Back to Journals » International Journal of Nanomedicine » Volume 16
Honokiol/Magnolol-Loaded Self-Assembling Lecithin-Based Mixed Polymeric Micelles (lbMPMs) for Improving Solubility to Enhance Oral Bioavailability
Authors Lin HL, Cheng WT, Chen LC, Ho HO , Lin SY, Hsieh CM
Received 6 November 2020
Accepted for publication 7 January 2021
Published 26 January 2021 Volume 2021:16 Pages 651—665
DOI https://doi.org/10.2147/IJN.S290444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Hong-Liang Lin,1 Wen-Ting Cheng,2 Ling-Chun Chen,3 Hsiu-O Ho,2 Shyr-Yi Lin,4,5 Chien-Ming Hsieh2
1School of Pharmacy, College of Pharmacy, Kaohsiung Medical University, Kaohsiung 80708, Taiwan, Republic of China; 2School of Pharmacy, College of Pharmacy, Taipei Medical University, Taipei 11031, Taiwan, Republic of China; 3Department of Biotechnology and Pharmaceutical Technology, Yuanpei University of Medical Technology, Hsinchu 30015, Taiwan, Republic of China; 4Division of Gastroenterology, Department of Internal Medicine, Wan Fang Hospital, Taipei Medical University, Taipei 11696, Taiwan, Republic of China; 5Department of General Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei 11031, Taiwan, Republic of China
Correspondence: Shyr-Yi Lin; Chien-Ming Hsieh 250 Wu-Hsing Street, Taipei 11031, Taiwan, Republic of China
Tel +886-2-27361661 ext 6112
Fax +886-2-23771942
Email [email protected]; [email protected]
Objective: This study was intended to utilize lecithin-based mixed polymeric micelles (lbMPMs) for enhancing the solubility and bioavailability of honokiol and magnolol to resolve the hindrance of their extreme hydrophobicity on the clinical applications.
Methods: Lecithin was selected to increase the volume of the core of lbMPMs, thereby providing a greater solubilization capacity. A series of amphiphilic polymers (sodium deoxycholate [NaDOC], Cremophor®, and Pluronic® series) were included with lecithin for screening and optimization.
Results: After preliminary evaluation and subsequentially optimization, two lbMPMs formulations composed of honokiol/magnolol:lecithin:NaDOC (lbMPMs[NaDOC]) and honokiol/magnolol:lecithin:PP123 (lbMPMs[PP123]) in respective ratios of 6:2:5 and 1:1:10 were optimally obtained with the mean particle sizes of 80– 150 nm, encapsulation efficacy (EEs) of > 90%, and drug loading (DL) of > 9.0%. These lbMPMs efficiently stabilized honokiol/magnolol in phosphate-buffered saline (PBS) at room temperature or 4 °C and in fetal bovine serum or PBS at 37 °C. PK study demonstrated that lbMPMs[NaDOC] showed much improvement in enhancing bioavailability than that by lbMPMs[PP123] for both honokiol and magnolol. The absolute bioavailability for honokiol and magnolol after intravenous administration of lbMPMs[NaDOC] exhibited 0.93- and 3.4-fold increases, respectively, compared to that of free honokiol and magnolol. For oral administration with lbMPMs[NaDOC], the absolute bioavailability of honokiol was 4.8%, and the absolute and relative bioavailability of magnolol were 20.1% and 2.9-fold increase, respectively.
Conclusion: Overall, honokiol/magnolol loaded in lbMPMs[NaDOC] showed an improvement of solubility with suitable physical characteristics leading to enhance honokiol and magnolol bioavailability and facilitating their wider application as therapeutic agents for treating human disorders.
Keywords: lecithin, mixed polymeric micelles, honokiol, magnolol, sodium deoxycholate, pluronic
Introduction
Honokiol (3,5′-diallyl-4,2′-dihydroxybiphenyl) and magnolol (5,5′-diallyl-2,2′-dihydroxybiphenyl) (shown in Figure 1) are the principal bioactive components of magnolia bark extract, which has long been used in traditional Chinese and Japanese medicine.1,2 Recently, they were demonstrated to have extensive and significant biological activities,3 including antioxidative,4 anti-angiogenesis,5 anti-inflammatory,6 antidepressant,7 and antitumor properties.8–10 Among these activities, the most beneficial effect of honokiol/magnolol is their suppression of the proliferation of various tumor cells. Studies reported that they exhibit anticancer properties by inhibiting proliferation, inducing differentiation and apoptosis, suppressing angiogenesis, countering metastasis, and reversing multidrug resistance.9,11–14 Although honokiol/magnolol showed antitumor activities against various tumor cells, their clinical use is limited by their insolubility, low bioavailability, and vascular irritation. Moreover, oral administration of honokiol/magnolol showed weak antitumor activity in vivo.15,16 The oral bioavailability of honokiol and magnolol in rats was reported to be only 5% due to extensive first-pass metabolism and low absorption.17 Therefore, there is a strong need to develop a novel oral delivery system to resolve the issues of poor oral bioavailability and high dosage problems.
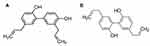 |
Figure 1 Chemical structures of (A) honokiol and (B) magnolol. |
Several novel drug delivery systems have been utilized to improve the bioavailability and anticancer effects of honokiol and magnolol. For example, a ligand-based (used folic acid as a targeted therapeutic ligand) surface modification of pH-sensitive honokiol nanoparticles has a high tumor inhibition rate.18 A stable honokiol-in-hydroxypropyl-β-cyclodextrin in liposomes was developed to release honokiol at slow, sustained speeds. Results of their pharmacokinetic (PK) study demonstrated significant retardation of elimination and prolongation of the residence time in the circulatory system.19 Furthermore, poly(ethylene glycol) (PEG)ylated honokiol liposomes as long-active liposomes exhibited significantly improved PKs in Balb/c mice and lengthened its elimination half-life (T1/2β) and the area under the plasma curve from time 0 to infinity (AUC0→∞).20 As a consequence, the antitumor efficacy of honokiol can be greatly enhanced in tumor-bearing mice. However, due to the high cost of production of PEG-distearoylphosphatidylethanolamine (DSPE) and the prevalence of anti-PEG antibodies after long-term administration of PEGylated liposomes, the use of PEG has many drawbacks that limit its application. A more cost-effective preparation and safer carrier for honokiol/magnolol is desired to further improve their oral bioavailability enabling their clinical applications.
