Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 12
HO-1 concentrations 24 hours after cardiac surgery are associated with the incidence of acute kidney injury: a prospective cohort study
Authors Magyar A, Wagner M, Thomas P, Malsch C, Schneider R, Störk S, Heuschmann PU, Leyh RG, Oezkur M
Received 12 February 2018
Accepted for publication 17 July 2018
Published 23 January 2019 Volume 2019:12 Pages 9—18
DOI https://doi.org/10.2147/IJNRD.S165308
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pravin Singhal
Attila Magyar,1,2 Martin Wagner,2–4 Phillip Thomas,1,2 Carolin Malsch,2 Reinhard Schneider,3 Stefan Störk,4,5 Peter U Heuschmann,2,4,6 Rainer G Leyh,1 Mehmet Oezkur1,2
1Department of Cardiovascular Surgery, University Hospital Würzburg, Würzburg, Germany; 2Institute of Clinical Epidemiology and Biometry, University of Würzburg, Würzburg, Germany; 3Division of Nephrology, Department of Medicine I, University Hospital Würzburg, Würzburg, Germany; 4Comprehensive Heart Failure Center, University of Würzburg, Würzburg, Germany; 5Division of Cardiology, Department of Medicine I, University Hospital Würzburg, Würzburg, Germany; 6Clinical Trial Center Würzburg, University Hospital Würzburg, Würzburg, Germany
Background: Acute kidney injury (AKI) is a serious complication after cardiac surgery that is associated with increased mortality and morbidity. Heme oxygenase-1 (HO-1) is an enzyme synthesized in renal tubular cells as one of the most intense responses to oxidant stress linked with protective, anti-inflammatory properties. Yet, it is unknown if serum HO-1 induction following cardiac surgical procedure involving cardiopulmonary bypass (CPB) is associated with incidence and severity of AKI.
Patients and methods: In the present study, we used data from a prospective cohort study of 150 adult cardiac surgical patients. HO-1 measurements were performed before, immediately after and 24 hours post-CPB. In univariate and multivariate analyses, the association between HO-1 and AKI was investigated.
Results: AKI with an incidence of 23.3% (35 patients) was not associated with an early elevation of HO-1 after CPB in all patients (P=0.88), whereas patients suffering from AKI developed a second burst of HO-1 24 hours after CBP. In patients without AKI, the HO-1 concentrations dropped to baseline values (P=0.031). Furthermore, early HO-1 induction was associated with CPB time (P=0.046), while the ones 24 hours later lost this association (P=0.219).
Conclusion: The association of the second HO-1 burst 24 hours after CBP might help to distinguish between the causality of AKI in patients undergoing CBP, thus helping to adapt patient stratification and management.
Keywords: acute kidney injury, cardiac surgery, heme oxygenase-1, cardiopulmonary bypass
Introduction
Surgery-associated acute kidney injury (AKI) is a common and serious complication after cardiac surgery, which increases the risk of mortality, morbidity as well as length of the hospital stay.1–6 The incidence of AKI associated with cardiac surgery may reach 50%,3,7–10 depending on the definition. The diagnosis of AKI is suggested by the Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group as an increase in serum creatinine (SCr) and/or a decrease in urine production.11 Even slight elevation of 0.3 mg/dL (AKI stage I) in SCr is considered as an independent predictor of in-hospital, 30-day as well as long-term mortality.12,13 Direct therapy for AKI is limited by the supportive strategy that includes fluid management, adequate drug dosing and dialysis for severe AKI stages.
Early prediction of AKI might allow an interventional therapeutic regime at the intensive care unit (ICU) before the damage of glomerular or renal tubular cells tends to become high grade or even irreversible. Furthermore, early distinction of the stages and causes of AKI might help in decision making in the ICU regarding catecholamine therapy, dialysis or fluid/drug management in early postoperative care.
The inducible isoform enzyme heme oxygenase-1 (HO-1) catalyzes the oxidative degradation of heme, producing CO, Fe2+ and biliverdin, which is converted to bilirubin by cytosolic biliverdin reductase. HO-1 is upregulated in proximal tubular cells as a response to oxidative stress and has protective anti-inflammatory and anti-oxidant effects.14 HO-1 was first recognized as a rapid and protective response in the context of rhabdomyolysis and heme pigment-induced AKI in animal models.14 The beneficial effects of HO-1 on oxidative stress and prevention of AKI have been reported in multiple animal models.15–17 In one small cohort in cardiac surgery, the association with AKI has been suggested in a clinical setting.18
In this prospective cohort study, we aimed to evaluate the course of HO-1 serum concentration after cardiac surgery and the association with AKI.
