Back to Journals » OncoTargets and Therapy » Volume 12
Homeobox B7 accelerates the cancer progression of gastric carcinoma cells by promoting epithelial–mesenchymal transition (EMT) and activating Src–FAK pathway
Authors Wu J, Long Z, Cai H , Yu S, Liu X
Received 13 December 2018
Accepted for publication 5 April 2019
Published 16 May 2019 Volume 2019:12 Pages 3743—3751
DOI https://doi.org/10.2147/OTT.S198115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Jianghong Wu,1,2 Ziwen Long,1,2 Hong Cai,1,2 Shengjia Yu,1,2 Xiaowen Liu1,2
1Department of Gastric Surgery, Fudan University Shanghai Cancer Center, Shanghai, 200032, People’s Republic of China; 2Department of Oncology, Shanghai Medical College of Fudan University, Shanghai 200032, People’s Republic of China
Aim: To study the carcinogenetic mechanism of HOXB7 in gastric cancer (GC) remains.
Methods: Two human GC cell lines — SGC7901 and SNU1 — were used for this study. SGC7901 cells were transfected with siRNA-HOXB7 (siHOXB7) to knock down HOXB7 expression, whereas, SNU1 cells were transduced with pCDNA3.1-HOXB7 to overexpress HOXB7. After transfection, cancer progression was assessed by determining cell proliferation, wound-healing process, cell cycle, apoptosis, invasion, and migration. The effect of HOXB7 on epithelial–mesenchymal transition (EMT) was measured by observing changes in F-actin cytoskeleton and evaluating the expression of EMT markers. p-Scr and p-FAK were evaluated to assess the mechanism.
Results: Knockdown of HOXB7 suppressed cell proliferation, alleviated the wound-healing process, inhibited cell migration and invasion, and arrested the cell cycle while promoting cell apoptosis, suggesting the tumor-suppressive effect of siHOXB7 in human GC cells. On the contrary, HOXB7 overexpression showed a tumor-promoting effect on human GC cells. Moreover, we confirmed an inhibitory effect of siHOXB7 on the EMT process by preventing epithelial cells from acquiring a mesenchymal phenotype and downregulating mesenchymal markers (vimentin, β-catenin, N-cadherin, Twist) while upregulating epithelial markers (E-cadherin). Our data revealed that HOXB7 was associated with Src/FAK and favored the activation of the Src–FAK pathway in human GC cells.
Conclusion: HOXB7 accelerated the malignancy of GC, by facilitating EMT and regulating the Scr–FAK pathway.
Keywords: gastric carcinoma, Homeobox B7, Epithelial-mesenchymal transition, Src, FAK
Introduction
Gastric cancer is the fifth-most frequent and second-most deadly malignancy in the world, with an estimated 952,000 new cases (6.8% of the total) in 2012 according to the GLOBOCAN network of the World Health Organization.1,2 Gastric carcinoma (GC) arises from the epithelium, accounting for 90%–95% of all GC cases.3 Recurrence and metastasis are frequent after treatment, contributing to the poor prognosis of this disease.4,5 Therefore, a better understanding of molecular mechanisms in GC progression is very necessary to guide therapeutic strategy to prevent this disease.
HOX genes contain HOX and non-HOX members, which encode a transcriptional family and usually function in morphogenesis and differentiation.6,7 HOX gene members have either tumor-suppressive (eg, HOXD10) or tumor-promoting effects (eg, HOXB5 and HOXB7), according to their abnormal expression pattern in human gastric tissue.7 Overexpression of HOXB7 predicts poor prognosis of GC and accelerates malignant properties of GC cells, probably through activation of Akt and phosphorylation of MAPK pathways.8,9 However, the specific mechanism by which HOXB7 functions in the cancer progression of GC remains largely unknown.
FAK binds to Src (an intracellular tyrosine kinase) to play a role in cancer progression.10 Src/FAK is activated and subsequently enhanced GC-cell viability, facilitates cell invasion and migration, and accelerated epithelial–mesenchymal transition (EMT).11,12 Prx1 (MHox) and Msx2, known as HOX-containing transcription factors, are regulated by FAK or Src.13,14 However, whether and how Src/FAK are involved in tumor-promoting effects of HOXB7 in human GC cells has scarcely been reported. In the present study, we established HOXB7 silencing in SGC7901 cells and HOXB7 overexpression in SNU1 cells. We confirmed that induction of EMT and activation of Src/FAK singling were the mechanisms by which HOXB7 exerts its oncogenic activities in human GC cells.