The use of micelles as a carrier for oral drug administration to overcome the low solubility and bioavailability of hydrophobic drugs has recently gained significant attention.21,22 The small size of micelles can also facilitate achieving a favorable biodistribution.23–26 The extravasation and accumulation of therapeutic agents in tumor sites can be achieved through an enhanced permeability and retention (EPR) effect.27,28 Previously, our research group successfully developed lecithin-based mixed polymeric micelles (lbMPMs) that combined amphiphilic polymers and lecithin to successfully encapsulate hydrophobic drugs to enhance their oral bioavailability.29,30 The design rationale of lbMPMs is to form a supporting lipid layer, which possesses better stability and encapsulates more of the hydrophobic drug than traditional mixed micelles leading to enhanced therapeutic efficacy. To resolve the problem of the low bioavailability of honokiol/magnolol, herein, the preparations of honokiol/magnolol-loaded lbMPMs, composed of lecithin and three amphiphilic polymers, namely sodium deoxycholate (NaDOC), Cremophor®, and a Pluronic® series, were investigated and characterized to find an optimal formulation. Physical characterizations, including particle size, physical stability, and drug-release profile, of optimal formulations, were evaluated and in vivo pharmacokinetics studies (intravenous and oral) were conducted to examine the enhancement efficiency of intravenous and oral bioavailability. The information obtained from the present study should provide a foundation for future clinical investigations.
Materials and Methods
Materials
Honokiol and magnolol were isolated and purified by professor Wang’s lab (graduate institute of pharmacognosy, Taipei Medical University) with a purity >95%. L-α-lecithin granules were supplied by Acros (Morris Plains, NJ, USA). Sodium deoxycholate (NaDOC), Pluronic® L121 (PL121), Pluronic® F108 (PF108), and Pluronic® P123 (PP123) were purchased from Sigma (St. Louis, MO, USA). Pluronic® F87 (PF87), Pluronic® F127 (PF127), and Pluronic® F68 (PF68), and Cremophor® ELP (PEG-35 Castor Oil, CELP) and Cremophor® RH40 (PEG-40 Hydrogenated Castor Oil, CRH40) were delivered by BASF (Hanover, Germany), and heparin (5000 IU/mL) was provided by China Chemical & Pharmaceutical (Hsinchu, Taiwan). All reagents for high-performance liquid chromatography (HPLC) or ultra-performance liquid chromatography (UPLC)/tandem mass spectrometric (MS)/MS analysis were of an HPLC or MS grade, and other reagents were analytical grade.
Preliminary Screening and Optimization of Honokiol/Magnolol-Loaded Lecithin-Based Mixed Polymeric Micelles (H/M-Loaded lbMPMs)
Preliminary screening of the influence of various amphiphilic polymers with/without the addition of lecithin on the formation of the micellar core for encapsulating honokiol/magnolol was examined with respect to the particles’ size and stability in this study. Honokiol/magnolol-loaded lbMPMs (H/M-loaded lbMPMs) were prepared using a thin film method as previously described.31 Briefly, honokiol/magnolol, lecithin, and another amphiphilic polymer (NaDOC; PF87, PF127, PF68, PL121, PF108, or PP123; CRH40 or CELP) in a predetermined ratio were added to 1 mL of a mixed solvent (methanol: dichloromethane, 3:7, v/v) in a round-bottom flask. The mixture was shaken for 30 s, sonicated for 1 min, and subsequently evaporated through rotary evaporation (Buchi, Rotavapor R124, Flawil, Switzerland) under reduced pressure to remove the solvent and obtain a thin film. Self-assembly of the thin films resulting in micelle formation was induced by adding 1 mL of deionized water and gently shaking the micellar solution until the thin film had completely dispersed. Non-encapsulated honokiol/magnolol (H/M) aggregates were removed by passing the solution through a 0.22-µm filter (Millipore, Billerica, MA, USA).
After preliminarily screening, those optimal amphiphilic polymers for formation of the H/M-loaded lbMPMs were further optimized with respect to those physical characteristics, namely the average particle size (PS) and polydispersity index (PI), zeta potentials (ZP), encapsulation efficacy (EE), and drug loading (DL), to determine optimal formulations to encapsulate honokiol/magnolol with a nano range of particle size and maximal EE and DL.
Characterization of Honokiol/Magnolol-Loaded lbMPMs
The average particle size and its size distribution of the optimal H/M-loaded lbMPMs were measured at a scattering angle of 90° with a Zetasizer (Nano-ZS) (Malvern Instruments, Worcestershire, UK) at 25 °C with the intensity autocorrelation of the sample being in a range of 5×104~106. The zeta potential was also examined utilized the same instrument of Zetasizer (Nano-ZS). The surface morphology of the optimal H/M-loaded lbMPMs was observed through transmission electron microscopy (TEM; Hitachi H-600, Tokyo, Japan).
Quantification of Encapsulated Honokiol/Magnolol
Honokiol/magnolol was analyzed using an HPLC method (Pump PU-980, Jasco, Tokyo, Japan) adapted from Yang et al.32 The honokiol/magnolol concentration was determined using an XBridgeTM C18 column (5 μm, 150 × 4.6 mm). The mobile phase was a mixture of acetonitrile and 0.5% acetic acid (4:6, v/v) at a flow rate of 1.0 mL/min and 30 °C. Furthermore, the column effluent was monitored using an ultraviolet detector (UV-975, Jasco) at a wavelength of 290 nm, and the HPLC method was validated to have an acceptable coefficient of variation for accuracy and precision. On determining the honokiol/magnolol concentration from the validated calibration curve, the EE and DL were calculated according to equations (1) and (2), respectively:
Encapsulation efficiency (EE, %) = WM/WI × 100 and (1)
Drug loading (DL, %) = WM/(WP + WM) × 100;(2)
where WM is the drug weight in micelles, WI is the weight of the initial feeding drug, and WP is the weight of the initial feeding polymers.
Examination of Encapsulation Stability
The encapsulation stability of two H/M-loaded lbMPMs composed of H/Ml:lecithin:NaDOC and honokiol/magnolol:lecithin:PP123 in respective ratios of 6:2:5 (designated as lbMPMs[NaDOC]) and 1:1:10 (designated as lbMPMs[PP123]) dispersed in dd water was assessed at room temperature and 4 °C under dark conditions. Furthermore, the encapsulation stability of both lbMPMs[NaDOC] and lbMPMs[PP123] in a simulated plasma was examined by mixing an equal volume of honokiol/magnolol-loaded lbMPMs and phosphate-buffered saline (PBS, 0.01 M, pH 7.4) or fetal bovine serum (FBS) and then was co-incubated in a 37 °C water bath. The mean particle size of the H/M-loaded lbMPMs as an indicator of the encapsulation stability without precipitation was analyzed at a predefined time-point.
