Back to Journals » OncoTargets and Therapy » Volume 13
HNF1A-AS1 Regulates Cell Migration, Invasion and Glycolysis via Modulating miR-124/MYO6 in Colorectal Cancer Cells
Authors Guo X, Zhang Y, Liu L, Yang W, Zhang Q
Received 16 September 2019
Accepted for publication 17 November 2019
Published 18 February 2020 Volume 2020:13 Pages 1507—1518
DOI https://doi.org/10.2147/OTT.S231249
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Leo Jen-Liang Su
Xiong Guo,1 Yang Zhang,1 Ling Liu,2 Weiming Yang,1 Qi Zhang3
1Colorectal and Anal Surgical Department, Xiangya Hospital Central South University, Changsha 410008, People’s Republic of China; 2Hepatobiliary and Enteric Surgery Research Center, Xiangya Hospital Central South University, Changsha 410008, People’s Republic of China; 3Department of Hepatobiliary and Pancreatic Surgery, Xiangya Hospital Central South University, Changsha 410008, People’s Republic of China
Correspondence: Qi Zhang
Department of Hepatobiliary and Pancreatic Surgery, Xiangya Hospital Central South University, No. 87, Xiangya Road, Kaifu District, Changsha 410008, People’s Republic of China
Tel +86 138 7594 9431
Email [email protected]
Background: Accumulating evidence determined that lncRNAs play multiple roles in cell progression in colorectal cancer (CRC). Long noncoding RNA (lncRNA) hepatocyte nuclear factor 1 homeobox A (HNF1A)-antisense RNA 1 (AS1) has been identified to affect cell growth and disease diagnosis in various cancers, including CRC. However, the underlying regulatory mechanism of HNF1A-AS1 in cell progression and glycolysis has not been fully explored in CRC.
Materials and Methods: The expression of HNF1A-AS1, microRNA-124 (miR-124) and Myosins of class VI (MYO6) was detected using reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The analysis of glucose consumption, lactate production and hexokinase 2 (HK2) protein level was used to assess glycolysis in cells. The protein level of HK2 and MYO6 was measured with Western blot. Cell migration and invasion were evaluated using the transwell assay. The relationship among HNF1A-AS1, miR-124 and MYO6 was determined via luciferase reporter and RNA immunoprecipitation (RIP) assay.
Results: In this study, we found that HNF1A-AS1 was upregulated in CRC tissues and cell lines. Functional experiments determined that reduction of HNF1A-AS1 or promotion of miR-124 inhibited cell migration and invasion as well as glycolysis in CRC cells. What’ more, luciferase reporter assay manifested that miR-124 was a target of HNF1A-AS1 and MYO6 was a target mRNA of miR-124 in CRC cells. Additionally, reverse experiments showed that the effects of si-HNF1A-AS1 on colorectal cancer cells were impaired by anti-miR-124 and the effects of high miR-124 expression on CRC cells were rescued by upregulating MYO6. HNF1A-AS1 regulated MYO6 expression via targeting miR-124 in CRC cells.
Conclusion: In this study, we first found that HNF1A-AS1 regulated cell migration, invasion and glycolysis via modulating miR-124/MYO6 in CRC cells.
Keywords: HNF1A-AS1, miR-124, MYO6, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, with high morbidity and mortality in China.1,2 Because advanced colorectal cancer still lacks effective diagnosis and treatment, it is necessary to further explore and analyze the pathogenesis and regulation mechanism of CRC to determine effective biomarkers.
At present, a large number of studies have shown that long noncoding RNAs (lncRNAs) are important regulators in cells and are widely involved in the occurrence and development of human diseases.3–9 LncRNAs are noncoding RNAs with a length of more than 200 nt, as ceRNAs, which further regulated the production of downstream proteins by regulating the miRNA/mRNA axis.10–13 Some lncRNAs have been reported to be differentially expressed in colorectal cancer tissues and cells, but their functions have been verified.14 For example, lncRNA DLEU1 promoted CRC cell progression through regulating KPNA3.15 Moreover, lncRNA CRNDE induced cell proliferation and chemoresistance in CRC.16 lncRNA BANCR has been determined to relate to CRC poor prognosis.17 However, the regulation mechanism of lncRNA in colorectal cancer has not yet been elucidated, and further exploration and research are needed.
