Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Honey and Chamomile Activate Keratinocyte Antioxidative Responses via the KEAP1/NRF2 System
Authors Ogawa T , Ishitsuka Y , Nakamura Y, Okiyama N , Watanabe R, Fujisawa Y , Fujimoto M
Received 8 July 2020
Accepted for publication 24 August 2020
Published 7 September 2020 Volume 2020:13 Pages 657—660
DOI https://doi.org/10.2147/CCID.S270602
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Tatsuya Ogawa, 1 Yosuke Ishitsuka, 1 Yoshiyuki Nakamura, 1 Naoko Okiyama, 1 Rei Watanabe, 1, 2 Yasuhiro Fujisawa, 1 Manabu Fujimoto 1, 2
1Department of Dermatology, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki, Japan; 2Department of Dermatology, Osaka University Graduate School of Medicine, Suita, Osaka, Japan
Correspondence: Yosuke Ishitsuka
Department of Dermatology, Faculty of Medicine, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki 305-8575, Japan
Tel +81-29-853-3128
Fax +81-29-853-3217
Email [email protected]
Introduction: The stratum corneum protects against the entry of pathogens, allergens, and irritants while preventing dehydration. The Kelch-like erythroid cell-derived protein with cap-n-collar homology-associated protein 1 (KEAP1)/NF-E2-related factor 2 (NRF2) system maintains skin barrier homeostasis. Aggregated evidence suggests that NRF2-mediated antioxidative response is hardwired into the stratified squamous epithelia. Honey and chamomile have long been regarded as natural antioxidants. Nonetheless, it is still unclear whether they activate the KEAP1/NRF2 system in the epidermis and could promote epidermal barrier recovery.
Methods: To address the abovementioned issue, we explored the antioxidative property of honey/chamomile extract by using non-cell-based KEAP1-inhibition assay and cultured human epidermal keratinocytes.
Results: Herein we report that the extract inhibited KEAP1-NRF2 interaction and induced keratinocyte production of antioxidant small proline-rich protein.
Conclusion: Our results may offer an opportunity to develop cosmetic products that boost NRF2-mediated antioxidative/antiaging, epidermis-intrinsic bio-responses.
Keywords: NRF2, honey, chamomile, keratinocyte
Introduction
The epidermis serves as a barrier between the body and the external environment and protects against dehydration and xenobiotic insults. Such a physical barrier is attributable to the specialized structure of cornified envelopes (CEs) formed beneath the keratinocyte plasma membrane.1 The major and ubiquitous CE protein components are loricrin and involucrin,1 while the minor, inducible CE protein components, such as small proline-rich proteins (SPRR) and late cornified envelope (LCE) proteins, cross-bridge other components and accommodates specific barrier requirements of different squamous epithelia.1
The Kelch-like erythroid cell-derived protein with cap-n-collar homology-associated protein 1 (KEAP1)/NF-E2-related factor 2 (NRF2) system forms the major node of environmental responses.2 In the steady-state, KEAP1 serves as an adaptor for the cullin 3-dependent E3 ubiquitin ligase and subjects NRF2 to proteasomal degradation in the cytosol. Upon exposure to redox-disruptive stimuli, KEAP1 undergoes conformational changes and unleashes NRF2 activity, resulting in the transactivation of various cytoprotective genes.2 The important observation from Keap1-deficient mice suggested that keratinization constitutes a critical tissue-intrinsic stress response of stratified squamous epithelia; excessive NRF2 activity leads to a progressive hyperkeratosis even in the inner squamous epithelia (esophagus/forestomach).2 Accordingly, NRF2 directly binds to the canonical cis-element antioxidant responsive element and induces the minor CE components Sprr2d, Sprr2h,3 or Lce1.4 The series of evidence suggests that the NRF2-mediated antioxidative responses strengthen skin barrier functions.
Both honey and chamomile have many health-promoting effects, including antioxidant activities, and one of the major modes of action is the NRF2 activation.5,6 However, little is known about their effects on the KEAP1/NRF2 system in keratinocytes and the skin barrier functions.
In this study, we used a screening kit for KEAP1 inhibitor and cultured neonatal human epidermal keratinocytes (HEKn) and investigated whether the extract from honey/chamomile activates the KEAP1/NRF2 system in vitro.