In vitro Release Studies
Drug release from H/M-loaded lbMPMs[NaDOC] and lbMPMs[PP123] was examined using the dialysis bag method, in which 0.01 M PBS containing 0.5% Tween 80 was used as the release medium.33 One milliliter of honokiol/magnolol-loaded lbMPMs or a free H/M solution (ie, honokiol/magnolol dissolved in dimethyl sulfoxide [DMSO]) diluted with water to yield a final concentration of 0.1 mg/mL was placed in a separate dialysis bag (MWCO 3500; Cellu-Sep® T1, Seguin, TX, USA). The dialysis bag was placed in a tube with the addition of 25 mL of a release medium and the whole tube then was placed at 37 °C shaker with a shaking rate of 100 rpm. At predetermined interval of 0.5, 1, 2, 3, 5, 7, 9, 12, 24, 48, and 72 h, the concentration of honokiol/magnolol released from the dialysis bag was analyzed using an HPLC method as described in Quantification of Encapsulated Honokiol/Magnolol. All measurements were conducted in triplicate. For comparison, honokiol/magnolol release from the free solution under the same conditions was assessed.
In vivo PK Studies
This study involved an animal experiment that was approved by the Institutional Animal Care and Use Committee of Taipei Medical University (approval no.: LAC-2013-0126) and conducted in compliance with the Animal Welfare Act in Taiwan. We used 8~10-week-old male Sprague-Dawley rats to investigate PK profiles of optimal H/M-loaded lbMPM formulations (6 mg/mL) and a free honokiol/magnolol solution (ie, honokiol/magnolol dissolved in CELP:Ethanol at a ratio of 1:1 and then diluted with sterile water to yield a final concentration of 6 mg/mL). Rats were administered a single intravenous (IV) dose of 20 mg/kg of H/M-loaded lbMPMs[NaDOC], lbMPMs[PP123], or free H/M solution (n = 3 for each group). Blood samples were collected in heparinized tubes from the jugular vein at 0.083, 0.166, 0.25, 0.5, 1, 2, 4, 7, 12, 24, 48, and 72 h after administration. In addition, rats were orally administered a single dose of 40 mg/kg of H/M-loaded lbMPMs[NaDOC], lbMPMs[PP123], or free H/M solution (n = 3 for each group). Blood samples were collected in heparinized tubes from the jugular vein at 0.083, 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, 24, 48, and 72 h after administration. All blood samples were immediately centrifuged at 3000 rpm for 15 min at 4 °C to obtain plasma, which was stored at −80 °C before the UPLC/MS/MS analysis.
The UPLC/MS/MS analysis was performed according to a method adapted from Sheng et al34 using the Waters ACQUITY UPLC and Xevo TQ MS system (Waters, Milford, MA, USA) equipped with an electrospray ionization (ESI) source. Separation was achieved using a BEH C18 column (2.1 mm ID × 50 mm, 1.7 μm; Waters). The system delivered a constant flow of 0.2 mL/min, and the mobile phase consisted of methanol and water with a gradient ratio, and an injection volume of 10 μL. During analyses, the ESI parameters were set as follows: capillary voltage of 3.6 kV in the negative mode, a desolvation temperature of 350 °C, cone gas flow of 100 L/h, and desolvation gas flow of 650 L/h.
PK parameters are presented as the mean and standard deviation (SD) from individual rats of each group and were estimated through a non-compartmental analysis. The terminal elimination rate constant (Ke) was estimated from the slope of the log-linear phase of a graph of the declining plasma concentration of honokiol/magnolol versus time. The half-life (T1/2) was calculated using the following equation: T1/2 = ln 2/Ke. Furthermore, the area under the plasma curve (AUC) from the beginning to the end point (AUC0–72) was calculated using the trapezoidal method. Summing AUC0→last and the concentration at the last measured point divided by Ke yielded AUC0→∞. Clearance (CL) was calculated by dividing the dose by AUC0→∞, and the distribution volume (V) was calculated by dividing CL by Ke.
Statistical Analysis
Data are presented as the mean ± standard deviation (SD). Student’s t-test was used to compare 2 groups, and more than 2 groups were compared using one-way ANOVA. A 2-tailed p value of <0.05 was considered significant.
Results
Preliminary Screening of Lecithin-Based Honokiol/Magnolol-Loaded lbMPMs
Preparatory procedures for hydrophobic drug-loaded amphiphilic polymeric micelles can be found in our earlier work.30,35,36 Similarly, the influence of various amphiphilic polymers on the formation of the micellar core for encapsulating honokiol/magnolol was preliminarily examined in this study. Surfactants play important roles in the precipitation of nanoparticles, in that they prevent agglomeration between particles, thus helping control the size of the nanoparticles. Various types of amphiphilic polymers with different values of the hydrophilic–lipophilic balance (HLB) and different proportions of drug and polymers play important roles in the particle size and stability of lbMPMs; thus, a range of single-factor experiments was performed to investigate different ratios of honokiol/magnolol and various polymers to prepare honokiol/magnolol-loaded lbMPM formulations. Results of preliminary screening are listed in Table 1 and Figure 2.
 |
Table 1 Preliminary Screening of Mixed Polymeric Micellar Formulations with Various Ratios of Different Amphiphiles (NaDOC, PP123, PF68, PF87, PF127, PF108, and PL121, CRH40 and CELP) and Lecithin |
 |
Figure 2 Particle sizes of honokiol/magnolol micelles formed using different amphiphiles and ratios. |
As shown in Table 1, the honokiol/magnolol formulation composed of H/M:L:NaDOC at a 1:0:5 weight ratio was unable to form micellar droplets. With an increasing amount of NaDOC of ratios of 10 to 20 (w/w), the mean size of micellar droplets increased from 170 to 636 nm. The addition of lecithin resulted in larger particle sizes, but a lower polydispersity index (PI) values. On the other hand, all of the H/M-loaded MPM formulations composed of H/M: L:Pluronic flake series at a 1:0:5 weight ratio were unable to form micellar droplets. When increasing the amount of amphiphiles at weight ratios of 10 to 20 (w/w), H/M-loaded lbMPMs utilizing Pluronic flake series containing 70% EO fragments (ie, PF87 and PF127) formed smaller micellar droplets compared to those of honokiol/magnolol-loaded lbMPMs using Pluronic® flake series containing 80% EO fragments (ie, PF68 and PF108). PF87 and PF127 micelles were more stable, but adding lecithin increased the particle size. For the Pluronic® liquid series, regardless of the ratio of PL121 and lecithin, no transparent micelle solution formed. When a lower PP123 concentration was used, micelles precipitated within 12 h even in the presence of lecithin. With an increase in the PP123 concentration, the particle size decreased and stability increased. PP123 facilitated micelle formation with a particle size of <200 nm and adding lecithin increased the size and stability (ie, no precipitation occurred at 12 h).
When using a low concentration of Cremophor as the amphiphile for H/M-loaded MPMs, micelles precipitated within 12 h even in the presence of lecithin. With an increase in the Cremophor concentration at weight ratios of 10 to 20, the particle size of micelles decreased. However, adding lecithin increased the particle size and reduced the EE.
Taken together, two amphiphilic polymers, NaDOC, and PP123, were found to be more appropriate to form H/M-loaded lbMPMs with acceptable particle size and stability. Those two amphiphilic polymers, NaDOC and PP123, were selected for further optimization.