The lncRNA HNF1A antisense RNA 1 (HNF1A-AS1), as an oncogene, was involved in cell progression and metabolism in many cancers, such as lung adenocarcinoma, colorectal carcinoma, oesophageal adenocarcinoma, nonsmall cell lung cancer, bladder cancer and hepatocellular carcinoma.18–23 Zhao et al demonstrated that induction of HNF1A-AS contributed to cell proliferation and metastasis through activating Wnt/β-catenin signaling pathway in osteosarcoma.24 In addition, lncRNA HNF1A-AS1 has been determined to be a novel diagnostic predictor for prognosis of cancers.25,26 However, the effect of HNF1A-AS1 on cell glycolysis has not been explained and the regulatory network HNF1A-AS1 remains to be further explored and analyzed.
In the present study, we investigated the functional effects of lncRNA HNF1A-AS1on the cell metabolism and glycolysis in CRC cells, as well as the potential regulatory mechanism.
Materials and Methods
Tissues, Cell Culture and Transfection
Forty pairs of CRC tissues and adjacent normal tissues were obtained from patients who were diagnosed at Xiangya Hospital Central South University. This experiment has been approved by the Ethics Committee of Xiangya Hospital Central South University. Informed written consents were acquired from patients.
CRC cell lines (SW620, HT-29, HCT116 and SW480) were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The immortalized colon cell (NCM460) was purchased from Rongbai (Shanghai, China). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS, Sciencell Research, Carlsbad, CA, USA) and 1% penicillin at 37°C in a humidified atmosphere of 5% CO2.
Small interfering RNA (siRNA) targeting HNF1A-AS1 (si-HNF1A-AS1), siRNA scrambled control (si-NC), pcDNA-HNF1A-AS1 (HNF1A-AS1), pcDNA-MYO6 (MYO6), pcDNA, miR-124 mimic (miR-212-3p), miRNA negative control (miR-NC), miR-124 inhibitor (anti-miR-212-3p) and inhibitor negative control (anti-miR-NC) were purchased from GenePharma Co. Ltd. (Suzhou, China). These vectors and oligos were transfected into SW620 and HCT116 cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from CRC cells and tissues using TRIzol reagent (Invitrogen) referring to the protocols supplied by the manufacturer. 1 ng of RNA was reversely transcribed into cDNA using PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian, China). For HNF1A-AS1 and MYO6 detection, One Step SYBR PrimeScript™ RTPCR Kit (Takara) was used to measure the relative expression of HNF1A-AS1 and MYO6. For miR-124 detection, TaqMan miRNA assays (Applied Biosystems, Carlsbad, CA, USA) were used to measure the relative expression of miR-124. All samples were performed on a CFX96 TouchTM Real-Time PCR system (Bio-Rad, Hercules, CA, USA). Glyceraldehyde phosphate dehydrogenase (GAPDH) and U6 were employed to normalize for mRNA and miRNA, respectively. The primer sequences were as follows: the primers for U6: forward, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ and reverse 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ. The primers for miR-124, forward, 5ʹ- GCCTAAGGCACGCGGTG-3ʹ and reverse, 5ʹ-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGGCATT −3ʹ. The primers for HNF1A-AS1: forward 5ʹ-TCAAGAAATGGTGGCTAT-3ʹ and reverse, 5ʹ- GATCTGAGACTGGCTGAA-3ʹ. The primers for MYO6: forward 5ʹ- GATGGAGCTGCACCCTGACA-3ʹ and reverse, 5ʹ- GCTCTCAATGGCGCTCTGAAG-3ʹ. The primers for GAPDH: forward 5ʹ-GGAGCGAGATCCCTCCAAAAT-3ʹ and reverse, 5ʹ-GGCTGTTGTCATACTTCTCATGG-3ʹ.
Western Blot Assay
Cells were lysed with RIPA Lysis Buffer (Thermo Fisher Scientific) and the protein concentrations were quantified by a bicinchoninic acid (BCA) Protein Assay Kit (Thermo Fisher Scientific). Protein samples were added onto sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were incubated with primary antibodies HK2 (1:2000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), MYO6 (1:2000 dilution; Santa Cruz Biotechnology) or GAPDH (1:1000 dilution; Santa Cruz Biotechnology) at 4°C overnight. After being washed in TBS, the membrane was incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature. The blots were detected by Clarity Western ECL substrate (Bio-Rad) and visualized with a ChemiDocTM MP Imaging System (Bio-Rad).
Glucose Consumption and Lactate Production Assay
The dose of cloxiquine (CLQ, 50 μM) with/without GW9662 (10 μM, PPAR gamma antagonist) was used to treat SW620 and HCT116 cells for 24 h. Glucose content was determined using a Glucose Assay kit (Rongsheng Biotechnology, Shanghai, China) according to the supplier’s instructions. The concentrations of intracellular lactate were analyzed with a lactate assay kit (Sigma, St Louis, MO, USA) following the protocols of the manufacturer.
