Back to Journals » Patient Preference and Adherence » Volume 12
Home administration of filgrastim (Nivestim™) in primary prophylaxis of chemotherapy-induced febrile neutropenia
Authors Otremba B , Hielscher C, Petersen V, Petrik C
Received 25 May 2018
Accepted for publication 4 September 2018
Published 16 October 2018 Volume 2018:12 Pages 2179—2186
DOI https://doi.org/10.2147/PPA.S168029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Burkhard Otremba,1 Carsten Hielscher,2 Volker Petersen,3 Christian Petrik4
1Oncological Practice Oldenburg/Delmenhorst, Oldenburg, Germany; 2Gynecology Competence Center Stralsund, Stralsund, Germany; 3Practice for Hematology and Oncology, Heidenheim, Germany; 4Pfizer Inc, Berlin, Germany
Background: The granulocyte-colony stimulating factor (G-CSF) biosimilar filgrastim (Nivestim™) reduces the duration and severity of neutropenia and the frequency of occurrence of febrile neutropenia (FN). Administration of this biosimilar filgrastim and the patient population receiving it at home have not been sufficiently documented in day-to-day medical practice. Insight into home administration may help optimize the management of FN in this setting, potentially at a reduced cost and patient burden vs hospital administration.
Materials and methods: This was a prospective, non-interventional, non-comparative, multisite study involving 171 patients across 29 sites treated with at least one dose of filgrastim. Mean age was 59.3 years, and most patients were female and G-CSF-naïve. The data collected originated from paper-based patient questionnaires and routine documentation by the treating physicians. The primary endpoint was the characterization of patients treated with filgrastim. Secondary endpoints were satisfaction with filgrastim, effectiveness, safety and tolerability, and compliance with prescription.
Results: Most patients had solid tumors (95.9%), mainly located in the breast, while 4.7% had malignant hematological disease. Solid tumors were recorded as grade 1 (7.9%), grade 2 (28.0%), grade 3 (45.7%), and grade 4 (3.0%), and the majority of patients classified at TNM Stages I and II. Many patients (71.0%) could self-inject filgrastim and 72.2% found the handling instructions “extremely straightforward and easy to understand” at least once. Nearly all (99.4%) patients found the syringes “easy to use” at least once and 91.7% were willing to continue home administration. The mean patient satisfaction score for home administration was 1.9±0.9, ranging from 1 (very satisfied) to 6 (absolutely dissatisfied). No cases of neutropenia were observed and only one event of FN occurred.
Conclusion: Home-based prophylaxis for FN with filgrastim was found to be effective, well tolerated, and well received by patients (ClinicalTrials.gov Identifier: NCT02956967).
Keywords: neutropenia, filgrastim, biosimilar, oncology
Introduction
Neutropenia is a common complication that frequently arises from chemotherapy in the treatment of cancer. It is a major risk factor for infection-related morbidity and, in some circumstances, can be life-threatening.1 There is the possibility that neutropenia may progress to febrile neutropenia (FN), defined by the European Society of Medical Oncology as “an oral temperature of >38.3°C or two consecutive readings of >38.0°C for 2 hours and an absolute neutrophil count (ANC) of <0.5×109/L, or expected to fall below 0.5×109/L”.2 FN is a severe neutropenic complication that places patients at high risk of increased mortality and is considered to be an oncologic emergency requiring hospitalization.3 The occurrence of FN is both dangerous and problematic as it frequently involves dose reductions to the chemotherapeutic regimen, and may delay treatment or impact the success of treatment, especially when treatment intent is curative or to prolong survival.1,4 Ultimately, FN undermines the patient’s response to chemotherapy or may jeopardize the success of any anti-neoplastic treatment.
In Europe, prophylactic treatment with granulocyte-colony stimulating factors (G-CSFs) is available, which promotes the proliferation, differentiation, and maturation of neutrophils. Current guidelines recommend the use of G-CSF as primary prophylaxis of chemotherapy-induced FN when the overall risk of FN among patients with non-myeloid malignancies receiving myelosuppressive chemotherapy is ≥20.0%.1,2,5 G-CSF lessens the risk of infectious complications and the consequent loss of therapeutic options, which may result from neutropenia.