Materials and Methods
Chemicals
The extract from honey/chamomile was provided by Yamada Bee Company, Inc. (Okayama, Japan). Briefly, lyophilized Japanese hyakka honey (10% W/V) and chamomile flower powder (1% W/V) was dissolved in distilled water and autoclaved (110 °C, 5 min). Fermentation was performed by incubating the extract with Lactobacillus kunkeei BPS402 at 30 °C for 40 hours. The lyophilized extract (2.5 mg/mL) was used for the experimentation.
Keap1: Nrf2 Inhibitor Screening Assay
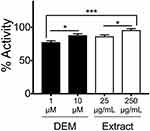
The Keap1: Nrf2 Inhibitor Screening Assay Kit (#72020; BPS Bioscience, San Diego, CA) was purchased, and KEAP1-inhibitory potential was determined by fluorescence polarization as per the manufacturer’s instruction. The change in fluorescent polarization was measured using a fluorescence reader (Varioskan LUX; Thermo Fisher Scientific, Waltham, MA). 250 μg/mL and 25 μg/mL of the extract with fermentation was used for this assay. Diethyl maleate ([DEM] D97703; Sigma-Aldrich, St. Louis, MO) was used as a positive control of the KEAP1 inhibitor.
Keratinocyte Culture
Human Epidermal Keratinocytes, neonatal ([HEKn] C0015C; Thermo Fisher Scientific) were maintained in EpiLifeTM medium (MEPI500CA; Thermo Fisher Scientific) with EpiLifeTM Defined Growth Supplement (s0125; Thermo Fisher Scientific).
Honey/Chamomile Extract Treatment
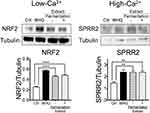
2×106 cells of HEKn were seeded in 100 mm dishes (Surface area; 55 cm2). Cells at 80% confluency were stimulated with 10 μg/mL of the extract with or without fermentation or 5 μM of tert-butylhydroquinone ([tBHQ] 112941; Sigma-Aldrich). For NRF2 detection, the whole-cell lysate was prepared using sodium dodecyl sulfate (SDS) extraction buffer (Tris-HCl [pH 6.8], 2% SDS, 0.86 M β-Mercaptoethanol and 10% glycerol) after 30 minutes of incubation. For SPRR2 detection, HEKn were incubated under the high Ca2+ condition (1.2 mM) for 96 hours. Differentiated HEKn were then treated with the extract or tBHQ, and the whole-cell lysate was dissolved in the extraction buffer after 24 hours of incubation.
Western Blotting
An equal amount of protein (10 μg) was subjected to SDS-PAGE on Mini-PROTEAN® TGXTM Precast Gels (4569036; Bio-Rad, Hercules, CA), and the proteins were transferred onto nitrocellulose membranes (10600001; GE Healthcare, Buckinghamshire, UK). The membranes were incubated overnight with following primary antibodies; anti-NRF2 (1:200; kindly provided by Dr. Dennis Roop),3,4 anti-SPRR (1:5000; kindly provided by Dr. Daniel Hohl),3,4 and anti-tubulin (1 μg/mL, 017–25031; FUJIFILM Wako Pure Chemical) followed by a 60 min incubation with HRP-labeled secondary antibodies against rabbit or mouse IgG (0.04 μg/mL, sc-2004 or sc-2005; Santa Cruz, Dallas, TX). Antibody binding was visualized and enhanced with SuperSignal West Dura Extended Duration Substrate (34075; Thermo Fisher, Waltham, MA) and an image analysis system (LAS4000 mini; Fujifilm, Tokyo, Japan). The bands’ intensities were quantified by densitometry, and the expression levels were normalized relative to tubulin.
Statistical Analysis
Data are expressed as means ± SEM. Comparisons were performed using one-way ANOVA (between each group) by the use of Prism 6 software (GraphPad, La Jolla, CA). In all studies, p < 0.05 was taken to indicate statistical significance.