Transwell Assay
For invasion assay, 2 ×103 cells were seeded in the upper chambers with 8-µm pores Matrigel-coated (BD Biosciences, San Jose, CA, USA) and cultured in concentration in DMEM. The lower chamber was added with DMEM containing 10% FBS as a chemoattractant. After incubation for 48 h at room temperature, cells in the upper chamber were scraped off with cotton swabs. Cells in the lower chamber were fixed with methanol and then stained by crystal violet for 30 min. For migration assay, cells were added onto the upper chambers without Matrigel-coated, other steps were similar to invasion assay. The migrated and invasive cells were calculated using a Countess automatic cell counter (Invitrogen).
Luciferase Reporter Assay
The fragments of HNF1A-AS1 containing the wild-type or mutated miR-124 binding sites were amplified and cloned into a pRL-TK (Promega, Madison, WI, USA), namely HNF1A-AS1-WT-Luc or HNF1A-AS1-MUT-Luc. In addition, the fragments of MYO6 containing the wild-type or mutated miR-124 binding sites were amplified and inserted into pRL-TK, namely MYO6-WT-Luc or MYO6-MUT-Luc. Then, these vectors were co-transfected with miR-124 or miR-NC into SW620 and HCT116 cells using Lipofectamine 2000 reagent (Invitrogen). The renilla luciferase control vector pRL-ubi-63E was a control vector using transfection reagent Lipofectamine 2000 (Invitrogen). After transfection for 48 h, cells were collected and the luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega).
RNA Immunoprecipitation (RIP) Assay
The EZ-Magna RIP Kit (Millipore) was used for RIP experiments according to the manual. Briefly, cells were collected by centrifugation and lysed in RIP buffer. Then, cells were incubated with human anti-Argonaute 2 (Ago2) antibody (Abcam) or normal mouse IgG (negative control, Sigma-Aldrich) overnight. Nonspecific binding was removed with proteinase K buffer (Abcam), and then the immunoprecipitated RNA was isolated. RT-qPCR was used to analyze the enrichment of HNF1A-AS1 or MYO6.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). Comparison between the two groups was analyzed using Student’s t-test and among three or more sets were calculated using LSD. All data were presented as means ± standard deviation (SD). P<0.05 was considered to be statistically significant.
Results
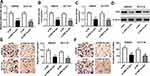
HNF1A-AS1 Was Upregulated in Colorectal Cancer Tissues and Cell Lines
To explore the expression of HNF1A-AS1 in CRC tissues and cell lines, we collected 40 pairs of colorectal cancer tissues and adjacent tissues as well as purchased four CRC cell lines (SW620, HT-29, HCT116 and SW480) and immortalized colon cells (NCM460). As shown in Figure 1A, the expression of HNF1A-AS1 in cancer tissues was significantly higher than that in normal tissues. In addition, we also found HNF1A-AS1 expression was upregulated in SW620, HT-29, HCT116 and SW480 cells compared with that in NCM460 cells (Figure 1B). Moreover, we selected two CRC lines (SW620 and HCT116), which have a relative higher expression of HNF1A-AS1 for the following experiments. Collectively, these results showed that HNF1A-AS1 was upregulated in CRC tissues and cell lines.
Knockdown of HNF1A-AS1 Inhibited Cell Migration and Invasion as Well as Glycolysis in Colorectal Cancer Cells
To determine the effects of HNF1A-AS1 on cell migration, invasion and glycolysis in CRC cells, we transfected two CRC cell lines (SW620 and HCT116) with siHNF1A-AS1 to construct CRC cells with lower HNF1A-AS1 expression than control cells (Figure 2A). As shown in Figure 2B and C, both relative glucose consumption and lactate production were obviously reduced in SW620 and HCT116 cells with HNF1A-AS1 knockdown compared with cells transfected with si-NC (Figure 2B and C). More than that, the expression of HK2 was also decreased in the si-HNF1A-AS1 group compared with that in the si-NC group of SW620 and HCT116 cells (Figure 2D). In addition, cell migration and invasion were significantly lower in SW620 and HCT116 transfected with si-HNF1A-AS1 than that in cells transfected with si-NC (Figure 2E and F). These data determined that knockdown of HNF1A-AS1 inhibited cell migration and invasion as well as glycolysis in CRC cells.