Optimization of Honokiol/Magnolol-Loaded lbMPMs
After screening as described above, with the use of lecithin and two amphiphilic polymers, NaDOC and PP123, were selected for further optimization to obtain honokiol/magnolol-loaded lbMPMs with a particle size (PS) in nano range and maximal encapsulation efficiency (EE) and drug loading (DL) values to improve their solubility hence resulting in the enhancement of bioavailability. Physical properties of those resultant mixed polymeric micelles (NaDOC and PP123) are listed in Table 2. As shown in Table 2, increasing the added amount of lecithin in the formulation of H/M:NaDOC=1:5 from 1 to 2 parts resulted in smaller PS and increasing DL, but increasing polydispersity index (PI) values and decreasing EE. Moreover, successively increasing the added amount of H-M in the formulation of L:NaDOC=2:5 from 2 to 6 parts resulted in PS gradually increasing, PI maintaining around 0.1–0.3, ZP progressively increasing, and drug loading proportionally increasing with a fair encapsulation efficiency. Results of optimization disclosed that lbMPMs with a H/M:L:NaDOC ratio of 6:2:5 (designated as lbMPMs[NaDOC]) was an optimal formulation, with a particle size of 118.4±2.1 nm, a PI of 0.186±0.022, a ZP of −63.7±1.23 mV, an EE of 96.41%, and a DL of 44.42%.
 |
Table 2 Optimization of Honokiol/Magnolol-Loaded Lecithin-Based Mixed Micelles Formed Using NaDOC and PP123 as Amphiphilic Polymers with Lecithin at Different Ratios |
Further illustrated in Table 2, increasing the added amount of lecithin in the formulations of H/M:PP123=1:5, 1:10, and 1:20 from 0 to 1 parts caused smaller PS and similar PI values. However, only for formulations of H/M:PP123=1:10 increased the added amount of lecithin from 0 to 1 part demonstrated to increase DL as a result of increasing EE from 69.1% to 99.5%. Results of optimization revealed that lbMPMs with a H/M:L:PP123 ratio of 1:1:10 (designated as lbMPMs[PP123]) was an optimal formulation with a particle size of 89.47±1.29 nm, a PI of 0.413±0.010, a ZP of 1.28±0.10 mV, an EE of 99.51%, and a DL of 9.81%. lbMPMs[PP123] was selected as the other one of the two optimal formulations for further evaluation.
TEM
TEM revealed that particle sizes were 80–150 nm for both optimal formulations (Figure 3A and B). A spherical and homogeneous morphology of micelles was observed for lbMPMs[NaDOC] and lbMPMs[PP123]. The sizes of micelles formed by lbMPMs[NaDOC] were more even than those formed by lbMPMs[PP123], and the result was consistent with that measured by the dynamic light scattering (DLS) method, as shown by lower PI values.
 |
Figure 3 TEM micrograph of lbMPMs[NaDOC] and lbMPMs[PP123]. (A) Honokiol/magnolol: lecithin: NaDOC in a ratio of 6:2:5. (B) Honokiol/magnolol: lecithin: PP123 in a ratio of 1:1:10. |
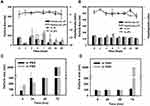
Encapsulation Stability
Honokiol/magnolol are hydrophobic polyphenol compounds and therefore are less soluble in aqueous solutions causing precipitation if they did not encapsulate in the micellar core. To evaluate whether the optimal formulations increased the encapsulation stability in micellar core, H/M-loaded lbMPMs[NaDOC] and lbMPMs[PP123] were, respectively, incubated with PBS and FBS and stored at various temperatures. The change of the mean particle size as an indicator of precipitation was determined at different time points as well as at different storage temperatures.
In PBS, the particle size of H/M-loaded lbMPMs[NaDOC] and lbMPMs[PP123] was ~80-150 nm and was stable for at least 56 days at both room temperature and at 4 °C (Figure 4A and B). When the incubation temperature was increased to 37 °C, the particle size of H/M-loaded lbMPMs[NaDOC] increased to >200 nm after 24 h of incubation and precipitated after 72 h of incubation, while no significant change occurred in the particle size of H/M-loaded lbMPMs[PP123] after 72 h of incubation.
At 37 °C in FBS, the same trend as in PBS was found for H/M-loaded lbMPMs[NaDOC], as the particle size increased to >200 nm after 24 h of incubation and then precipitated after 72 h of incubation (Figure 4C). As for H/M-loaded lbMPMs[PP123], the size did not exceed 200 nm until 72 h of incubation (Figure 4D).
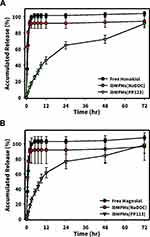
In vitro Release Studies
Figure 5 shows drug-release profiles of lbMPMs[NaDOC] and lbMPMs[PP123] (at a drug load of 0.1 mg/mL) which were slower than that of the free honokiol/magnolol solution. Free honokiol/magnolol were depleted in about 9 h, as no sign of the cumulative amount was further detected after 9 h of the eluting test. However, once the chemicals were well encapsulated in lbMPMs, slower release profiles were detected; in particular, a much-slower release rate was found when Pluronic® P123 was employed for encapsulation, ie, reaching ~62% of release for lbMPMs[NaDOC], compared to ~10% for lbMPMs[PP123] in the first 1 h. Similar release profiles were observed for honokiol and magnolol, as both were considerably reduced from lbMPMs[PP123].
In vivo PK Studies
PK profiles as shown by Figure 6 demonstrated that the plasma honokiol concentrations after IV administration of H/M-loaded lbMPMs[NaDOC] were higher than those of free honokiol/magnolol and H/M-loaded lbMPMs[PP123]. The main PK parameters of magnolol and honokiol after IV administration calculated by a non-compartmental analysis are shown in Tables 3 and 4. PK parameters demonstrated that the AUC0-72 of honokiol for lbMPMs[NaDOC] was 1.57-fold higher than that of the free honokiol/magnolol group. However, there was no significant difference in plasma honokiol concentrations between H/M-loaded lbMPMs[PP123] and free honokiol/magnolol groups. lbMPMs[NaDOC] had lower elimination rates and longer retention times of honokiol/magnolol (t1/2=4.01±0.70 h).
 |
Table 3 Summary of Pharmacokinetic Parameters for Honokiol Following Intravenous and Oral Administration of Lecithin-Based Mixed Polymeric Micelles (NaDOC and PP123) and Free Honokiol-Magnolol (n=3) |
 |
Table 4 Summary of Pharmacokinetic Parameters for Magnolol Following Intravenous and Oral Administration of Lecithin-Based Mixed Polymeric Micelles (NaDOC and PP123) and Free Honokiol-Magnolol (n=3) |
PK results in Figure 7 and Tables 3 and 4 show the plasma honokiol and magnolol concentrations after oral administration of free honokiol/magnolol, H/M-loaded lbMPMs[NaDOC] and lbMPMs[PP123], respectively. The maximal concentrations (Cmax) of H/M-loaded lbMPMs[NaDOC] and lbMPMs[PP123] were higher than that of free honokiol/magnolol (Figure 7). (Note: the plasma honokiol concentration in the free H/M group was too low to calculate these parameters.) The AUC0–72 of honokiol in the H/M-loaded lbMPMs[NaDOC] group was higher than those of free H/M and lbMPMs[PP123], while no significant difference was found for the AUC0–72 of magnolol in the H/M-loaded lbMPMs[PP123] group compared to that of the free honokiol/magnolol group.