Results
Honey/Chamomile Extract Inhibits KEAP1–NRF2 Binding
KEAP1 represses NRF2 activity under normal physiological conditions; NRF2 is constitutively ubiquitinated via the adaptor protein KEAP1 and subjected to proteasome-mediated degradation.2 Oxidative stresses are sensed by cysteine residues of KEAP1 protein, whose conformational changes lead to NRF2 activation.2 To examine whether honey/chamomile extract inhibits KEAP1 activity, we used a commercially available non-cell-based screening kit for KEAP1 inhibitors. The extract with fermentation exhibited as strong KEAP1 inhibitory activity as an electrophile diethyl maleate (DEM) did in a dose-dependent manner (Figure 1).
Honey/Chamomile Extract Enhances NRF2 and SPRR2 Expression
Upregulation of NRF2 and antioxidative SPRRs profoundly contributes to tissue-intrinsic skin protective responses.1 Modification of KEAP thiols and stabilization of NRF2 progress rapidly, whereas the induction of cytoprotective genes takes a whole day. Furthermore, SPRR2 is induced during the differentiation of epidermal keratinocytes in which Ca2+ plays an important role.3 To investigate whether the extract enhances NRF2 and SPRR2 expression, HEKn were incubated in the presence of the extract under low or high Ca2+ condition, and tBHQ was used as a positive control of the NRF2 activator.2 The whole cell lysate was collected after 30 minutes or 24 hours of incubation. Incubation with the extract induced greater stabilization of NRF2 and SPRR2 expression compared to that with medium alone (Figure 2). Those effects were not affected by the fermentation.
Discussion/Conclusion
Because of the profound contributions of NRF2 in redox homeostasis, cytoprotection, and anti-inflammatory effects, many efforts have been made to develop NRF2-inducing chemicals.2 Honey and chamomile are derived from the natural source and have already been the ingredients of daily skincare products. We herein demonstrated for the first time that honey and chamomile are the potent inducers of NRF2-mediated keratinocyte antioxidative responses, which could promote the recovery from disrupted skin barrier.1 However, as previous studies indicate, excess NRF2 activation could cause epidermal hyperproliferation/hyperkeratosis2 or allergic contact dermatitis.7 Therefore, careful attention should be paid to the aesthetic use of honey and chamomile. We hope that our results will contribute to a better understanding of the effects of NRF2 on keratinocytes and the development of novel cosmetic products.
Acknowledgment
We appreciate Yamada Bee Company, Inc. for providing us the honey/chamomile extract.
Disclosure
This research was supported in part by the following JSPS KAKENHI Grant, Grant-in-Aid for Research Activity Start-up (16H06663 to YI), and Early-Career Scientists (18K16018 to YI). The authors state they have no conflicts of interest for this work.
References
1. Ishitsuka Y, Roop DR. Loricrin: past, present, and future. Int J Mol Sci. 2020;21(7):2271. doi:10.3390/ijms21072271
2. Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98(3):1169–1203. doi:10.1152/physrev.00023.2017
3. Huebner AJ, Dai D, Morasso M, et al. Amniotic fluid activates the nrf2/keap1 pathway to repair an epidermal barrier defect in utero. Dev Cell. 2012;23(6):1238–1246. doi:10.1016/j.devcel.2012.11.002
4. Ishitsuka Y, Huebner AJ, Rice RH, et al. Lce1 family members are Nrf2-target genes that are induced to compensate for the loss of loricrin. J Invest Dermatol. 2016;136(8):1656–1663. doi:10.1016/j.jid.2016.04.022
5. Ranneh Y, Akim AM, Hamid HA, Khazaai H, Fadel A, Mahmoud AM. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-kappaB and p38 MAPK. Nutr Metab (Lond). 2019;16:15. doi:10.1186/s12986-019-0341-z
6. Bhaskaran N, Srivastava JK, Shukla S, Gupta S. Chamomile confers protection against hydrogen peroxide-induced toxicity through activation of Nrf2-mediated defense response. Phytother Res. 2013;27(1):118–125. doi:10.1002/ptr.4701
7. Ogawa T, Ishitsuka Y, Nakamura Y, et al. NRF2 augments epidermal antioxidant defenses and promotes atopy. J Immunol. 2020;205(4):907–914. doi:10.4049/jimmunol.2000274
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.