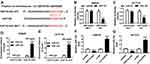
miR-124 Was a Target of HNF1A-AS1
To further investigate the potential regulatory mechanisms of HNF1A-AS1 in CRC cells, we sought potential target miRNAs for HNF1A-AS1 through DIANA tools and found that miR-124 had a reverse complement to 3ʹUTR of HNF1A-AS1 (Figure 3A). Therefore, we constructed HNF1A-AS1-WT-Luc (binding sites of wild type) or HNF1A-AS1-MUT-Luc (binding sites of mutate) vectors. Luciferase reporter assay determined that luciferase activity of HNF1A-AS1-WT-Luc in SW620 and HCT116 cells were remarkably reduced by miR-124, while the luciferase activity of HNF1A-AS1-MUT-Luc has not changed (Figure 3B and C). Otherwise, RIP assay was applied to further validate the interaction between miR-124 and HNF1A-AS1, the results showed that HNF1A-AS1 was significantly enriched in the RIP-Ago2 than RIP-IgG in SW620 and HCT116 cells (Figure 3D and E). Meanwhile, we analyzed the expression of miR-124 in SW620 and HCT116 cells with HNF1A-AS1-overexpression or HNF1A-AS1-down-expression via RT-qPCR (Figure 3F and G). The results showed that upregulated HNF1A-AS1 inhibited miR-124 expression while downregulated HNF1A-AS1 promoted miR-124 expression in SW620 and HCT116 cells. Based on the above results, we ensured that miR-124 was a target miRNA of HNF1A-AS1.
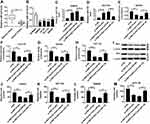
The Effects of Downregulated HNF1A-AS1 on Colorectal Cancer Cells Were Impaired by Silenced miR-124
To further understand the function of miR-124 in CRC cells, we analyzed the expression of miR-124 in four CRC cell lines (SW620, HT-29, HCT116 and SW480) by RT-qPCR and observed that miR-124 expression was significantly reduced in CRC tissues and cells compared to immortalized colon cells (NCM460) (Figure 4A and B). As shown in Figure 4B and C, we constructed SW620 and HCT116 cells with a lower expression of miR-124 through transfection with anti-miR-124. Furthermore, the si-HNF1A-AS and anti-miR-124 were co-transfected into SW620 and HCT116 cells for functional rescue experiments. We found that si-HNF1A-AS transfection significantly induced the expression of miR-124, which the promoted impact was alleviated by anti-miR-124 transfection in SW620 and HCT116 cells. Additionally, both relative glucose consumption and lactate production were inhibited by downregulating HNF1A-AS, while this inhibitory effect was weakened by anti-miR-124 transfection in SW620 and HCT116 cells (Figure 4D–G). In addition, the reduction of HNF1A-AS inhibited the expression of HK2, which was rescued by anti-miR-124 transfection in SW620 and HCT116 cells (Figure 4H). Strikingly, cell migration and invasion were suppressed by reduction of HNF1A-AS, while they were relieved by inhibition of miR-124 in SW620 and HCT116 cells (Figure 4I–M). Thus, these data determined that silencing of miR-124 could reverse the si-HNF1A-AS-mediated inhibited effects on cell migration, invasion and glycolysis in SW620 and HCT116 cells.
miR-124 Directly Targeted MYO6
We all know that miRNAs regulate the expression of downstream target genes. To further investigate the regulatory network of HNF1A-AS, we predicted the potential target genes of miR-124 by DIANA tools and found that MYO6 has a complementary sequence to miR-124 (Figure 5A). MYO6-WT-Luc or MYO6-MUT-Luc vector was constructed and co-transfected into SW620 and HCT116 cells with miR-NC or miR-124. Luciferase reporter assay analysis showed that luciferase activity of MYO6-WT-Luc was apparently decreased after co-transfection with miR-124, but no significant change was observed in luciferase activity of MYO6-MUT-Luc (Figure 5B and C). RIP assay also determined that MYO6 expression was enriched in the RIP-Ago2 than RIP-IgG in SW620 and HCT116 cells (Figure 5D and E). Additionally, the expression of MYO6 was inhibited by the upegulation of miR-124, while promoted by downregulation of miR-124 in SW620 and HCT116 cells (Figure 5F and G). The above results indicated that MYO6 was the target gene of miR-124 in CRC cells.