Methods
Cell culture and treatment
SGC7901 and SNU1 cells (Cell Bank of Shanghai Biology Institute, Shanghai, China) were cultured in a medium of RPMI-1640 (HyClone), FBS (Thermo Fisher Scientific), and penicillin (Solarbio) at 89:10:1 (v:v) at 37°C under 5% CO2 until in logarithmic growth.
To verify the involvement of Src and FAK in oncogenic activities of HOXB7, after transfection SGC7901 and SNU1 cells were treated with 4 nM PF573228 (869288-64-2; Aladdin) or 10 nM PP2 (EMD Chemicals), and then cultured the same way.
Small interfering RNAs
Three sequences of siRNAs targeting HOXB7 (NM_004502.3) were designed: siHOXB7-1, 5ʹ-GAGUAACUUCCGGAUCUAC-3ʹ (based on position 464–486); siHOXB7-2, 5ʹ-GGAUAUUAUCUACCUGUUC-3ʹ (based on position 1,027–1,049); and siHOXB7-3, 5ʹ-UCAGGAAACUCAAAUCGAA-3ʹ (based on position 827–849). siHOXB7 (5 μL, 100 pmol) was transfected into SGC7901 cells using the Lipofectamine 2000 reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. Meanwhile, aspecific siRNA (siNC) was used as a negative control.
HOXB7 overexpression
The designed primers of the human HOXB7 gene (NCBI NM_004502.3) were 5ʹ- CCCAAGCTTATGAGTTCATTGTATTATGCGAATA-3ʹ (forward) and 5ʹ- CCGGAATTCTCACTCTTCCTCTTCCTCCTCTGCT-3ʹ (reverse), which were cloned into the pCDNA3.1+ (Addgene). HOXB7-expressing and control vectors were generated by DH5a cells (Transgene) as previously described, 1.5 μg of which was transfected into SNU1 cells via Lipofectamine 2000.
Quantitative real-time PCR
After transduction, total RNA from SGC7901 and SNU1 cells was extracted via trizol regent (1596-026; Invitrogen). First-strand cDNA was synthesized using a RevertAid First Stand cDNA-synthesis kit (K1622; Fermentas). mRNA levels of HOXB7, E-cadherin, N-cadherin, vimentin, Twist, and β-catenin were determined using an SYBR green PCR mix (Thermo Fisher Scientific) on an ABI Prism 7300 SDS system (Applied Biosystems). GAPDH served as the internal control for normalization. Primer sequences are listed in Table 1
 | Table 1 Primers used in RT-PCR analysis |
Western blot analysis
Total protein levels in lysis supernatant of SGC7901 and SNU1 were determined with a BCA protein assay kit (Thermo Fisher Scientific). Total protein (25 μg) was separated on 10% and 15% SDS-PAGE. Electrophoretically pure was transferred onto nitrocellulose membranes (Millipore),and incubated with primary antibodies at 4°C overnight, followed by a secondary antibody for another hour at 25°C. Immunoreactive bands were analyzed with an electrochemiluminescence system (GE Healthcare).
Primary antibodies used in our study were: anti-HOXB7 (Abcam ab168466), anti-Src (Cell Signaling Technology [CST] 2108), anti-p-Src-Y416 (CST 2101), anti-FAK (CST 3285), antip-FAK-Y397 (CST 8556), anti-FAK-phospho-Y576+Y577 (CST 3281), GAPDH (CST 5174), anti-E-cadherin (CST 14472), anti-N-cadherin (CST 4061), antivimentin (CST 5741), anti-Twist (Abcam Ab49254), anti-β-catenin (CST 8480). Secondary antibodies used in our study were horseradish peroxidase–conjugated (Beyotime).