The G-CSF biosimilar filgrastim (Nivestim™, Pfizer Europe MA EEIG, Brussels, Belgium) is licensed for the prophylaxis and treatment of FN, and is successfully used in oncology to reduce the duration and severity of neutropenia.1,6–8 Three large-scale studies have shown primary prophylaxis with G-CSF to significantly reduce the incidence of FN in cytotoxic chemotherapy.9–11
To date, home administration of filgrastim and the patient population typically receiving it at home have not been sufficiently documented in day-to-day medical practice. Gaining insight into the home administration of filgrastim is important as it is likely to inform how to optimize prophylaxis and treatment of FN within this environment, potentially with reduced patient burden vs hospital administration.
The aim of this study was to observe patients at risk of FN treated with filgrastim at home (as opposed to in the hospital) as primary prophylaxis against chemotherapy-induced neutropenia. This non-interventional study was conducted in Germany and was designed to expand the knowledge about the patient population taking filgrastim at home; the training and convenience concerning the handling of syringes; the safety and effectiveness of the medication; and the incidence, duration, and intensity of FN with filgrastim.
Materials and methods
Study design
This was a prospective, non-interventional, non-comparative, multisite study that observed patients in the home setting receiving filgrastim for primary prophylaxis against chemotherapy-induced FN.
Medical care with filgrastim followed the routine clinical practice of the respective participating study site. According to product information12 and routine practice, the dosage should consist of 0.5 MU (5 μg)/kg/day subcutaneously, with the first injection administered no sooner than 24 hours after cytotoxic chemotherapy and daily therapy continuing until the post-nadir neutrophil granulocyte count returns to the normal range.
A paper questionnaire concerning home treatment was provided to patients for completion for each treatment cycle with filgrastim. All data collected originated from the routine documentation from the treating physicians and from paper-based patient questionnaires. Data were collected at the enrollment visit and, subsequently, at visits after completion of each cycle of chemotherapy along with daily filgrastim treatment, until a total of three cycles were recorded. The maximum observation period was 6 months for each patient. Permanent discontinuation of filgrastim treatment mandated withdrawal from the study. The enrollment period for the study ran from 1st July 2015 until 30th September 2016. The study was reviewed and approved by the Freiburg Ethics Commission International, an independent ethics committee.
Patients
Patients were ≥18 years of age with a solid tumor or a malignant hematological tumor, had been prescribed cytotoxic chemotherapy, and were starting primary prophylactic treatment using filgrastim either to shorten the duration of neutropenia or to prevent the occurrence of chemotherapy-induced FN.
Patients also were either G-CSF-naïve or had not been treated with G-CSF in the 3 months before enrollment and were excluded if they had chronic myeloid leukemia or myelodysplastic syndrome, were hypersensitive to one of the excipients of the drug, were not undergoing chemotherapy, or were being treated with G-CSF curatively or as secondary prophylaxis. All patients provided written informed consent to participate.
Endpoints and outcome measures
The primary endpoint was the characterization of patients treated with filgrastim, which included their demography, medical history, type of malignant disease (tumor), chemotherapy data, and clinical and laboratory data preceding the first treatment with filgrastim.
The secondary endpoints were satisfaction with filgrastim, effectiveness, safety and tolerability of filgrastim, and compliance with prescription. Patient satisfaction was assessed by questions concerning the training for home administration, the person performing the injection, evaluation of filgrastim packaging, handling instructions, manageability of syringes, and patients’ overall rating of satisfaction with filgrastim. Effectiveness was evaluated by ANC, incidence of neutropenia and FN, and frequency of infections. The tolerability and safety of filgrastim were assessed by the patients’ rating of tolerability at the injection site, painfulness of the needle insertion into the skin, overall tolerability of the subcutaneous injection, and adverse drug reactions. Compliance with prescription was evaluated by the investigators’ assessment of whether filgrastim was administered as prescribed (according to the treatment data recorded in the patients’ questionnaires). Reasons for not using filgrastim as prescribed were also documented.
The physicians’ documentation was evaluated for the primary endpoint (“characterization of patients treated with filgrastim at home”) and the secondary endpoints regarding effectiveness of filgrastim and compliance with the prescription. The patients’ questionnaires were evaluated for the secondary endpoint “patients’ satisfaction with filgrastim packaging, manual and handling”. The secondary endpoint “tolerability and safety of filgrastim” was assessed by the physicians’ records and patients’ ratings on questionnaires.