Patients and methods
Patient population
We enrolled in this prospective cohort study 165 patients between April and December 2014. Adult patients undergoing elective cardiac surgical procedure involving cardiopulmonary bypass (CPB) (coronary artery bypass graft [CABG], valve surgery [reconstruction, replacement] or combined CABG and valve surgery, and surgery of the thoracic aorta) were included. Patients signed informed consent form. Patients with chronic kidney disease (CKD) stage 3 or higher, ie, eGFR <30 mL/min, with signs of active infection (clinical assessment), women during pregnancy and lactation, as well as patients on COMT inhibitors, MAO inhibitors or on immunosuppression were excluded. Patient enrollment was at least 24 hours prior to surgery. Demography, medical history, medication, details of physical examination, anesthesia details, surgical data and the postoperative data including the hospital discharge were collected by the study personnel and were completed by the Comprehensive Heart Failure Center DataWarehouse.19 The study protocol was approved by the ethics committee at the Medical Faculty of the University of Würzburg (Vote 302/13) and the data protection officer at the University Hospital Würzburg.
Biomarker measurements
We collected blood samples at a predefined time schedule (preoperatively; at admission in ICU; 6, 24, and 48 hours after surgery; and at discharge from hospital). These time points were defined by local clinical routine; samples were collected by treating staff, while further processing was performed by study personnel. All samples were processed and stored at −80°C. For biomarker analyses, serum samples were thawed at +4°C and HO-1 (Enzo LifeSciences, Ann Arbor, MI, USA). Neutrophil gelatinase-associated lipocalin (NGAL; BioPorto Diagnostics A/S, 2900 Hellerup, Denmark) concentrations were measured by ELISA following the manufacturer’s guides. HO-1 was measured at anesthesia induction (baseline), at admission in the ICU and on postoperative day 1 (24 hours postsurgery), while NGAL was measured at baseline and at admission in the ICU.
Definition of end points and outcomes
The primary end point of the study was the incidence of AKI within 48 hours after surgery. The severity of postoperative AKI is defined as any of the following: 1. SCr increase by ≥0.3 mg/dL within 48 hours; or 2. increase in SCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or 3. urine volume <0.5 ml/kg/h for 6 hours. The stages of AKI within 48 hours were defined as SCr increase ≥0.3 mg/dL or of 1.5–1.99 times baseline as (stage 1), SCr increase of 2.0–2.9× baseline (stage 2) and SCr increase ≥3.0 times baseline or SCr >4 mg/dL or dialyses (stage 3). Secondary end points were the length of stay in ICU and hospital, time on mechanical ventilation and in-hospital mortality.
EuroScore II is a mortality risk calculator for cardiac surgical patients considering age, gender, pulmonary disease, extracardiac arteriopathy, neurological status, renal status, cardiac status as well as the complexity of the planned procedure.20 The EuroScore II is therefore a reliable score to rate the preoperative status of the patient. CPB time represents the duration of the operation in which the myocardium suffers from oxidative stress.21 Furthermore, it represents a sound surrogate marker for the course of the operation and is an independent predictor of mortality and morbidity after cardiac surgery.22
Statistical analyses
Statistical tests were performed with IBM SPSS 23 Statistics-Software. Continuous variables were described as medians with interquartile range. Categorical variables were presented as numbers and percentages. Normally distributed variables were compared regarding AKI yes/no by t-test, parametric variables by Mann–Whitney U test. Fisher’s exact or the chi-squared test was used for categorical values. Correlations were evaluated by Pearson’s rank correlation test. Univariate and adjusted multivariate logistic regression analyses were performed to investigate associations between predictors of development of AKI including clinically relevant variables and renal biomarkers. Variables were included in the multivariable analysis using a backward stepwise approach with criteria of P<0.05 for inclusion and P>0.10 for removal from the model. Areas under the curve of receiver operating characteristic (ROC) were analyzed to assess the cutoff value for postoperative HO-1 levels for predicting AKI. The optimal HO-1 cutoff level was defined as the value that provided the highest sensitivity and specificity for predicting AKI. An explorative, two-sided P-value of less than 0.05 was considered significant for all results.
Results
Patients’ characteristics
Of the enrolled 165 patients, 15 were excluded due to withdrawal of consent and change of surgical procedure to off-pump surgery. The patients’ characteristics stratified by development of AKI are displayed in Table 1.