Discussion
Honokiol and magnolol have shown multiple potential pharmacological effects in several preclinical models. In recent studies, they displayed diverse biological activities, including anti-arrhythmic, anti-inflammatory, antioxidative, anti-depressant, anti-thrombocytic, and anxiolytic activities.4,11,37,38 In the past few years, the anticancer properties of honokiol/magnolol have also been a focus, emphasizing their tremendous potential as anticancer agents. However, owing to their high lipophilicity, they cannot be dispersed in water, which makes oral and IV administration difficult and hinders their clinical use in exerting maximal therapeutic activities against various diseases. Previously, our group developed a protocol for lbMPM drug delivery systems that combines lecithin and amphiphilic polymers to successfully encapsulate hydrophobic drugs to enhance their oral bioavailabilities.30,36 Taking advantage of the unique strengths of lbMPMs, the present study attempted to optimize and characterize combinations of lecithin with amphiphilic polymers to develop suitable H/M-loaded micelles for future applications.
The particle size (PS), polydispersity index (PI), EE, and DL of lbMPMs were optimally studied for their influences on the physical stability and release of encapsulated drugs. From the results of a preliminary screening (Table 1), the particle size of the mixed micelles decreased when the drug/polymer ratio changed from 1:5 to 1:20 in the absence of lecithin. After adding lecithin to the micellar systems, an increased particle size was found, and in some cases, it further caused precipitation, particularly for amphiphiles using the Pluronic flake series (PF68, PF108, PF87, and PF127) with higher HLB values and Cremophor (data not shown). These polymers with higher hydrophilic properties (HLB) were incompatible with the hydrophobic lecithin, causing honokiol/magnolol-loaded lbMPMs to precipitate. The relatively high hydrophilicity of these amphiphilic polymers (HLB >10), therefore, might have had a lower ability to incorporate a hydrophobic drug and form micelles. Although the critical micellar concentration (CMC) of PL121 is considered the lowest and this surfactant can self-assemble into micelles, the poly(propylene oxide) (PPO) fragment is excessively long and has limited space for loading the hydrophobic honokiol-magnolol. Therefore, the high HLB value and the short hydrophilic chain of PL121 allowed the micelles to easily aggregate.39–42
Stable lbMPMs were formed when combining lecithin with the NaDOC and PP123 mixed micelles groups. PI values were lower than those of micelles formed using other amphiphilic polymers, indicating an even distribution. In addition, their drug loading (listed in Table 2) was also slightly higher after adding lecithin, eg, from 6.29% to 9.81% for the PP123 group. EE increased with an increasing amount of lecithin, because the addition of lecithin into the micellar system increased the volume of the hydrophobic region of the micelles, thereby providing more space for the hydrophobic drug to be solubilized.43 Inserting lecithin into the micelles formed lbMPMs, with a possible transition of polyoxyethylene-polyoxypropylene chains from a brush to a mushroom conformation as previously suggested, thus decreasing the particle size.44 The results demonstrated that lbMPMs comprised of two polymers (NaDOC and PP123) offered benefits of both polymers, which could compensate for each other’s limitations. Additionally, the presence of lecithin facilitated the absorption of the encapsulated drug and helped stabilize the micellar system with an optimal combination ratio.
The physical characteristics of the optimal mixed polymeric micelles containing NaDOC and PP123 are listed in Table 2 and show that their particle sizes were 80–150 nm with a high drug EE (>90%) and DL (>9%). (solubilization capacity: >6 mg/mL; data not shown). The tremendous potential of honokiol/magnolol as anticancer agents has been emphasized, and a small particle size of the delivery system is beneficial for passive targeting to tumor tissues through the EPR effect, cellular uptake, and intracellular trafficking. Therefore, it is hoped that the small size of the formulated honokiol/magnolol micelles can increase the blood circulation time and avoid the reticuloendothelial system.
To study honokiol/magnolol encapsulation stability, we incubated honokiol/magnolol (free and H/M-loaded lbMPMs) in PBS at room temperature, 4 °C and 37 °C, and in FBS at 37 °C and determined the change of particle sizes over time (Figure 4). Regardless of whether honokiol/magnolol was incubated in PBS or FBS, the time when the particle size reached <200 nm was longer for PP123 than that for NaDOC. The CMC value of PP123 was lower than that of NaDOC, revealing higher stability of Pluronic® P123 in FBS. The lack of adherence of plasma proteins to Pluronic® micelles was possibly due to the poly(ethylene oxide) (PEO) units in Pluronic®, which retard protein adsorption. According to the encapsulation stability test, H/M-loaded lbMPMs[PP123] were more stable than those using lbMPMs[NaDOC]; therefore, the in vitro release rate of H/M-loaded lbMPMs[PP123] was slower. In this study, the release of honokiol/magnolol from lbMPMs[PP123] was slightly slower compared to that from lbMPMs[NaDOC] and occurred to a lesser extent. This can probably be attributed to the “core-shell” nanostructure of honokiol/magnolol entrapped in lbMPMs[PP123] in water, which allowed the slow-release behaviors of drug-loaded micelles in vitro. This demonstrates that honokiol/magnolol entrapped in lbMPMs[PP123] were able to be sustainably released, thus preventing their clearance by the systemic circulation.