Adverse events (AEs) were collected and recorded throughout the study and coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 19.0. Any AEs reported during the study were classified according to MedDRA system organ class and preferred term and/or based on Common Terminology Criteria for Adverse Events grading (version 4.0), causality to filgrastim according to the reporter, and seriousness.
Statistical analysis
Descriptive statistics summarized continuous variables, and means of counts and percentages summarized the categorical variables.
Results
Of 180 screened patients, 171 across 29 sites were treated with at least one dose of filgrastim and constituted the full analysis set (FAS). Considering the descriptive nature of this study, this number of patients was regarded as adequate for the collection of data representative for everyday home application. Of these, 123 patients (71.9%) completed without premature discontinuation, ie, they completed at least three cycles. Of the 48 patients (28.1%) who did not complete the study, the two most common reasons for not completing three treatment cycles were termination of treatment with filgrastim (physician decision, 18 patients, 10.5%) and termination of chemotherapy for a reason not related to neutropenia (10 patients, 5.8%) (Table 1). Possible reasons for discontinuation of chemotherapy could include FN, neutropenia, or a reason not related to neutropenia. The possible reasons for filgrastim discontinuation could include poor tolerability or effectiveness, physician decision, requested by patient, or the prescription of a different product. Poor effectiveness was never selected as the primary reason for discontinuation of filgrastim. There were three patients whose primary reason for study discontinuation was an AE. The AEs were leukopenia (which may indicate poor efficacy), back pain, and chest pain. Patients in the FAS were observed for a mean of 55.9 days (SD±23.4 days); the minimum duration was 2 days and maximum duration was 162 days.
  | Table 1 Reasons for treatment discontinuation |
Characterization of patients
Patient characteristics are summarized in Table 2. The mean age was 59.3±11.4 years (range 32–85; median 60.0 years). Most patients were female and G-CSF-naïve at enrollment. Among the female patients, 106 (74.1%) were ≤65 years of age and 37 (25.9%) were aged >65 years. Among the male patients, 13 (46.4%) were ≤65 years of age and 15 (53.6%) were aged >65 years. All eight patients pretreated with G-CSF were females.
  | Table 2 Patient characteristics at baseline |
Prior to receiving filgrastim therapy as part of this study, the mean body mass index was 26.0±5.5 kg/m2 (overweight range), mean systolic blood pressure was 133.7±13.7 mmHg, mean diastolic blood pressure was 82.1±9.7 mmHg, mean neutrophil count was 3.4±3.0 (109/L), and mean C-reactive protein was 3.9±3.9 (109/L). The majority of patients had solid tumors (164 patients, 95.9%), which were mostly located in the breast (Table 2), while eight (4.7%) patients had malignant hematological disease. Tumors were recorded as grade 1 (low grade, well differentiated, 7.9%), grade 2 (intermediate grade, moderately differentiated, 28.0%), grade 3 (high grade, poorly differentiated, 45.7%), grade 4 (high grade, undifferentiated, 3.0%), missing (15.2%), and the majority of patients classified at TNM Stages I and II. The mean time since diagnosis of solid tumor was 1.0±2.9 years with a minimum of 0 years and a maximum of 27 years.
Fifty-one (29.8%) patients in the FAS had received prior chemotherapy or non-chemotherapeutic antineoplastic therapy. Ninety-five (55.6%) patients received chemotherapeutic/antineoplastic treatment as adjuvant therapy and 52 (30.4%) patients received it as curative therapy. The most frequent regimens for chemotherapeutic/antineoplastic treatment were, in the first cycle, a combination of cyclophosphamide + epirubicin (72 [42.1%] patients, N=171), a combination of cyclophosphamide + docetaxel + epirubicin (10 [5.8%] patients), and a combination of carboplatin + paclitaxel (5 [2.9%] patients). Only four (2.3%) patients received concomitant radiotherapy in at least one cycle of chemotherapy and only seven (4.1%) patients received concomitant prophylaxis with antibiotics in at least one cycle of chemotherapy.
In the FAS, 73 (42.7%) patients had previous or ongoing significant comorbidities (Table 3). The most frequent comorbidities, in 55 (32.2%) patients, were cardiovascular diseases, occurring in more than half of patients >65 years of age and in less than a quarter aged ≤65 years. One other factor aggravating FN, ie, mucositis, was reported for one patient.