Immunofluorescence
After transfection, SGC7901 and SNU1 cells were mounted on slides, fixed with 4% formaldehyde for 30 minutes, and then permeabilized using 0.5% Triton X-100 (T8200; Solarbio) for 10min. After blocking with 1% BSA (A8010; Solarbio) for 30 minutes, cells were incubated with a mouse monoclonal anti-F-actin antibody (Ab205; Abcam) at 4°C overnight, followed by a secondary antibody (Beyotime) for 30 minutes at 37°C in the dark. DAPI (C1002; Beyotime) was used for cell-nuclei staining. Confocal laser-scanning microscopy (FV1000, Olympus) was used for location detection.
Coimmunoprecipitation assay
To study whether HOXB7 was associated with Scr and FAK, after transfection 100 μg total protein in cell-lysis supernatant was added to protein G-agarose beads (Roche) and then immunoprecipitated with anti-HOXB7 (Abcam), anti-Src (Millipore), anti-FAK (Abcam), or control IgG antibody overnight at 4°C. Protein levels of HOXB7, Scr, and FAK in immunocomplexes precipitated were assessed using Western blot as mentioned earlier. Meanwhile, the same amount of protein in each group was reserved for input control.
Cell-proliferation and -apoptosis assay
Proliferation of SGC7901 and SNU1 cells was assessed using CCK8. Briefly, cells (3×103/well) were cultured at 37°C overnight in a 96-well culture plate, and after treatment assay plates were incubated with CCK8 working solution (10 mL/well) and serum-free cultured medium (90 mL/well). After incubation for 0, 12, 24, 48, and 72 hours, OD450 values were measured with a microplate reader (Bio-Rad).
Apoptosis of SGC7901 and SNU1 cells was assessed using an annexin V–FITC apoptosis-detection kit (Beyotime). Briefly, cells (3×105/well) were cultured at 37°C for 24 hours in a six-well culture plate, and after treatment cells (5×104–105) were stained with 5 μL annexin V–FITC for 15 minutes in the dark at 4°C, followed by 5 μL PI for another 15 minutes. Fluorescence microscopy (BD,Biosciences) was used for analysis. Early-apoptosis cells were annexin+–PI–, and presented in the lower-right quadrant.
Cell-migration and -invasion assay
For the migration assay, SGC7901 and SNU1 cells (5×104) were seeded in the upper chamber in 0.2 mL serum-free RPMI 1640, whereas 0.7 mL RPMI 1640 with 10% FBS was added to the lower chamber and cultured for 24 hours. For the invasion assay, membranes were precoated with Matrigel (80 μL 356234; Corning) to simulate a matrix barrier, and cells were treated as mentioned previously and then cultured for 48 hours. After removal of unmigrated cells in the upper chamber, cells passing through the membrane were formaldehyde (4%)-fixed and crystal violet (0.05%)-stained and numbers counted using light microscopy (100× magnification).
Wound-healing assay
After treatment, SGC7901 and SNU1 cells (8×105) were plated in a culture dish (35 mm2) and grown to confluence. A 10 μL pipette tip (Gilson) was used to create a linear wound through the cell monolayer. After that, cells were washed with PBS and then cultured in complete medium. Photographs were taken at 0, 24, and 48 hours postwounding (100× magnification).
Statistical analysis
Mean values and SEM were calculated from triplicate experiments. Data are given as means ± SEM. Student’s t-test was used for comparisons between two groups.
Results
Roles of HOXB7 in cancer progression of human GC cells
To study the carcinogenesis of HOXB7 in GC in vitro, the GC cell lines SGC7901 and SNU1 were transfected with siRNA-HOXB7 and pCDNA3.1-HOXB7, respectively. Malignant progress of human GC cells was assessed by measuring cell viability, cell-cycle progression, apoptosis, migration, invasion, and wound-healing process.
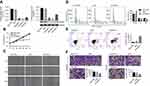
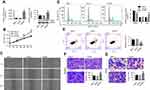
As shown in Figure 1A, mRNA and protein expression of HOXB7 were obviously reduced in siRNA-HOXB7 groups (siHOXB7-1, siHOXB7-2, and siHOXB7-3), with the maximum effect being obtained in the siHOXB7-2 group when compared withthe siNC group. As such, we chose siHOXB7-2 for the following experiments. Figure 2A shows that HOXB7 overexpression significantly enhanced mRNA and protein levels of HOXB7 when compared with the control vector, demonstrating successful establishment of HOXB7 overexpression in SNU1 cells.