Patients experiencing AKI within 48 hours after surgery were older (70 [65–77] vs 65 [59–75] years, P=0.015) and more likely to report on stroke or transient ischemic attack (TIA) in the medical history (P=0.013). EuroScore II tended to be higher without reaching statistical significance in patients with AKI (1.78 [1.15–3.50] vs 1.63 [0.89–2.77], P=0.055). Moreover, the time on CPB (109 [88–127] vs 110 [66–270], P=0.04) was longer in patients with AKI as compared to those without AKI. Patients with AKI also spent more time on mechanical ventilation (14 [11–23] vs 12 [10–15] hours, P=0.006) and in the ICU (55 [22–96] vs 23 [21–44] hours, P=0.001) and were also at high risk of in-hospital death (14.3% vs 0%, P=0.001).
Preoperative SCr levels did not differ between patients who developed AKI compared with patients who did not (AKI: 1.00 [0.78–1.23] vs no-AKI: 0.90 [0.75–1.02] mg/dL; P=0.535; Table 2, Figure 1). Neither preoperative serum NGAL nor HO-1 levels differed between patients who subsequently developed AKI and those who did not (Table 2, Figures 2 and 3). Both markers were higher at ICU arrival as compared to baseline (P<0.001 for NGAL and HO-1) (Table 2, Figures 2 and 3). NGAL levels were significantly higher in patients who developed AKI within 48 hours (960 [614–1,262] pg/mL vs 600 [461–783] pg/mL, P<0.001), while HO-1 levels did not differ (P=0.88) at this time point (Table 2, Figures 2 and 3). In patients developing AKI, an additional increase of serum HO-1 levels 24 hours after surgery was observed, whereas in patients who subsequently did not develop AKI, serum HO-1 levels decreased (AKI: 12.75 [8.69–34.35] ng/mL vs no AKI: 8.30 [4.13–15.60] ng/mL, P=0.031; Table 2 and Figures 2 and 3). In total, 45 patients (30%) developed AKI during the first 7 days after surgery and three patients required renal replacement therapy (Table 3). The severity of AKI is described in Table 3.
  | Table 3 AKI stages within 48 hours and 7 days after surgery Abbreviations: AKI, acute kidney injury; RRT, renal replacement therapy. |
The results of univariate and multivariate logistic regression analyses are presented in Table 4. We analyzed the relationship of clinical relevant variables (age, EuroScore II, hypertension, diabetes, chronic obstructive pulmonary disease, peripheral artery disease, stroke in medical history, CPB time, deep hypothermic circulatory arrest, mechanical ventilation time, noradrenalin dose, resuscitation, hemodynamic instability, rethoracotomy and transfusions) and serum HO-1 levels with AKI. We found an independent relationship of serum HO-1 levels 24 hours after surgery with the development of AKI (P=0.043, OR 1.053, 95% CI: 1.002–1.107).
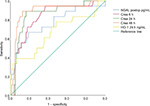
CPB time of all patients was associated with HO-1 levels at ICU arrival (P=0.046, correlation coefficient=0.236) but not with HO-1 levels at 24 hours after surgery (P=0.219). Furthermore, HO-1 levels at 24 hours after surgery in patients with AKI were associated with EuroScore II (P=0.048, correlation coefficient=0.181). Using ROC analyses, we provided a comparison of AUC between creatinine, NGAL postoperatively and HO-1 at 24 hours after surgery (Figure 4). Data are summarized in Table 5. By contrast, diagnostic accuracy for HO-1 at 24 hours after cardiac surgery was more reliable with a sensitivity of 75% and specificity of 85.8% for predicting AKI stage 2/3 after 48 hours (AUC 0.802, SE=0.102, 95% CI: 0.602–1.000, P=0.004; Figure 5).
Discussion
In the present study, we investigated data from a prospective cohort study of 150 adult cardiac surgical patients. Our results indicate that HO-1 kinetics differ in patients developing AKI within 48 hours from those who do not. All patients – independent from subsequent incidence of AKI – showed an HO-1 increase immediately after the operation. While AKI patients showed a second burst 24 hours after surgery, HO-1 levels in patients without AKI decreased almost to baseline values. In multivariate analyses, HO-1 levels at 24 hours after surgery remained associated with AKI within 48 hours. HO-1 showed a stronger association with higher stages of AKI (stages 2 and 3) compared with the AKI stage 1 in the measurement 24 hours after the surgery. Further investigations showed that NGAL concentrations increased early after surgery and were associated with the incidence of AKI within 48 hours as well.