Patient satisfaction
Satisfaction was evaluated from the patient questionnaires for 169 patients. A total of 95 (56.2%) patients (or their supporting persons) received training in the handling of syringes in at least one cycle of treatment, which was rated as useful by 84.2% of patients (15 [15.8%] patients missing). Most patients performed the injections themselves at least once (71.0%), followed by relatives (24.9%), and oncologists or the oncologists’ assistants (18.9%). Occasionally, injections were administered by general practitioners, internists or similar (3.6%), employees of nursing services at home visits (2.4%), and hospital staff (2.4%).
Most patients (144 [85.2%]) rated the packaging as “easy” to handle at least once, whereas only a few (4 [2.4%]) rated it as “complicated” at least once.
Following training, the majority of patients (122 [72.2%]) rated the handling instructions as “extremely straightforward and easy to understand” at least once, and 29 (17.2%) found, at least once, that the product could be used easily without consulting the instructions for use. Only 18 (10.7%) patients had to re-read the instructions several times before they could use the product, and only two (1.2%) patients rated the handling instructions as “incomprehensible” according to at least one questionnaire.
Almost all patients (99.4%) found the syringes “easy to use” at least once, whereas no patient ever rated the use of syringes as “complicated”. A total of 155 (91.7%) patients were willing to continue with home administration of filgrastim.
The average patient satisfaction score was 1.9±0.9, based on a scale ranging from 1 (very satisfied) to 6 (absolutely dissatisfied), indicating that patients were satisfied with home administration, consistent with the large proportion of patients willing to continue home administration. Minimum and maximum values were 1 and 6, respectively, indicating a broad interpatient variability in rating.
Effectiveness
Effectiveness was evaluated based on the ANC nadir and ANC at the end of the treatment with filgrastim in each cycle, the incidence of neutropenia and FN, and the frequency of infections.
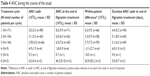
Mean ANC increases between 3.3 and 6.2×109/L were observed during filgrastim treatment in the first three cycles (Table 4). No cases of neutropenia were observed and only one event of FN occurred (in a female patient aged ≤65 years and G-CSF-naïve). Fifteen events of infection were recorded in 14 patients. Twelve infections in 11 patients were non-serious and three were serious infections in three patients. Non-serious infections were bronchitis (n=2), upper respiratory tract infection (n=2), cystitis, infection (includes original terms “unclear infection”, “infection with unclear focus”, “infection without clear focus”), herpes virus, influenza, lung infection, oral candidiasis, skin infection, and vulvovaginal mycotic infection (all n=1 each). Serious infections were defined as any serious AEs within the MedDRA system organ class “infections and infestations”, and included diverticulitis (n=1) and infection (n=2). Infections occurred mainly in the subgroup of patients aged ≤65 years (n=119), ie, 13 events in 12 (10.1%) patients. In patients aged >65 years (n=52), two infections in two (3.8%) patients were recorded. Infections occurred only in the subgroup of G-CSF-naïve patients (n=163).
Safety and tolerability
Many patients rated the tolerability of filgrastim at the injection site as “very good” (107 [63.3%]) or “good” (106 [62.7%]) at least once (Table 5). For 53 (31.4%) patients, needle insertion into the skin was pain-free, while 128 (75.7%) patients felt only “a small prick” and 42 (24.9%) a “light stitch” according to at least one questionnaire. The overall tolerability of the injection was assessed as “good” at least once by 117 (69.2%) patients and as “satisfactory” at least once by 20 (11.8%) patients.
Adverse drug reactions (ADRs) recorded from the FAS are summarized in Table 6. Forty ADRs in 34 (19.9%) patients were recorded. Five ADRs in five (2.9%) patients were regarded as serious (SADRs); these were FN, leukocytosis, chest pain, bone pain, and general pain. Eight ADRs led to discontinuation of filgrastim but no AE or ADR had a fatal outcome.
  | Table 6 Summary of ADRs |
Compliance
Most patients received injections according to the prescription. For the first treatment cycle, only 10 (5.8%) patients did not apply filgrastim according to the prescription. The reason for non-compliance is not known for one patient.
The reasons for prescription deviation included the following: forgetting to inject – five (2.9%) patients; patient discontinued treatment on investigator advice – two (1.2%) patients; the patient independently decided to discontinue treatment with filgrastim – two (1.2%) patients.