With IV administration, free honokiol and magnolol were found to have rapid rates of distribution followed by slower rates of elimination in Sprague Dawley rats.45 In our study, the PK characters of the isomers after IV administration indicated similar in vivo PK behaviors (eg AUC0–∞: 15.24 ± 8.95 vs 12.29 ± 2.08; t1/2: 1.22 ± 0.78 vs 1.29 ± 0.57). The AUC, CL, and V of honokiol and magnolol tended to be similar between the two rat groups. The pattern of the plasma concentration–time curves demonstrated a rapid distribution phase followed by a slower elimination phase, which is consistent with what is reported in the literature.45,46
Previous in vitro release and stability study results revealed that lbMPMs[PP123] were more stable and exhibited slower release rates compared to lbMPMs[NaDOC] groups. lbMPMs[PP123] were expected to be sustainably released in vivo, thus preventing clearance by the systemic circulation. Nevertheless, this was not the case, as PK results suggested that H/M-loaded lbMPMs[NaDOC] had longer t1/2 and larger AUC values than those of lbMPMs[PP123] regardless of IV or oral administration. As is well known, the particle size and PI are the main physicochemical attributes that influence stability, EE, the drug-release profile, biodistribution, mucoadhesion, and cellular uptake.47 The rate of uptake by immune system cells increases with an increase in the size of the nanocarrier. In general, the lowest possible PI is preferred in order to obtain more reliable and repeatable in vivo blood clearance PKs and subsequent biodistribution results.48 Therefore, a possible explanation for such a high elimination rate found in the lbMPMs[PP123] group was the high PI value (0.413±0.010) which caused a dramatic increase in being taken up by phagocytes, which resulted in a shorter period of circulation in vivo.
t1/2 and AUC levels were found to be significantly higher in lbMPMs[NaDOC] compared to the respective free drug groups. AUC levels of honokiol and magnolol for lbMPMs[NaDOC] were nearly equal (0.93) and 3.41-fold increases compared with those of free honokiol and magnolol, respectively. H/M-loaded lbMPMs[NaDOC] were detected for a long duration (after 24 h). These results suggested that H/M-loaded lbMPMs[NaDOC] had greatly improved absorption, but the elimination of honokiol/magnolol was retarded. These results implied that H/M loaded micelles could allow honokiol/magnolol to circulate in the blood for a longer time, inducing better therapeutic effects.
In the oral PK study, the oral bioavailability of honokiol and magnolol was reported to be only 5% due to extensive first-pass metabolism and low absorption.17 We also found a low bioavailability of free honokiol and magnolol in rats from our study (bioavailability: 0% and 9% respectively for the free honokiol and magnolol group). It is worth noting that the plasma concentration of free honokiol after oral administration was too low to accurately calculate the PK parameters. These experimental results are very similar to test results from other groups.49,50 Honokiol is a weakly acidic substance and is unstable in an alkaline environment such as the intestines at pH 8.4~8.6. The low bioavailability of honokiol in rats could presumably be due to its structural transformation, which might be related to pH and intestinal flora catabolism in the gastrointestinal tract.51 Another study by Liu et al also showed poor absorption in the gastrointestinal tract;49 about half of the honokiol was quickly removed from the body after oral administration. Using lbMPMs[NaDOC], the oral bioavailability of honokiol/magnolol was significantly increased. This was clearly demonstrated by the significant increase in t1/2 and AUC levels for the lbMPMs[NaDOC] groups compared to the respective free honokiol and magnolol groups. Moreover, the relative bioavailability was dramatically enhanced, which was a 2.91-fold increase compared to the free magnolol group.
On the other hand, unsatisfactory results were obtained from the lbMPMs[P123] groups. There was no significant difference in plasma honokiol and magnolol concentrations between the lbMPMs[P123] and free drug groups. The low oral bioavailability of honokiol and magnolol could not be improved using lbMPMs[PP123] in our study. As is well known, in vitro drug-release testing is a powerful and useful method for determining product quality and sometimes to evaluate the clinical performance of dosage forms. However, in our experiments, the in vitro release did not correlate well with in vivo bioavailability. Higher bioavailability but higher in vitro drug-release results were observed for lbMPMs[NaDOC], while lbMPMs[PP123] demonstrated lower bioavailability and a slower in vitro drug-release profile. Furthermore, there was a little concern about the cytotoxicity of PP123 against MCF-7 observed in a previous study showing that the IC50 value for blank LMPM (composed of lecithin, PP123, and DSPE-PEG2K at a w/w ratio of 1:17.5:2.5) was >200 μM.30 Although this IC50 value was not significantly too high, it was still a potential concern for in vivo safety if the local concentration in tissues or organs cumulated higher than this concentration.
The reasons for such a higher absorption for lbMPMs[NaDOC] may be as follows. First, the small particle size of lbMPMs provides a large interfacial surface area for drug absorption, therefore inducing higher bioavailability. Second, bile salts may act as an absorption enhancer by increasing the permeability of honokiol and magnolol across the gastrointestinal mucosa.52,53 Thus, the rapid and effective transmembrane transport of lbMPMs[NaDOC] led to much-higher bioavailability. In addition, clear secondary peaks were observed in the plasma concentration–time curves at 2 and 1.5 h for honokiol and magnolol, respectively, when H/M loaded lbMPMs[NaDOC] groups were orally administered. This might be due to the effect of bile salts via the enterohepatic circulation,54,55 which is attributed to the phenomenon of enterohepatic recycling, and may be an important consideration for interpreting the higher bioavailability.
Overall, lbMPMs[NaDOC] formulations exhibited significantly improved and superior bioavailability profiles regardless of IV or oral administration. The micelle biodistribution mainly depends on components of the hydrophilic shell causing the micelles to stabilize and interact with plasma proteins and cell membranes. Moreover, because of their amphiphilic characteristics, the encapsulated polymers used here have surfactant properties and boost the stability and biocompatibility of micelles. These favorable oral PK profiles may resolve the high dose problems associated with promising natural products for various medicinal applications.
Conclusions
The current study involved developing and characterizing H/M-loaded lbMPMs for improving the bioavailability of honokiol/magnolol. Optimal formulations of H/M:L:NaDOC (lbMPMs[NaDOC]) and H/M:L:PP123 (lbMPMs[PP123]) were 6:2:5 and 1:1:10, respectively, providing a particle size of 80–150 nm, an EE of >90%, and a DL of >9.0%. Among two optimal formulations, lbMPMs[NaDOC] significantly improved the bioavailability of honokiol and magnolol in comparison with that for lbMPMs[PP123] for both IV and oral administrations. It was concluded that lbMPMs improved the solubility, encapsulation stability, and bioavailability of honokiol/magnolol, and represent a suitable delivery system for these hydrophobic drugs. Therefore, increasing honokiol/magnolol bioavailability using lbMPMs can allow honokiol/magnolol to become prominent therapeutic agents for treating cancer and other various disorders.