AKI, as defined by the most recent guidelines, has a very strict definition as a rise in SCr of ≥0.3 mg/dL within 48 hours.11 Although a slight increase of SCr within 48 hours might not get noticed by the treating physicians in absence of a significant change of urine production, AKI stage 1 impacts the prognosis of a patient.6,8,9,23–25 Not only regarding an increased in-hospital mortality – as also confirmed in our cohort – but also the long-term prognosis of the patients suffering from AKI demonstrated a higher rate in re-hospitalization, mortality and heart failure.6,8,9,23–25
HO-1 is upregulated in proximal tubular cells in response to oxidative stress and has protective anti-inflammatory and anti-oxidant effects.14 In rodent models, it has been associated with AKI.15 In cardiac surgery patients, it was associated with AKI after surgery in a very small sample of 74 patients,18 which was a substudy of a clinical trial using ACE inhibitors to prevent inflammatory reactions after cardiac surgery. ACE inhibitors have been described to increase HO-1 expression and might be a bias in the prior study.26 Although in both studies the measurements were performed at the same time points, the course of HO-1 levels differed. While we observed one burst of HO-1 post-CPB, the earlier cohort showed a decrease in HO-1. Furthermore, we showed a decrease of HO-1 in patients without AKI, which was not shown in the first study.
In our cohort, elevation of HO-1 serum concentrations after CPB was positively associated with the CPB time. The ischemia–reperfusion stress of the cross-clamping and diastolic arrest represent an intensive oxidative stress triggering HO-1 production in different tissues.21,27,28 CPB time is a strong surrogate marker for the surgical complexity of the procedure itself and a complicated course during the operation.22 The longer a patient remains on CPB, the more oxidative stress is generated – not only in the myocardium but also in other sensitive tissues of the organism.21,22,28,29 Still the HO-1 elevation 24 hours after the surgery is a specific signal of renal stress. The first HO-1 induction does not differ between patients with or without AKI within the next 48 hours. Thus, we assume that the renal stress caused by the CPB might not be the only cause for AKI in our cohort. Patients with prolonged CPB times tend to have higher incidences of cardiogenic shock, anemia, bleeding, arrhythmia and pulmonary complications – all possible triggers of AKI for themselves.22,29–32 Our results might suggest that not the direct oxidative stress on the kidneys due to CPB is the causative for AKI, but the secondary upcoming complications and consequences of a long CPB duration.30,32 HO-1 levels 24 hours after surgery are not any longer associated with the CPB time but with the incidence of AKI. This second burst of HO-1 suggests that renal stress leading to AKI might be caused by, eg, inappropriate fluid management, diuretic treatment, appearance of cardiac shock or cardiac arrhythmias. Our results suggest that the time after surgery might be crucial for the development of AKI. Therefore, attention and targeted interventions to prevent AKI should be favorable during the postoperative phase, as already shown in literature.36
HO-1 measurements 24 hours after surgery were associated with the EuroScore II. EuroScore II is a risk score for cardiovascular surgery taking comorbidities, demography and other factors into consideration and providing a good sum up of all relevant operative risk factors.20 Thus, our results suggest that the renal oxidative stress might be caused or influenced by the comorbidities of the patient, as described previously in recent literature34,35,37,38
Being a rather late marker after cardiac surgery, HO-1 seems to be not helpful in clinical routine for early diagnostic issues after cardiac surgery compared to other biomarkers. Concurring with the literature we observed a statistically significant difference in HO-1 between AKI and no-AKI patients 24 hours after surgery.18 However, NGAL serum concentrations differed between the groups already after the CPB in our cohort, also according with the literature.39,41 But even NGAL does not provide an advantage over SCr to be of predictive value. Biomarkers for detection of acute kidney injury induced after the tubular damage has occurred are unsuitable for early diagnostics because the treatment options after the renal damage are limited.41 Some biomarkers, such as tissue inhibitor of metalloproteinase two and insulin-like growth factor binding protein 7 ([TIMP-2]*[IGFBP7]), seem to provide some significant timing advantage.41–44 In our cohort, HO-1 carried some benefits over other markers. In contrast to NGAL, HO-1 was very specific for AKI.40,45 The HO-1 serum concentrations 24 hours after surgery had a specificity of over 91% for AKI. By contrast, NGAL concentrations in serum increase due to oxidative stress in other organs as well and are less specific for AKI.41 On the other hand, this association might be caused by the high cutoff level, and HO-1 elevation could be caused by general oxidative stress. ([TIMP-2]*[IGFBP7] has a very high sensitivity of up to 90% in literature but has a rather low specificity (about 60%).44,46 With its high specificity, HO-1 might gather additional information about AKI especially in combination with other markers.