Our results showed that siHOXB7-2 significantly decreased cell proliferation (Figure 1B), inhibited the wound-healing process (Figure 1C), retarded the cell cycle at the G0/G1 phase (Figure 1D), promoted cell apoptosis (Figure 1E), and reduced cell migration and invasion (Figure 1, F and G) when compared with siNC, suggesting that knockdown of HOXB7 inhibited cancer progression of SGC7901 cells. Conversely, HOXB7 overexpression obviously increased cell proliferation (Figure 2B), promoted the wound-healing process (Figure 2C), induced cell-cycle progression (Figure 2D), inhibited cell apoptosis (Figure 2E), and enhanced cell migration and invasion (Figure 2F, G) compared to the control, suggesting that upregulation of HOXB7 contributed to the cancer progression of SNU1 cells.
HOXB7 regulated EMT in human GC cells
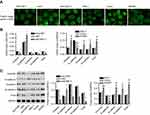
Cytoskeleton rearrangement is fundamental for cells to obtain enhanced properties of motility and invasiveness, and is also recognized as a feature of the mesenchymal phenotype. To study whether HOXB7 induced EMT in human GC cells, F-actin cytoskeleton of cells transfected with siHOXB7-2 or HOXB7 overexpression were observed. Images from immunocytofluorescence showed that F-actin filaments spread over the entire cytoplasm, and the cell boundary was clear without detectable flamellipodia and filopodia in siHOXB7-2 when compared with siNC, displaying a stable F-actin cytoskeleton with adhesive contacts of junctions belonging to epithelial-like cells (Figure 3A). In contrast, HOXB7 overexpression induced enrichment of F-actin filaments on cell membrane and an increase in lamellipodia and filopodia on the cell boundary, which suggested an unstable F-actin cytoskeleton with loosened connectivity between filaments belonging to interstitial-like cells (Figure 3A).
To substantiate the roles of HOXB7 in regulating EMT, after transfection expression of several EMT markers (E-cadherin, N-cadherin, vimentin, Twist, and β-catenin) were assessed. Figure 3, B and C shows that siHOXB7-2 significantly decreased vimentin, β-catenin, N-cadherin, and Twist (mesenchymal markers) while increasing E-cadherin (epithelial marker) when compared with siNC; however, HOXB7 overexpression resulted in the opposite, confirming a promoted effect of HOXB7 on EMT.
HOXB7 was associated with Src and FAK in human GC cells
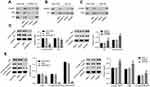
Coimmunoprecipitation was performed to study the interaction among HOXB7, FAK, and Src in human GC cells. As shown in Figure 4A, when protein samples were precipitated using HOXB7 antibody, HOXB7, FAK, and Src were significantly reduced by siHOXB7-2 and enhanced by HOXB7. Figure 4B shows that when protein samples were precipitated using Src antibody, Src was significantly reduced by siHOXB7-2 and enhanced in the HOXB7 group, suggesting that Src was associated with HOXB7 in human GC cells. Figure 4C shows that when protein samples were precipitated using FAK antibody, FAK was significantly reduced by siHOXB7-2 and enhanced by HOXB7, suggesting that FAK was associated with HOXB7 in human GC cells.
Effect of HOXB7 on phosphorylation of Src and FAK within human GC cells
To study the effect of HOXB7 on the activation of Src and FAK, after transfection protein levels of total Src, p-Src (Y416), total FAK, p-FAK (Y397), and p-FAK (Y576/577) were assessed. Figure 4, D and E indicates that siHOXB7-2 significantly decreased total Src, p-Src (Y416), total FAK, p-FAK (Y397), and p-FAK (Y576/577) compared to siNC, suggesting that siHOXB7-2 inhibited the expression of Src and FAK, retarding their activation. Conversely, HOXB7 overexpression exerted an opposite effect.