The Effects Induced by Overexpression of miR-124 on Colorectal Cancer Cells Were Rescued by Upregulated MYO6
To further clarify the regulatory mechanisms of miR-124 and MYO6 in CRC, the rescue experiments were performed on SW620 and HCT116 cells. First, we measured the expression of MYO6 in CRC tissues and cell lines (SW620, HT-29, HCT116 and SW480), and the results showed that MYO6 was highly expressed in CRC tissues and cells (Figure 6A and B). In the following experiment, miR-124 mimics and pcDNA-MYO6 were co-transfected into SW620 and HCT116 cells. As depicted in Figure 6B and C, promotion of miR-124 inhibited MYO6 expression, while upegulating MYO6 could reverse this effect in SW620 and HCT116 cells. Furthermore, relative glucose consumption, lactate production and HK2 expression were obviously reduced by miR-124 transfection, which was impaired by the induction of MYO6 in SW620 and HCT116 cells (Figure 6D–H). More than that, the inhibited effects of high miR-124 expression on cell migration and invasion in SW620 and HCT116 cells were rescued by improving MYO6 expression (Figure 6I–M). These results showed that the induction of MYO6 could impair the inhibited effects of high miR-124 expression on cell metastasis and glycolysis in CRC cells.
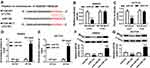
HNF1A-AS1 Regulated MYO6 Expression Through miR-124 in Colorectal Cancer Cells
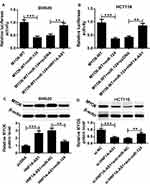
In order to further explore the regulatory network among HNF1A-AS1, miR-124 and MYO6. Luciferase reporter methods and Western blot assay were conducted. We found that the luciferase activity was significantly reduced in MYO6-WT-Luc + miR-124 group compared with that in the MYO6-WT-Luc group. In addition, compared with MYO6-WT-Luc+miR-124 + pcDNA group, luciferase activity was notably increased in MYO6-WT-Luc + miR-124 + HNF1A-AS1 group in SW620 and HCT116 cells (Figure 7A and B). Not only that, the analysis of Western blot showed that induction of HNF1A-AS1 improved the protein level of MYO6, which was inhibited by the promotion of miR-124 in SW620 and HCT116 cells (Figure=7C and D). Therefore, these results indicated that HNF1A-AS1 regulated the expression of MYO6 through miR-124 in colorectal cancer cells.
Discussion
In this paper, we found that lncRNA HNF1A-AS1 was highly expressed in CRC tissues and cells, and further functional experiments showed that silenced HNF1A-AS1 reduced the cell invasion and metastasis abilities of CRC cells. And it was found for the first time that low expression of lncRNA HNF1A-AS1 inhibited intracellular glycolysis in tumor cells. In the research on the regulation mechanism of lncRNA HNF1A-AS1, luciferase reporter assay determined that miR-124 was a target of HNF1A-AS1 and MYO6 was a target gene of miR-124. Therefore, we speculated that lncRNA HNF1A-AS1 affected cell migration, invasion and glycolysis by regulating miR-124/MYO6 axis in CRC cells. Rescue experiments further confirmed our speculation. In this paper, we first put forward lncRNA HNF1A-AS1 regulates MYO6 expression by sponging miR-124 to affect the cell glycolysis process in CRC cells, which significantly improved our understanding of the regulatory mechanisms in CRC.
Cell invasion and migration are necessary biological processes for tumor cells, marking their abilities to migrate and spread.27,28 In clinical research, inhibiting and blocking cell migration and invasion are important processes in the treatment of cancer. Many noncoding RNAs, such as lncRNA and miRNA, as important regulatory factors of cell metabolism, were closely involved in cell progression for various cancers, including invasion and migration.29–31 For example, lncRNA UCA1 impacted cell invasion and migration through regulating FOXO3 via sponging miR-96 in pancreatic cancer.32 LncRNA ZFAS1 was upregulated in rheumatoid arthritis and contributed to cell invasion and migration through the reduction of miR-27a.33 Moreover, lncRNA SNHG16 promoted cell migration, invasion and proliferation through modulating miR-520d-3p/STAT3 axis in hemangioma endothelial cells.34 Lin et al reported that HIFIA-AS2 promoted cell progression and EMT in CRC cells via regulation of miR-129-5p/DNMP3A.35 In our study, we also found that knockdown of HNF1A-AS1 or promotion of miR-124 inhibited CRC cell migration and invasion. Furthermore, we found that miR-124 was a target of HNF1A-AS1, and MYO6 was a target of miR-124. Accumulating evidence suggested that miR-124 had multiple roles in cell progression of cancers, such as brain tumor and CRC.36,37 A previous study determined that miR-124 was downregulated in CRC and inhibited cell proliferation and drug resistance.37–39 More than that, MYO6 has been verified to highly express in many cancers, such as prostate cancer, CRC, breast cancer and gastric cancer, which also participated in cell progression.40–43 In our study, consistent with a previous study, miR-124 expressed low and MYO6 expressed high in CRC tissues and cells, and rescue experiments confirmed the effects of HNF1A-AS1 on cell migration and invasion through targeting miR-124/MYO6 axis.