Discussion
The aim of this observational study in a German cohort was to characterize the patient population who receive filgrastim in a home-administration setting and to provide an insight into the patients’ experience and their evaluation of manageability and tolerability.
The observed patient population consisted of 171 patients, the majority of whom were aged ≤65 years, primarily female, and predominantly G-CSF-naïve. Most patients had solid tumors, with the most frequent being breast cancer. The largest percentage of solid tumors was recorded as grade 3, but there was great diversity in the stages of cancer experienced. A large proportion of patients had not received any chemotherapy or non-chemotherapeutic antineoplastic therapy prior to inclusion.
Overall, 42.7% of participants had previous or ongoing significant comorbidities, the most frequent of which was cardiovascular disease. Documenting the presence of comorbidities during treatment of chemotherapy-associated FN is important because the literature suggests that the frequency of comorbidities has an impact on the rate of mortality and the length and costs of hospitalization.3 Kuderer et al noted that cancer patients with FN had a 2.6% risk of mortality where there were no major comorbidities. However, this risk increased to 10.3% in those with one major comorbidity and to ≥21.4% in those with more than one major comorbidity.3
Most patients could administer the injections themselves, found the packaging easy to handle, and were willing to continue home administration. The mean patient satisfaction score was 1.9±0.9, indicating that, on average, patients were satisfied with home administration.
Filgrastim was found to be an effective therapy in this setting, with only one event of FN and a low incidence of infections. The ANC increases are consistent with an effective treatment of neutropenia, although the outcome varied considerably between patients.
Adverse events may be related to the patients’ underlying disease or the chemotherapeutic/antineoplastic treatment they were receiving. Considering the possible variety of underlying diseases and chemotherapeutic/antineoplastic agents, and the absence of a control group, safety was focused on ADRs rather than AEs. The low number of ADRs confirmed the safety of filgrastim in home administration without new safety signals. The overall rating of tolerability being “good” is consistent with the relatively low number of observed SADRs, ADRs, and discontinuations, and with previous studies demonstrating the favorable safety and tolerability profile of filgrastim.13
Although costs were not assessed in the present study, the use of G-CSFs for primary prophylaxis of FN in chemotherapy patients has the potential to reduce direct medical costs associated with FN by reducing rates of hospitalization, serious infections, and the use of broad-spectrum antibiotics. In a recent real-world study comparing a filgrastim biosimilar with reference filgrastim in patients with non-myeloid cancers undergoing chemotherapy, for example, the incidence of FN was equivalent between groups, but with substantial costs for the small number of patients who did develop FN.14 Another study of a filgrastim biosimilar in patients with soft-tissue sarcoma receiving chemotherapy found it as effective as originator filgrastim in primary prophylaxis, reducing costs and the need for hospitalization.15 Various studies have also compared the cost-effectiveness of short-acting and long-acting G-CSFs. Some studies suggest that long-acting G-CSFs such as pegfilgrastim can be cost-effective and cost-saving in situations where maintaining treatment dose intensity is thought to be necessary to provide optimal long-term disease control.16 A US real-world study examined the comparative cost using an interactive model to simulate the relative cost for one chemotherapy cycle from 1 to 14 days in patients with non-myeloid malignancies treated with myelosuppressive chemotherapy. The study concluded that short-acting G-CSF products are used fewer than 14 days per chemotherapy cycle in clinical practice and, under these circumstances, are more cost-efficient than pegfilgrastim.17 A simulation study also demonstrated the potential cost savings of a biosimilar filgrastim compared with originator filgrastim and long-acting pegfilgrastim across the European G5 countries, finding biosimilar filgrastim to offer the greatest cost savings of the three treatments.18
Switching from long-acting to short-acting G-CSFs was associated with a cost saving to the annual US health-plan budget of over $5.5 million or more than $5.5 per member per year following an overall 7.6% utilization shift from long-acting to short-acting products.19 If short-acting G-CSFs can be administered effectively in a home setting, then further cost savings may be achievable, and this possibly warrants further investigation.
Limitations
Limitations of the present study include the low number of patients in some of the subgroups (particularly the subgroup pretreated with G-CSF), which limits the informative value of the respective data. The majority of patients were females and suffering from gynecological tumors, mostly of the breast and, therefore, receiving adjuvant treatment. This might also limit the value of the data, as these patients may be different from patients with more advanced malignant diseases.