Acknowledgments
This work was financially supported by the Ministry of Science and Technology, Taiwan. (MOST 109-2221-E-038-001-MY3) and the Jin-lung-yuan Foundation (2019 to 2020).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shen -C-C, Ni C-L, Shen Y-C, et al. Phenolic Constituents from the Stem Bark of Magnolia officinalis. J Nat Prod. 2009;72(1):168–171. doi:10.1021/np800494e
2. Ong CP, Lee WL, Tang YQ, Yap WH. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers. 2019;12(1):1. doi:10.3390/cancers12010048
3. Yang C, Zhi X, Xu H. Advances on Semisynthesis, Total Synthesis, and Structure-Activity Relationships of Honokiol and Magnolol Derivatives. Mini Rev Med Chem. 2016;16(5):404–426. doi:10.2174/1389557516666151120115558
4. Zhao C, Liu Z-Q. Comparison of antioxidant abilities of magnolol and honokiol to scavenge radicals and to protect DNA. Biochimie. 2011;93(10):1755–1760. doi:10.1016/j.biochi.2011.06.012
5. Wang T, Chen W, Wu J. H2-P, a honokiol derivative, exerts anti-angiogenesis effects via c-MYC signaling pathway in glioblastoma. J Cell Biochem. 2018;119(4):3142–3148. doi:10.1002/jcb.26462
6. Lu X, Lu X, Zhang Z, Lv LH. Preparation and Characterization of Honokiol Nanosuspensions and Preliminary Evaluation of Anti-Inflammatory Effect. AAPS PharmSciTech. 2020;21(2):62. doi:10.1208/s12249-019-1602-x
7. Zhang B, Wang -P-P, Hu K-L, et al. Antidepressant-Like Effect and Mechanism of Action of Honokiol on the Mouse Lipopolysaccharide (LPS) Depression Model. Molecules. 2019;24(11):2035. doi:10.3390/molecules24112035
8. Banik K, Ranaware AM, Deshpande V, et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol Res. 2019;144:192–209. doi:10.1016/j.phrs.2019.04.004
9. Zhu M, Li B, Ma H, et al. Synthesis and in vitro antitumor evaluation of honokiol derivatives. Bioorg Med Chem Lett. 2020;30(2):126849. doi:10.1016/j.bmcl.2019.126849
10. Chei S, Oh H-J, Song J-H, Seo Y-J, Lee K, Lee B-Y. Magnolol Suppresses TGF-β-Induced Epithelial-to-Mesenchymal Transition in Human Colorectal Cancer Cells. Front Oncol. 2019;9:752. doi:10.3389/fonc.2019.00752
11. Shen J, Ma H, Zhang T, et al. Magnolol Inhibits the Growth of Non-Small Cell Lung Cancer via Inhibiting Microtubule Polymerization. Cell Physiol Biochem. 2017;42(5):1789–1801. doi:10.1159/000479458
12. Shen L, Zhang F, Huang R, Yan J, Shen B. Honokiol inhibits bladder cancer cell invasion through repressing SRC-3 expression and epithelial-mesenchymal transition. Oncol Lett. 2017;14(4):4294–4300. doi:10.3892/ol.2017.6665
13. Sengupta S, Nagalingam A, Muniraj N, et al. Activation of tumor suppressor LKB1 by honokiol abrogates cancer stem-like phenotype in breast cancer via inhibition of oncogenic Stat3. Oncogene. 2017;36(41):5709–5721. doi:10.1038/onc.2017.164
14. Tang H, Zhang Y, Li D, et al. Discovery and synthesis of novel magnolol derivatives with potent anticancer activity in non-small cell lung cancer. Eur J Med Chem. 2018;156:190–205. doi:10.1016/j.ejmech.2018.06.048
15. Yamaguchi N, Satoh-Yamaguchi K, Ono M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine. 2009;16(4):369–376. doi:10.1016/j.phymed.2008.12.021
16. Kang JS, Lee KH, Han MH, et al. Antiinflammatory activity of methanol extract isolated from stem bark of Magnolia kobus. Phytother Res. 2008;22(7):883–888. doi:10.1002/ptr.2386
17. Lin S-P, Tsai S-Y, Lee Chao P-D, Chen Y-C, Hou Y-C. Pharmacokinetics, bioavailability, and tissue distribution of magnolol following single and repeated dosing of magnolol to rats. Planta Med. 2011;77(16):1800–1805. doi:10.1055/s-0030-1271159
18. Yu R, Zou Y, Liu B, Guo Y, Wang X, Han M. Surface modification of pH-sensitive honokiol nanoparticles based on dopamine coating for targeted therapy of breast cancer. Colloids Surf B Biointerfaces. 2019;177:1–10. doi:10.1016/j.colsurfb.2019.01.047
19. Wang XH, Deng LY, Cai LL, et al. Preparation, characterization, pharmacokinetics, and bioactivity of honokiol-in-hydroxypropyl-β-cyclodextrin-in-liposome. J Pharm Sci-Us. 2011;100(8):3357–3364. doi:10.1002/jps.22534
20. Wang X-H, Cai -L-L, Zhang X-Y, et al. Improved solubility and pharmacokinetics of PEGylated liposomal honokiol and human plasma protein binding ability of honokiol. Int J Pharmaceut. 2011;410(1–2):169–174. doi:10.1016/j.ijpharm.2011.03.003
21. Yang B, Ni X, Chen L, et al. Honokiol-loaded polymeric nanoparticles: an active targeting drug delivery system for the treatment of nasopharyngeal carcinoma. Drug Deliv. 2017;24(1):660–669. doi:10.1080/10717544.2017.1303854
22. Godugu C, Doddapaneni R, Singh M. Honokiol nanomicellar formulation produced increased oral bioavailability and anticancer effects in triple negative breast cancer (TNBC). Colloids Surf B Biointerfaces. 2017;153:208–219. doi:10.1016/j.colsurfb.2017.01.038
23. Gong J, Chen M, Zheng Y, Wang S, Wang Y. Polymeric micelles drug delivery system in oncology. J Control Release. 2012;159(3):312–323. doi:10.1016/j.jconrel.2011.12.012
24. Lu Y, Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm. 2013;453(1):198–214. doi:10.1016/j.ijpharm.2012.08.042
25. Jones M, Leroux J. Polymeric micelles – a new generation of colloidal drug carriers. Eur j Pharm Biopharm. 1999;48(2):101–111. doi:10.1016/S0939-6411(99)00039-9
26. Yokoyama M. Polymeric micelles as a new drug carrier system and their required considerations for clinical trials. Expert Opin Drug Del. 2010;7(2):145–158. doi:10.1517/17425240903436479
27. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65(1–2):271–284. doi:10.1016/S0168-3659(99)00248-5
28. Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74(1–3):47–61. doi:10.1016/S0168-3659(01)00309-1
29. Chen Y-C, Su C-Y, Jhan H-J, Ho H-O, Sheu M-T. Physical characterization and in vivo pharmacokinetic study of self-assembling amphotericin B-loaded lecithin-based mixed polymeric micelles.. Int J Nanomedicine. 2015;10:7265–7274. doi:10.2147/IJN.S95194