More than that, glycolysis provides energy for the metabolism of tumor cells and guarantees the proliferation and development of tumor cells. Lactate production and glucose consumption are important indexes for the determination of glycolysis. Additionally, the glycolytic enzyme hexokinase 2 (HK2) is one of the important aerobic glycolytic mediating factors in cells.44–46 Increasing the expression of HK2 could promote cell glycolysis and release more energy for cell development.47,48 Various lncRNAs were related to glycolysis in cancers, such as lncRNA Ftx, lncRNA p23154, lncRNA MIF and lncRNA MALAT1.49–52 The study of Li et al suggested that lncRNA UCA1 induced glycolysis in bladder cancer cells, and promoted lactate production and glucose consumption through upregulating HK2 via activation of mTOR-STAT3/miR-143 pathway.48 In this study, inhibition of HNF1A-AS1 or promotion of miR-124 decreased cell glycolysis. While inhibition of miR-124 or promotion of MYO6 could weaken the suppressive effect of reduction of HNF1A-AS1 or promotion of miR-124 on glycolysis, respectively. Therefore, lncRNA HNF1A-AS1 regulated cell glycolysis in CRC cells through targeting the miR-124/MYO6 axis.
Conclusion
In this study, we first found that HNF1A-AS1 regulated cell migration, invasion and glycolysis via modulating miR-124/MYO6 in CRC cells, providing a new regulatory network and a new therapeutic target of CRC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Baena R, Salinas P. Diet and colorectal cancer. Maturitas. 2015;80(3):258–264. doi:10.1016/j.maturitas.2014.12.017
2. Zhao P, Dai M, Chen W, et al. Cancer trends in China. Jpn J Clin Oncol. 2010;40(4):281–285. doi:10.1093/jjco/hyp187
3. Cao J. The functional role of long non-coding RNAs and epigenetics. Biol Proced Online. 2014;16(1):42. doi:10.1186/1480-9222-16-11
4. Jiang C, Li X, Zhao H, et al. Long non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer. 2016;15(1):62. doi:10.1186/s12943-016-0545-z
5. Kitagawa M, Kitagawa K, Kotake Y, et al. Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci. 2013;70(24):4785–4794. doi:10.1007/s00018-013-1423-0
6. Li Z, Yu X, Shen J. ANRIL: a pivotal tumor suppressor long non-coding RNA in human cancers. Tumour Biol. 2016;37(5):5657–5661. doi:10.1007/s13277-016-4808-5
7. Sun K, Wu D, Chen S, et al. LncRNA MEG3 inhibit endometrial carcinoma tumorigenesis and progression through PI3K pathway. Apoptosis. 2017;22(12):1543–1552. doi:10.1007/s10495-017-1426-7
8. Qin J, Ning H, Yao Z, et al. LncRNA Uc.173 is a key molecule for the regulation of lead-induced renal tubular epithelial cell apoptosis. Biomed Pharmacother. 2018;100:101–107. doi:10.1016/j.biopha.2018.01.112
9. Xiao-Yun HE, Chun-Lin OU, Xiao YH, et al. Inhibitory effect of saxagliptin on high glucose-induced overexpression of LncRNA-MALAT1 in endothelial cells. J Med Postgrad. 2016;29(9):902–905.