After cardiac surgery, serum NGAL levels have been shown to increase early within 2 hours and have already been proven to be predictive of the duration and severity of AKI after cardiac surgery.39 Recently, NGAL has been investigated extensively and appeared to be one of the most promising early biomarkers after renal injury.40 A meta-analysis including 19 studies with 2,500 patients was performed to estimate the diagnostic and therapeutic accuracy of NGAL in AKI.33 In conclusion, NGAL was found to be a useful early predictor of AKI with prognostic value for clinical end points. Unfortunately, substantial extrarenal NGAL generation in response to systemic stress can increase NGAL level in the absence of AKI,39 which partly limits its value in diagnostic accuracy.
Although the treating physicians were kept blinded regarding HO-1 and NGAL measurements, we address other limitations of the present study. A limited sample size prevents extensive multivariate adjustments, but this study still investigated the largest prospective cohort on HO-1 in cardiac surgery. Additionally, with the predefined exclusion criteria in our cohort, we could not study patients with preexisting advanced CKD and cannot comment or extrapolate on patients with CKD grade G4 or higher according to the KDIGO classification. Further information could be gathered by measuring HO-1 in urine as well, but this was not depicted in the study protocol. Finally, there is a potential treatment bias, because our protocol did not involve predefined approaches of treatment in case of postoperative AKI.
Conclusion
HO-1 measurements 24 hours after cardiac surgery are associated with AKI within 48 hours after surgery. Early measurements at ICU admission do not show any association with AKI. Further research on this marker in larger cohorts is important to characterize the entire value and determinants of the HO-1 kinetics. Clinical usefulness of this marker has yet to be investigated.
Acknowledgment
This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding program Open Access Publishing.
Disclosure
The authors report no conflicts of interest in this work.
References
Fung MM, O’Connor DT. Complex renal traits: role of adrenergic genetic polymorphism. J Am Soc Nephrol. 2009;20(6):1172–1174. | ||
Susantitaphong P, Cruz DN, Cerda J, et al; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–1493. | ||
Pickering JW, James MT, Palmer SC. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis. 2015;65(2):283–293. | ||
Wu VC, Wu CH, Huang TM, et al; NSARF Group. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25(3):595–605. | ||
Cooper DS, Claes D, Goldstein SL, et al. Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury (FRAIL-AKI). Clin J Am Soc Nephrol. 2016;11(1):21–29. | ||
Pickering JW, Blunt IR, Than MP. Acute Kidney Injury and mortality prognosis in Acute Coronary Syndrome patients: A meta-analysis. Nephrology (Carlton). 2018;23(3):237–246. | ||
Li SY, Chen JY, Yang WC, Chuang CL. Acute kidney injury network classification predicts in-hospital and long-term mortality in patients undergoing elective coronary artery bypass grafting surgery. Eur J Cardiothorac Surg. 2011;39(3):323–328. | ||
Olsson D, Sartipy U, Braunschweig F, Holzmann MJ. Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circ Heart Fail. 2013;6(1):83–90. | ||
Kandler K, Jensen ME, Nilsson JC, Møller CH, Steinbrüchel DA. Acute kidney injury is independently associated with higher mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(6):1448–1452. | ||
Reents W, Hilker M, Börgermann J, et al. Acute kidney injury after on-pump or off-pump coronary artery bypass grafting in elderly patients. Ann Thorac Surg. 2014;98(1):9–14. | ||
Khwaja A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clinical practice. 2012;120:(4):c179–c184. | ||
Dardashti A, Ederoth P, Algotsson L, Brondén B, Bjursten H. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg. 2014;147(2):800–807. | ||
Machado MN, Nakazone MA, Maia LN. Prognostic value of acute kidney injury after cardiac surgery according to kidney disease: improving global outcomes definition and staging (KDIGO) criteria. PLoS One. 2014;9(5):e98028. | ||
Nath KA, Balla G, Vercellotti GM, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90(1):267–270. | ||
Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995;48(4):1298–1307. | ||
Shimizu H, Takahashi T, Suzuki T, et al. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit Care Med. 2000;28(3):809–817. | ||
