratitis with scleral contact lenses. Eye Ear Nose Throat Mon. 1967;46(11):1406–1414.
139. Severinsky B, Behrman S, Frucht-Pery J, Solomon A. Scleral contact lenses for visual rehabilitation after penetrating keratoplasty: long term outcomes. Contact Lens Anterior Eye. 2014;37(3):196–202. doi:10.1016/j.clae.2013.11.001
140. Wang J, Fonn D, Simpson TL, Jones L. Precorneal and pre- and postlens tear film thickness measured indirectly with optical coherence tomography. Investig Opthalmol Vis Sci. 2003;44(6):2524. doi:10.1167/iovs.02-0731
141. García-Marqués JV, García-Lázaro S, Martínez-Albert N, Cerviño A. Meibomian glands visibility assessment through a new quantitative method. Graefe’s Arch Clin Exp Ophthalmol. 2021;259(5):1323–1331. doi:10.1007/s00417-020-05034-7
142. Dhallu SK, Huarte ST, Bilkhu PS, Boychev N, Wolffsohn JS. Effect of scleral lens oxygen permeability on corneal physiology. Optom Vis Sci. 2020;97(9):669–675. doi:10.1097/OPX.0000000000001557
143. Bergmanson JPG, Ezekiel DF, Van der worp E. Scleral contact lenses and hypoxia. Contact Lens Anterior Eye. 2015;38(3):145–147. doi:10.1016/j.clae.2015.03.007
144. Fisher D, Collins MJ, Vincent SJ. Fluid reservoir thickness and corneal edema during open-eye scleral lens wear. Optom Vis Sci. 2020;97(9):683–689. doi:10.1097/OPX.0000000000001558
145. Fisher D, Collins MJ, Vincent SJ. Fluid reservoir thickness and corneal oedema during closed eye scleral lens wear. Contact Lens Anterior Eye. 2021;44(1):102–107. doi:10.1016/j.clae.2020.08.002
146. Guillon NC, Godfrey A, Hammond DS. Corneal oedema in a unilateral corneal graft patient induced by high Dk mini-scleral contact lens. Contact Lens Anterior Eye. 2018;41(5):458–462. doi:10.1016/j.clae.2018.05.004
147. Gonzalez-Meijome JM, Cervino A, Peixoto-de-matos SC, Madrid-Costa D, Jorge J, Ferrer-Blasco T. “In situ” corneal and contact lens thickness changes with high-resolution optical coherence tomography. Cornea. 2012;31(6):633–638. doi:10.1097/ICO.0b013e31823f0905
148. Michaud L, Vincent S. Scleral lens and hypoxia: a balanced approach. Contact Lens Spectr. 2019;34:38–42.
149. Schornack MM, Lin MC. Physiology of a scleral lens fit. Contact Lens Spectr. 2019;34:32–37.
150. Bergmanson JPG. Clinical ocular anatomy and physiology. Texas Eye Res Technol Cent. 2009;45:652–734.
151. Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi:10.1016/S0039-6257(97)00119-7
152. Fadel D, Kramer E. Indications and potential contraindications to scleral lens wear. Cont Lens Anterior Eye. 2018;42:92–103. doi:10.1016/j.clae.2018.10.024
153. Kramer EG, Vincent SJ. Intraocular pressure changes in neophyte scleral lens wearers: a prospective study. Contact Lens Anterior Eye. 2020;43(6):609–612. doi:10.1016/j.clae.2020.05.010
154. Cheung SY, Collins MJ, Vincent SJ. The impact of short-term fenestrated scleral lens wear on intraocular pressure. Contact Lens Anterior Eye. 2020;43(6):585–588. doi:10.1016/j.clae.2020.02.003
155. McMonnies CW, Boneham GC. Experimentally increased intraocular pressure using digital forces. Eye Contact Lens Sci Clin Pract. 2007;33(3):124–129. doi:10.1097/01.icl.0000247637.71618.26
156. Johnstone M, Martin E, Jamil A. Pulsatile flow into the aqueous veins: manifestations in normal and glaucomatous eyes. Exp Eye Res. 2011;92(5):318–327. doi:10.1016/j.exer.2011.03.011
157. Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24(5):612–637. doi:10.1016/j.preteyeres.2004.10.003
158. Mollan SP, Wolffsohn JS, Nessim M, et al. Accuracy of Goldmann, ocular response analyser, Pascal and TonoPen XL tonometry in keratoconic and normal eyes. Br J Ophthalmol. 2008;92(12):1661–1665. doi:10.1136/bjo.2007.136473
159. Shahnazi KC, Isozaki VL, Chiu GB. Effect of scleral lens wear on central corneal thickness and intraocular pressure in patients with ocular surface disease. Eye Contact Lens Sci Clin Pract. 2020;46(6):341–347. doi:10.1097/ICL.0000000000000670
160. Macedo-de-araújo RJ, McAlinden C, van der Worp E, González-Méijome JM. Improvement of vision and ocular surface symptoms with a scleral lens after microbial keratitis. Eye Contact Lens Sci Clin Pract. 2021;47(8):480–483. doi:10.1097/ICL.0000000000000794
161. Shorter E, Harthan J, Nau A, et al. Dry eye symptoms in individuals with keratoconus wearing contact lenses. Eye Contact Lens Sci Clin Pract. 2021;47(9):515–519. doi:10.1097/ICL.0000000000000802
162. Baran I, Bradley JA, Alipour F, Rosenthal P, Le H-G, Jacobs DS. PROSE treatment of corneal ectasia. Cont Lens Anterior Eye. 2012;35(5):222–227. doi:10.1016/j.clae.2012.04.003
163. Baudin F, Chemaly A, Arnould L, et al. Quality-of-life improvement after scleral lens fitting in patients with keratoconus. Eye Contact Lens Sci Clin Pract. 2021;47(9):520–525. doi:10.1097/ICL.0000000000000821
164. Kreps EO, Pesudovs K, Claerhout I, Koppen C. Mini-scleral lenses improve vision-related quality of life in keratoconus. Cornea. 2021;40(7):859–864. doi:10.1097/ICO.0000000000002518
165. El Bahloul M, Bennis A, Chraïbi F, Abdellaoui M, Benatiya I. Scleral contact lenses: visual outcomes and tolerance. A prospective study about 98 eyes. J Fr Ophtalmol. 2021;44(4):549–558. doi:10.1016/j.jfo.2020.08.016
166. Baali M, Belghmaidi S, Ahammou H, Belgadi S, Hajji I, Moutaouakil A. Évaluation de la qualité de vie des patients équipés en verres scléraux à l’aide d’une version marocaine du NEI-VFQ 25. J Fr Ophtalmol. 2018;41(3):201–205. doi:10.1016/j.jfo.2017.09.011
167. Macedo-de-araújo RJ, van der Worp E, González-Méijome JM. A one-year prospective study on scleral lens wear success. Contact Lens Anterior Eye. 2019. doi:10.1016/j.clae.2019.10.140
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.