HOXB7 exerted oncogenic activities via activating Src and FAK in human GC cells
To study whether activation of Src and FAK were the mechanism by which HOXB7 functioned in the cancer progression of human GC cells, SNU1 cells transfected with HOXB7 were treated with PP2 (a specific Src inhibitor) or PF573228 (an FAK inhibitor), and then protein levels of total Src, p-Src (Y416), total FAK, p-FAK (Y397), and p-FAK Y576/577) were assessed. In this part, cancer progression was assessed by measuring cell migration and invasion and the wound-healing process. As shown in Figure 5, A and B, we confirmed the inhibitory effect of PF573228 and PP2 on the activation of Src and FAK in SUN1 cells, as evidenced by remarkably reduced expression of p-Src (Y416), p-FAK (Y397), and p-FAK (Y576/577) when compared with their corresponding controls. PF573228 suppressed the activation of Src and FAK (Figure 5A), reduced migration and invasion (Figure 5C and D) and retarded the wound-healing process (Figure 5G) in SNU1 cells transfected with HOXB7 overexpression. Interestingly, similar changes were also observed with additional PP2 treatment (Figure 5, B, E, F, and H), suggesting the direct involvement of Src and FAK in carcinogenesis of HOXB7 in human GC cells.
Discussion
HOXB7 contributes to cancer progression in GC cells.8,9 In this study, we confirmed the tumor-suppressive effects of knockdown of HOXB7 in GC cells, as evidenced by inhibited cell proliferation (Figure 1B), arrested cell cycle (Figure 1D), enhanced cell apoptosis (Figure 1E), reduced cell migration and invasion (Figure 1F, G), and inhibited wound-healing abilities (Figure 1C). Conversely, overexpression of HOXB7 in SNU1 cells led to the opposite (Figure 2), demonstrating the tumor-promoting effect of HOXB7 in human GC cells.
EMT is crucial for human GC cells to obtain migratory and invasive abilities.15 Our data suggested that HOXB7 decreased the expression of E-cadherin (epithelial marker); however, it increased the expression of N-cadherin and vimentin (mesenchymal markers), which was consistent with another study.8 Also, in this study the promotion effect of HOXB7 on other mesenchymal markers (such as β-catenin and Twist) was also observed in GC cells (Figure 3B and C), substantiating the promoting effect of HOXB7 on EMT at a molecular level. Evidence suggests that F-actin remodeling is an upstream regulator of EMT in metastatic cancer cells, and thus the effect of HOXB7 on F-actin remodeling in GC cells was assessed.16 Our data firstly suggested that HOXB7 promoted SNU1 cells to switch to mesenchymal form by disorganization of F-actin architecture (Figure 3A), implying that HOXB7 had a strong effect on EMT phenotypes of GC cells.
Src and FAK interact physically and functionally and regulate malignant responses of GC cells.10–12,17 FAK is phosphorylated directly by Src in its Y576 and Y577 forms.10 Msx2, a HOX-containing transcription factor, increases the activity of Src and its downstream FAK, and subsequently induces EMT in mouse mammary epithelial cells.13 Herein, we found that HOXB7 was closely associated with Src and FAK in human GC cells (Figure 4, A–C), and our data confirmed that siHOXB7 prevented the activation of Src and FAK signaling through reducing the expression of p-Src (Y416), p-FAK (Y397), and p-FAK (Y576/577); however, HOXB7 resulted in the opposite (Figure 4, D and E), demonstrating the direct regulation of HOXB7 on Src/FAK signaling in human GC cells.
To substantiate whether Src/FAK signaling was the mechanism by which HOXB7 regulated cancer progression of human GC in vitro, GC cells with HOXB7 overexpression were treated with PP2 (a specific Src inhibitor18) and PF-573228 (an FAK inhibitor that impairs FAK on the activation loop induced by Src)19. Our data suggested that HOXB7-induced malignant progress of GC cells and activation of the Src–FAK pathway were significantly reversed by additional PP2 or PF573228 treatment (Figure 5), substantiating that there was an activation loop between Src and FAK, which was the mechanism whereby HOXB7 exerted its oncogenic activities in human GC cells.
In conclusion, HOXB7 facilitated EMT and promoted the acquisition of mesenchymal properties in human GC cells. Mechanistically, HOXB7 promoted oncogenic activities of human GC cells via the Src–FAK pathway.