Blydt-Hansen TD, Katori M, Lassman C, et al. Gene transfer-induced local heme oxygenase-1 overexpression protects rat kidney transplants from ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14(3):745–754. | ||
Billings FT, Billings FT, Yu C, Byrne JG, Petracek MR, Pretorius M. Heme Oxygenase-1 and Acute Kidney Injury following Cardiac Surgery. Cardiorenal Med. 2014;4(1):12–21. | ||
Kaspar M, Ertl M, Fette G, et al. Data Linkage from Clinical to Study Databases via an R Data Warehouse User Interface. Experiences from a Large Clinical Follow-up Study. Methods Inf Med. 2016;55(4):381–386. | ||
Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–744; discussion 744–745. | ||
Miyamoto TA, Miyamoto KJ. Oxidative stress during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001;121(3):598–599. | ||
Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(6):814–822. | ||
Brown JR, Parikh CR, Ross CS, et al; Northern New England Cardiovascular Disease Study Group. Impact of perioperative acute kidney injury as a severity index for thirty-day readmission after cardiac surgery. Ann Thorac Surg. 2014;97(1):111–117. | ||
Elmistekawy E, McDonald B, Hudson C, et al. Clinical impact of mild acute kidney injury after cardiac surgery. Ann Thorac Surg. 2014;98(3):815–822. | ||
Silver SA, Harel Z, McArthur E, et al. 30-Day Readmissions After an Acute Kidney Injury Hospitalization. Am J Med. 2017;130(2):163–172.e4. | ||
Yim HE, Kim JH, Yoo KH, Bae IS, Hong YS, Lee JW. Spironolactone and enalapril differentially up-regulate the expression of VEGF and heme oxygenase-1 in the neonatal rat kidney. Pediatr Res. 2011;69(5 Pt 1):378–383. | ||
Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70(3):432–443. | ||
Zakkar M, Guida G, Suleiman MS, Angelini GD. Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longev. 2015;2015:189863. | ||
Nissinen J, Biancari F, Wistbacka JO, et al. Safe time limits of aortic cross-clamping and cardiopulmonary bypass in adult cardiac surgery. Perfusion. 2009;24(5):297–305. | ||
Basran S, Frumento RJ, Cohen A, et al. The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesth Analg. 2006;103(1):15–20. | ||
Cˇanádyová J, Zmeko D, Mokrácˇek A. Re-exploration for bleeding or tamponade after cardiac operation. Interact Cardiovasc Thorac Surg. 2012;14(6):704–707. | ||
Peretto G, Durante A, Limite LR, Cianflone D. Postoperative arrhythmias after cardiac surgery: incidence, risk factors, and therapeutic management. Cardiol Res Pract. 2014;2014:615987. | ||
Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A; NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024. | ||
Angeloni E, Melina G, Benedetto U, et al. Metabolic syndrome affects midterm outcome after coronary artery bypass grafting. Ann Thorac Surg. 2012;93(2):537–544. | ||
Vellinga S, Verbrugghe W, De Paep R, Verpooten GA, Janssen van Doorn K. Identification of modifiable risk factors for acute kidney injury after cardiac surgery. Neth J Med. 2012;70(10):450–454. | ||
Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43(11):1551–1561. | ||
Gaudino M, Luciani N, Giungi S, et al. Different profiles of patients who require dialysis after cardiac surgery. Ann Thorac Surg. 2005;79(3):825–829. | ||
Karkouti K, Beattie WS, Wijeysundera DN, et al. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg. 2005;129(2):391–400. | ||
Haase M, Bellomo R, Devarajan P, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg. 2009;88(1):124–130. | ||
Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012;50(9):1505–1517. | ||
Malhotra R, Siew ED. Biomarkers for the Early Detection and Prognosis of Acute Kidney Injury. Clin J Am Soc Nephrol. 2017;12(1):149–173. | ||
Vijayan A, Faubel S, Askenazi DJ, et al; American Society of Nephrology Acute Kidney Injury Advisory Group. Clinical Use of the Urine Biomarker [TIMP-2] x [IGFBP7] for Acute Kidney Injury Risk Assessment. Am J Kidney Dis. 2016;68(1):19–28. | ||
Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9(3):e93460. | ||
Wetz AJ, Richardt EM, Wand S, et al. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care. 2015;19:3. | ||
Singer E, Markó L, Paragas N, et al. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf). 2013;207(4):663–672. | ||
Gunnerson KJ, Shaw AD, Chawla LS, et al; Sapphire Topaz investigators. TIMP2•IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J Trauma Acute Care Surg. 2016;80(2):243–249. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.