10. Zhang Y, Zhang X, Chen T, et al. Circular intronic long noncoding RNAs. Mol Cell. 2013;51(6):792–806. doi:10.1016/j.molcel.2013.08.017
11. Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109. doi:10.1016/j.bbagrm.2014.08.012
12. Wu Q, Meng W, Jie Y, et al. LncRNA MALAT1 induces colon cancer development by regulating miR‐129‐5p/HMGB1 axis. J Cell Physiol. 2018;233(9):6750–6757. doi:10.1002/jcp.26383
13. Zhang G, Li S, Lu J, et al. LncRNA MT1JP functions as a ceRNA in regulating FBXW7 through competitively binding to miR-92a-3p in gastric cancer. Mol Cancer. 2018;17(1):87. doi:10.1186/s12943-018-0829-6
14. Zhu H, Yu J, Zhu H, et al. Identification of key lncRNAs in colorectal cancer progression based on associated protein–protein interaction analysis. World J Surg Oncol. 2017;15(1):153. doi:10.1186/s12957-017-1211-7
15. Liu T, Han Z, Li H, et al. LncRNA DLEU1 contributes to colorectal cancer progression via activation of KPNA3. Mol Cancer. 2018;17(1):118. doi:10.1186/s12943-018-0873-2
16. Han P, Li J, Zhang B, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR-181a-5p-mediated regulation of Wnt/β-catenin signaling. Mol Cancer. 2017;16(1):9. doi:10.1186/s12943-017-0583-1
17. Shen X, Bai Y, Luo B, et al. Upregulation of lncRNA BANCR associated with the lymph node metastasis and poor prognosis in colorectal cancer. Biol Res. 2017;50(1):32. doi:10.1186/s40659-017-0136-5
18. Wu Y, Liu H, Shi X, et al. The long non-coding RNA HNF1A-AS1 regulates proliferation and metastasis in lung adenocarcinoma. Oncotarget. 2015;6(11):9160–9172. doi:10.18632/oncotarget.3247
19. Zhu W, Zhuang P, Song W, et al. Knockdown of lncRNA HNF1A-AS1 inhibits oncogenic phenotypes in colorectal carcinoma. Mol Med Rep. 2017;16(4):4694–4700. doi:10.3892/mmr.2017.7175
20. Zhang G, An X, Zhao H, et al. Long non-coding RNA HNF1A-AS1 promotes cell proliferation and invasion via regulating miR-17-5p in non-small cell lung cancer. Biomed Pharmacother. 2018;98:594–599. doi:10.1016/j.biopha.2017.12.080
21. Feng Z, Wang B. Long non-coding RNA HNF1A-AS1 promotes cell viability and migration in human bladder cancer. Oncol Lett. 2018;15(4):4535–4540. doi:10.3892/ol.2018.7878
22. Ding C, Yin C, Chen S, et al. The HNF1α-regulated lncRNA HNF1A-AS1 reverses the malignancy of hepatocellular carcinoma by enhancing the phosphatase activity of SHP-1. Mol Cancer. 2018;17(1):63. doi:10.1186/s12943-018-0813-1
23. Yang X, Song JH, Cheng Y, et al. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63(6):881–890. doi:10.1136/gutjnl-2013-305266
24. Zhao H, Hou W, Tao J, et al. Upregulation of lncRNA HNF1A-AS1 promotes cell proliferation and metastasis in osteosarcoma through activation of the Wnt/β-catenin signaling pathway. Am J Transl Res. 2016;8(8):3503–3512.
25. Gong W, Tian M, Qiu H, et al. Elevated serum level of lncRNA-HIF1A-AS1 as a novel diagnostic predictor for worse prognosis in colorectal carcinoma. Cancer Biomarkers. 2017;20(4):417–424. doi:10.3233/CBM-170179
26. Zhuang C, Zheng L, Wang P. Prognostic role of long non-coding RNA HNF1A-AS1 in Chinese cancer patients: a meta-analysis. Onco Targets Ther. 2018;11:5325–5332. doi:10.2147/OTT.S163575
27. Liotta LA. Tumor invasion and metastases—role of the extracellular matrix: rhoads memorial award lecture. Cancer Res. 1986;46(1):1–7.
28. Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114(Pt 15):2713–2722.
29. Eades G, Zhang Y, Li Q, et al. Long non-coding RNAs in stem cells and cancer. World J Clin Oncol. 2014;5(2):134–141. doi:10.5306/wjco.v5.i2.134
30. Xu S, Kong D, Chen Q, et al. Oncogenic long noncoding RNA landscape in breast cancer. Mol Cancer. 2017;16(1):129. doi:10.1186/s12943-017-0696-6
31. Chaofeng TU. The interaction between lncRNA and microRNA contributes to tumor. Chin J Biochem Mol Biol. 2013;29(11):1029–1034.