30. Chen L-C, Chen Y-C, Su C-Y, Hong C-S, Ho H-O, Sheu M-T. Development and characterization of self-assembling lecithin-based mixed polymeric micelles containing quercetin in cancer treatment and an in vivo pharmacokinetic study.. Int J Nanomedicine. 2016;11:1557–1566. doi:10.2147/IJN.S103681
31. Chen L-C, Chen Y-C, Su C-Y, Wong W-P, Sheu M-T, Ho H-O. Development and Characterization of Lecithin-based Self-assembling Mixed Polymeric Micellar (saMPMs) Drug Delivery Systems for Curcumin. Sci Rep. 2016;6(1):37122. doi:10.1038/srep37122
32. Yang K-Y, Lin L-C, Tseng T-Y, Wang S-C, Tsai T-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J Chromatogr B Anal Technol Biomed Life Sci. 2007;853(1–2):183–189. doi:10.1016/j.jchromb.2007.03.010
33. Zhao L, Shi Y, Zou S, Sun M, Lil L, Zhai ZG. Formulation and in vitro Evaluation of Quercetin Loaded Polymeric Micelles Composed of Pluronic P123 and D-a-Tocopheryl Polyethylene Glycol Succinate. J Biomed Nanotechnol. 2011;7(3):358–365. doi:10.1166/jbn.2011.1298
34. Sheng Y-L, Xu J-H, Shi C-H, et al. UPLC-MS/MS-ESI assay for simultaneous determination of magnolol and honokiol in rat plasma: application to pharmacokinetic study after administration emulsion of the isomer. J Ethnopharmacol. 2014;155(3):1568–1574. doi:10.1016/j.jep.2014.07.052
35. Chang C-E, Hsieh C-M, Huang S-C, Su C-Y, Sheu M-T, Ho H-O. Lecithin-Stabilized Polymeric Micelles (LsbPMs) for Delivering Quercetin: pharmacokinetic Studies and Therapeutic Effects of Quercetin Alone and in Combination with Doxorubicin. Sci Rep. 2018;8(1):17640. doi:10.1038/s41598-018-36162-0
36. Su C-Y, Liu -J-J, Ho Y-S, et al. Development and characterization of docetaxel-loaded lecithin-stabilized micellar drug delivery system (L sb MDDs) for improving the therapeutic efficacy and reducing systemic toxicity. Eur J Pharm Biopharm. 2018;123:9–19. doi:10.1016/j.ejpb.2017.11.006
37. Xu Q, Yi L-T, Pan Y, et al. Antidepressant-like effects of the mixture of honokiol and magnolol from the barks of Magnolia officinalis in stressed rodents. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):715–725. doi:10.1016/j.pnpbp.2007.11.020
38. Han H-K, Van Anh LT. Modulation of P-glycoprotein expression by honokiol, magnolol and 4-O-methylhonokiol, the bioactive components of Magnolia officinalis.. Anticancer Res. 2012;32(10):4445–4452.
39. Abdelbary GA, Tadros MI. Brain targeting of olanzapine via intranasal delivery of core–shell difunctional block copolymer mixed nanomicellar carriers: in vitro characterization, ex vivo estimation of nasal toxicity and in vivo biodistribution studies. Int J Pharm. 2013;452(1–2):300–310. doi:10.1016/j.ijpharm.2013.04.084
40. Pepić I, Lovrić J, Hafner A, Filipović-Grčić J. Powder form and stability of Pluronic mixed micelle dispersions for drug delivery applications. Drug Dev Ind Pharm. 2014;40(7):944–951. doi:10.3109/03639045.2013.791831
41. Zhao Y, Li Y, Ge J, Li N, Li L-B. Pluronic-poly (acrylic acid)-cysteine/Pluronic L121 mixed micelles improve the oral bioavailability of paclitaxel. Drug Dev Ind Pharm. 2014;40(11):1483–1493. doi:10.3109/03639045.2013.829487
42. Lee ES, Oh YT, Youn YS, et al. Binary mixing of micelles using Pluronics for a nano-sized drug delivery system. Colloids Surf B Biointerfaces. 2011;82(1):190–195. doi:10.1016/j.colsurfb.2010.08.033
43. Alkan‐Onyuksel H, Ramakrishnan S, Chai H-B, Pezzuto JM. A mixed micellar formulation suitable for the parenteral administration of taxol. Pharm Res. 1994;11(2):206–212. doi:10.1023/A:1018943021705
44. Rex S, Zuckermann MJ, Lafleur M, Silvius JR. Experimental and Monte Carlo simulation studies of the thermodynamics of polyethyleneglycol chains grafted to lipid bilayers. Biophys J. 1998;75(6):2900–2914. doi:10.1016/S0006-3495(98)77732-X
45. Tsai T-H, Chou C-J, Cheng F-C, Chen C-F. Pharmacokinetics of honokiol after intravenous administration in rats assessed using high performance liquid chromatography. Journal of Chromatography B: Biomedical Sciences and Applications. 1994;655(1):41–45. doi:10.1016/0378-4347(94)00031-X
46. Tsai TH, Chou CJ, Chen CF. Disposition of magnolol after intravenous bolus and infusion in rabbits.. Drug Metab Dispos. 1994;22(4):518–521.
47. Azhar Shekoufeh Bahari L, Hamishehkar H. The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers; A Comparative Literature Review. Adv Pharm Bull. 2016;6(2):143–151. doi:10.15171/apb.2016.021
48. Danaei M, Dehghankhold M, Ataei S, et al. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics. 2018;10(2):2. doi:10.3390/pharmaceutics10020057
49. Liu Y, Wang D, Yang G, Shi Q, Feng F. Comparative pharmacokinetics and brain distribution of magnolol and honokiol after oral administration of Magnolia officinalis cortex extract and its compatibility with other herbal medicines in Zhi-Zi-Hou-Po Decoction to rats. Biomed Chromatogr. 2016;30(3):369–375. doi:10.1002/bmc.3557
50. Xu F, Liu Y, Zhang Z, Song R, Dong H, Tian Y. Rapid simultaneous quantification of five active constituents in rat plasma by high-performance liquid chromatography/tandem mass spectrometry after oral administration of Da-Cheng-Qi decoction. J Pharm Biomed Anal. 2008;47(3):586–595. doi:10.1016/j.jpba.2008.02.005
51. Hattori M, Endo Y, Takebe S, Kobashi K, Fukasaku N, Namba T. Metabolism of magnolol from Magnoliae Cortex. II. Absorption, metabolism and excretion of (ring-14C)magnolol in rats.. Chem Pharm Bull (Tokyo). 1986;34(1):158–167. doi:10.1248/cpb.34.158
52. Wilson FA. Intestinal transport of bile acids.. Am J Physiol. 1981;241(2):G83–G92. doi:10.1152/ajpgi.1981.241.2.G83
53. Sallee VL, Dietschy JM. Determinants of intestinal mucosal uptake of short- and medium-chain fatty acids and alcohols. J Lipid Res. 1973;14(4):475–484. doi:10.1016/S0022-2275(20)36881-4
54. Li X, Yuan Q, Huang Y, Zhou Y, Liu Y. Development of silymarin self-microemulsifying drug delivery system with enhanced oral bioavailability. AAPS PharmSciTech. 2010;11(2):672–678. doi:10.1208/s12249-010-9432-x
55. Wu W, Wang Y, Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2006;63(3):288–294. doi:10.1016/j.ejpb.2005.12.005
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.