The number of patients was low for treatment cycles 4–6, due to a large proportion already completing the documentation required in the first three cycles of their treatment, making comparisons with cycles 1–3 difficult. A further limitation is the use of ANC as a measure of effectiveness. In this study, the ANC was collected at its trough value during each cycle (ANC nadir) and at the end of each treatment cycle. As the study relied on routine data provided by the sites, the ANC may not have been monitored as frequently as required for the precise determination of the nadir. Therefore, this effectiveness variable should be interpreted with caution.
Conclusion
Collecting information on the application of filgrastim at home expands the knowledge base on the patient population and provides information on training and convenience concerning the handling of syringes; safety and effectiveness of medication; and the incidence, duration, and intensity of FN. In this study, home-based filgrastim treatment was effective, well tolerated, and well received by patients.
Acknowledgments
This study was funded by Hospira Inc., which was acquired by Pfizer Inc in September 2015. Medical writing support was provided by David Sunter, PhD, of Engage Scientific Solutions and was funded by Pfizer Inc.
Disclosure
B Otremba has nothing to disclose regarding financial relationships or other conflicts of interest; C Hielscher has received honoraria from Pfizer, Roche and Celgene; V Petersen has nothing to disclose regarding financial relationships or other conflicts of interest; C Petrik is an employee of Pfizer Inc and holds stock in Pfizer.
References
Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32. | ||
Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2016;27(suppl 5):v111–v118. | ||
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266. | ||
Wang L, Baser O, Kutikova L, Page JH, Barron R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2015;23(11):3131–3140. | ||
Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2015;33(28):3199–3212. | ||
Crawford J, Armitage J, Balducci L, et al. Myeloid growth factors. J Natl Compr Canc Netw. 2013;11(10):1266–1290. | ||
Crawford J, Caserta C, Roila F, ESMO Guidelines Working Group. Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol. 2010;21(Suppl 5):v248–v251. | ||
Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187–3205. | ||
Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325(3):164–170. | ||
Pettengell R, Gurney H, Radford JA, et al. Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin’s lymphoma: a randomized controlled trial. Blood. 1992;80(6):1430–1436. | ||
Trillet-Lenoir V, Green J, Manegold C, et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer. 1993;29A(3):319–324. | ||
Nivestim™ (filgrastim) [summary of product characteristics]; 2017. Available from: https://www.medicines.org.uk/emc/medicine/23174. Accessed March 5, 2017. | ||
Waller CF, Semiglazov VF, Tjulandin S, Bentsion D, Chan S, Challand R. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie. 2010;33(10):504–511. | ||
Schwartzberg LS, Lal LS, Balu S, et al. Clinical Outcomes of Treatment with Filgrastim Versus a Filgrastim Biosimilar and Febrile Neutropenia-Associated Costs Among Patients with Nonmyeloid Cancer Undergoing Chemotherapy. J Manag Care Spec Pharm. 2018:1–9. | ||
Bongiovanni A, Monti M, Foca F, et al. Recombinant granulocyte colony-stimulating factor (rG-CSF) in the management of neutropenia induced by anthracyclines and ifosfamide in patients with soft tissue sarcomas (NEUSAR). Support Care Cancer. 2017;25(1):111–117. | ||
Eldar-Lissai A, Cosler LE, Culakova E, Lyman GH. Economic analysis of prophylactic pegfilgrastim in adult cancer patients receiving chemotherapy. Value Health. 2008;11(2):172–179. | ||
James E, Trautman H, Szabo E, Tang B. Comparative cost-efficiency analysis of granulocyte colony-stimulating factors for use in chemotherapy patients in the United States. Blood. 2017;130(Suppl 1):4667. | ||
Sun D, Andayani TM, Altyar A, Macdonald K, Abraham I. Potential cost savings from chemotherapy-induced febrile neutropenia with biosimilar filgrastim and expanded access to targeted antineoplastic treatment across the European Union G5 countries: a simulation study. Clin Ther. 2015;37(4):842–857. | ||
James E, Trautman H, Szabo E, Tang B. Budget impact analysis of switching chemotherapy patients using granulocyte colony-stimulating factors (G-CSFs) from pegfilgrastim to short-acting G-CSFs in the United States [Abstract 4668]. American Society of Hematology. 2017. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.