32. Zhou Y, Chen Y, Ding W, et al. LncRNA UCA1 impacts cell proliferation, invasion, and migration of pancreatic cancer through regulating miR‐96/FOXO3. IUBMB Life. 2018;70(4):276–290. doi:10.1002/iub.v70.4
33. Ye Y, Gao X, Yang N. LncRNA ZFAS1 promotes cell migration and invasion of fibroblast-like synoviocytes by suppression of miR-27a in rheumatoid arthritis. Hum Cell. 2018;31(1):14–21. doi:10.1007/s13577-017-0179-5
34. Zhao W, Fu H, Zhang S, et al. LncRNA SNHG16 drives proliferation, migration, and invasion of hemangioma endothelial cell through modulation of miR‐520d‐3p/STAT3 axis. Cancer Med. 2018;7(7):3311–3320. doi:10.1002/cam4.2018.7.issue-7
35. Lin J, Shi Z, Yu Z, et al. LncRNA HIF1A-AS2 positively affects the progression and EMT formation of colorectal cancer through regulating miR-129-5p and DNMT3A. Biomed Pharmacother. 2017;98:433. doi:10.1016/j.biopha.2017.12.058
36. Silber J, Lim DA, Petritsch C, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6(1):14. doi:10.1186/1741-7015-6-14
37. Taniguchi K, Sugito N, Kumazaki M, et al. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett. 2015;363(1):17–27. doi:10.1016/j.canlet.2015.03.026
38. Zhou L, Xu Z, Ren X, et al. MicroRNA-124 (MiR-124) inhibits cell proliferation, metastasis and invasion in colorectal cancer by downregulating Rho-Associated Protein Kinase 1(ROCK1). Cell Physiol Biochem. 2016;38(5):1785–1795. doi:10.1159/000443117
39. Zhu J, Zhang R, Yang D, et al. Knockdown of long non-coding RNA XIST inhibited doxorubicin resistance in colorectal cancer by upregulation of miR-124 and downregulation of SGK1. Cell Physiol Biochem. 2018;51(1):113–128. doi:10.1159/000495168
40. Wang Z, Ying M, Wu Q, et al. Overexpression of myosin VI regulates gastric cancer cell progression. Gene. 2016;593(1):100–109. doi:10.1016/j.gene.2016.08.015
41. Wang D, Zhu L, Liao M, et al. MYO6 knockdown inhibits the growth and induces the apoptosis of prostate cancer cells by decreasing the phosphorylation of ERK1/2 and PRAS40. Oncol Rep. 2016;36(3):1285–1292. doi:10.3892/or.2016.4910
42. Wang H, Wang B, Zhu W, et al. Lentivirus-mediated knockdown of myosin VI inhibits cell proliferation of breast cancer cell. Cancer Biother Radiopharm. 2015;30(8):330–335. doi:10.1089/cbr.2014.1759
43. You W, Tan G, Sheng N, et al. Downregulation of myosin VI reduced cell growth and increased apoptosis in human colorectal cancer. Acta Biochim Biophys Sin (Shanghai). 2016;48(5):430–436. doi:10.1093/abbs/gmw020
44. Gershon TR, Crowther AJ, Tikunov AP, et al. Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer Metab. 2013;1(1):2. doi:10.1186/2049-3002-1-2
45. Ros S, Schulze A. Glycolysis back in the limelight: systemic targeting of HK2 blocks tumor growth. Cancer Discov. 2013;3(10):1105–1107. doi:10.1158/2159-8290.CD-13-0565
46. Wang H, Wang L, Zhang Y, et al. Inhibition of glycolytic enzyme hexokinase II (HK2) suppresses lung tumor growth. Cancer Cell Int. 2016;16(1):9. doi:10.1186/s12935-016-0280-y
47. Wolf A, Agnihotri S, Micallef J, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313–326. doi:10.1084/jem.20101470
48. Li Z, Li X, Wu S, et al. Long non‐coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR–STAT3/microRNA143 pathway. Cancer Sci. 2014;105(8):951–955. doi:10.1111/cas.2014.105.issue-8
49. Kong X, Hu S, Sun Z, et al. Regulation of aerobic glycolysis by long non-coding RNAs in cancer. Biochem Biophys Res Commun. 2016;479(1):28–32. doi:10.1016/j.bbrc.2016.09.007
50. Li X, Zhao Q, Qi J, et al. lncRNA Ftx promotes aerobic glycolysis and tumor progression through the PPARγ pathway in hepatocellular carcinoma. Int J Oncol. 2018;53(2):551–566. doi:10.3892/ijo.2018.4418
51. Liu W, Zhang Q, Zhang J, et al. Long non-coding RNA MALAT1 contributes to cell apoptosis by sponging miR-124 in parkinson disease. Cell Biosci. 2017;7(1):19. doi:10.1186/s13578-017-0147-5
52. Luo F, Liu X, Ling M, et al. The lncRNA MALAT1, acting through HIF-1α stabilization, enhances arsenite-induced glycolysis in human hepatic L-02 cells. Biochim Biophys Acta. 2016;1862(9):1685–1695. doi:10.1016/j.bbadis.2016.06.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.