Institutional animal care and use committee statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Acknowledgments
This study was supported by grant 81502027 from the National Natural Science Foundation of China and grant 17411963200 from the Shanghai Committee of Science and Technology Funds.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Goetze OT, Al-Batran SE, Chevallay M, Mönig S. Multimodal therapy in locally advanced gastric cancer. Updates Surg. 2018;70(2):173–179. doi:10.1007/s13304-018-0539-z
2. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi:10.2147/CMAR.S149619
3. Wesołowska M, Pawlik P, Jagodziński PP. The clinicopathologic significance of estrogen receptors in human gastric carcinoma. Biomed Pharmacother. 2016;83:314–322. doi:10.1016/j.biopha.2016.06.048
4. Giampieri R, Del Prete M, Cantini L, et al. Optimal management of resected gastric cancer. Cancer Manag Res. 2018;10:1605–1618. doi:10.2147/CMAR.S151552
5. Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: current status of diagnosis and treatment. Cancers. 2013;5(1):48–63. doi:10.3390/cancers5010048
6. Tschopp P, Duboule D. A genetic approach to the transcriptional regulation of hox gene clusters. Annu Rev Genet. 2011;45(1):145–166. doi:10.1146/annurev-genet-102209-163429
7. Joo MK, Park -J-J, Chun HJ. Impact of homeobox genes in gastrointestinal cancer. World J Gastroenterol. 2016;22(37):8247–8256. doi:10.3748/wjg.v22.i37.8247
8. Cai JQ, Xu XW, Mou YP, Chen K, Pan Y, Wu D. Upregulation of HOXB7 promotes the tumorigenesis and progression of gastric cancer and correlates with clinical characteristics. Tumour Biol. 2016;37(2):1641–1650. doi:10.1007/s13277-015-3948-3
9. He X, Liu Z, Xia Y, et al. HOXB7 overexpression promotes cell proliferation and correlates with poor prognosis in gastric cancer patients by inducing expression of both AKT and MARKs. Oncotarget. 2017;8(1):1247–1261. doi:10.18632/oncotarget.13604
10. Bolós V, Gasent JM, Lópeztarruella S, Grande E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther. 2010;2010(default):83–97. doi:10.2147/OTT.S6909
11. Lai IR, Chu PY, Lin HS, et al. Phosphorylation of focal adhesion kinase at Tyr397 in gastric carcinomas and its clinical significance. Am J Pathol. 2010;177(4):1629–1637. doi:10.2353/ajpath.2010.100172
12. Yu Z, Li Z, Wang C, et al. Oncostatin M receptor, positively regulated by SP1, promotes gastric cancer growth and metastasis upon treatment with Oncostatin M. Gastric Cancer. 2019. doi:10.1007/s10120-019-00934-y
13. Di BM, Ginsburg E, Plant J, Strizzi L, Salomon DS, Vonderhaar BK. Msx2 induces epithelial-mesenchymal transition in mouse mammary epithelial cells through upregulation of Cripto-1. J Cell Physiol. 2010;219(3):659–666.
14. Mckean DM, Sisbarro L, Ilic D, et al. FAK induces expression of Prx1 to promote tenascin-C. J Cell Biol. 2003;161(2):393–402. doi:10.1083/jcb.200302126
15. Xue Z, Wu X, Chen X, et al. Mesenchymal stem cells promote epithelial to mesenchymal transition and metastasis in gastric cancer though paracrine cues and close physical contact. Journal of Cellular Biochemistry. 2015;116(4):618–627. doi:10.1002/jcb.25013
16. Shankar J, Nabi IR. Actin cytoskeleton regulation of epithelial mesenchymal transition in metastatic cancer cells. Plos One. 2015;10(3):e0119954. doi:10.1371/journal.pone.0119954
17. Okamoto W, Okamoto I, Yoshida T, et al. Identification of c-Src as a potential therapeutic target for gastric cancer and of MET activation as a cause of resistance to c-Src inhibition. Mol Cancer Ther. 2010;9(5):1188–1197. doi:10.1158/1535-7163.MCT-10-0002
18. Aponte M, Jiang W, Lakkis M, et al. Activation of platelet-activating factor receptor and pleiotropic effects on tyrosine phospho-EGFR/Src/FAK/paxillin in ovarian cancer. Cancer Research. 2008;68(14):5839–5848. doi:10.1158/0008-5472.CAN-07-5771
19. Kong DB, Chen F, Sima N. Focal adhesion kinases crucially regulate TGFβ-induced migration and invasion of bladder cancer cells via Src kinase and E-cadherin. Onco Targets Ther. 2017;10:1783–1792. doi:10.2147/OTT.S122463
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.