Back to Journals » Drug Design, Development and Therapy » Volume 14
HLJ2 Effectively Ameliorates Colitis-Associated Cancer via Inhibition of NF-κB and Epithelial–Mesenchymal Transition
Authors Song H , Tang X, Li X, Wang Y, Deng A, Wang W, Zhang H, Qin H, Wu LQ
Received 28 May 2020
Accepted for publication 2 September 2020
Published 15 October 2020 Volume 2020:14 Pages 4291—4302
DOI https://doi.org/10.2147/DDDT.S262806
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Huachen Song, Xiaonan Tang, Xiang Li, Yufei Wang, Anjun Deng, Wenjie Wang, Haijing Zhang, Hailin Qin, LianQiu Wu
State Key Laboratory of Bioactive Substances and Functions of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, People’s Republic of China
Correspondence: Haijing Zhang; LianQiu Wu Tel +86 10-63031589
Fax +86 10-63035779
Email [email protected]; [email protected]
Introduction: Colitis-associated cancer (CAC) accounts for approximately 15% of IBD patient mortalities. However, currently available anti-CAC drugs possess many disadvantages including safety, specificity and side effects. Therefore, the development of novel anti-CAC compounds is imperative. HLJ2 was a monomeric compound synthesized by our institute and reported to have an effect on ulcer colitis.
Methods: In vivo the AOM/DSS-induced CAC model was used to evaluate the effects of HLJ2 on ameliorating CAC symptoms, immunohistochemical analysis was used to analyze the pathological damage to colons and epithelial–mesenchymal transition was for changes of cytokines. In vitro, flow cytometric analysis, immunofluorescence and Western blot were used to detect the inhibition effect of HLJ2 on nuclear factor-κB and epithelial–mesenchymal transition in TGF-β 1-stimulated SW480 cells.
Results: In the AOM/DSS animal model, HLJ2 was demonstrated to inhibit the secretion of inflammatory cytokines and nuclear factor-κB, levels of tumorigenesis-related proteins including snail, and finally inhibited a key step in metastasis, epithelial–mesenchymal transition. In vitro, HLJ2 was also shown to inhibit nuclear factor-κB and epithelial–mesenchymal transition in TGF-β 1-stimulated SW480 cells in accordance with in vivo results. Meanwhile, the nuclear factor-κB inhibitor could interrupt the effect of HLJ2 on epithelial–mesenchymal transition.
Discussion: HLJ2 may ameliorate CAC through inhibiting nuclear factor-κB and then downstream epithelial–mesenchymal transition. The combination of the obvious improvement in effects on CAC without obvious side effects suggests that HLJ2 could be developed as a potential CAC therapeutic candidate.
Keywords: inflammatory bowel disease, colitis-associated cancer, EMT
Introduction
Colon cancer is one of the fastest-growing malignancies occurring in recent years. Colorectal cancer prevalence has shown to be the second highest.1,2 Human colon cancer includes familial colorectal polyps syndrome, hereditary nonpolyposis colon cancer, sporadic colon cancer, and colitis-associated cancer (CAC). The CAC pathology, the course of disease development and treatments all differ from other types of colon cancer.3
CAC is currently considered to be one of the leading causes of death in inflammatory bowel disease (IBD) patients. As early as 1925, it had been reported that ulcerative colitis (UC) was closely associated with CAC, and that the risk of colorectal cancer developing from UC was greater than that of normal adults in IBD patients.4 It is suggesting that continuous inflammation in the colon promotes the transformation of colonic epithelial cells. Intestinal inflammation easily relapses and the colon is more likely to become cancerous.5,6 Treatments targeted at may provide new strategies for CAC.7
The CAC pre-lesion microenvironment is often accompanied by a large number of inflammatory cells and chemokine infiltration, and its tumor malignancy has been closely tied to the degree of infiltration.8 Numerous cytokines act as signaling molecules in the intestinal immune system and have been demonstrated to participate in the disruption of the normal state of controlled inflammation. The nuclear factor-κB (NF-κB) pathway has been demonstrated to play a critical role in numerous pathological activities in CAC, primarily through the regulation of the expression of immune and inflammatory mediators, including interleukin-6 (IL-6) and transforming growth factor-beta (TGF-β) secretion.9–11 Furthermore, TGF-β represents one of the most critical induction factors involved in the regulation of epithelial–mesenchymal transition (EMT).12 EMT is a critical process during the progression of “inflammation-cancer” associated with CAC. When undergoing EMT, epithelial cells lose their cell polarity, their ability to attach to the basement membrane, and acquire the ability to achieve high levels of migration, invasion, anti-apoptosis, etc.
Over recent years, the active ingredients extracted from traditional Chinese medicine have played a specific role in CAC treatment. HLJ2 was extracted from berberine and then modified, and previous results have demonstrated that it possessed a clear anti-UC effect.13 Due to the close relationship between UC and CAC, we hypothesized that HLJ2 may also function to ameliorate the occurrence and development of CAC.
The above evidence suggest that inflammatory cytokines and related proteins contribute to EMT and then function to promote the transition of “inflammation-cancer”, which plays a critical role in the process of CAC. In this study, we utilized the azoxymethane (AOM)/dextran sulphate sodium (DSS) induced the CAC model to study the effect of HLJ2 on CAC. HLJ2 had a good effect on CAC mice and effectively alleviated the tumor burden of CAC mice. Then, we further explored the anti-CAC mechanism of HLJ2 surrounding NF-κB and EMT in the “inflammation-cancer” process in vivo and in vitro.
Materials and Methods
Reagents
DSS was purchased from MP Biomedicals (Santa Ana, CA, USA), AOM from Sigma-Aldrich (St. Louis, MO, USA), capecitabine tablets from Chia TaiTianqing Pharmaceutical Co. Ltd (Nanjing, China), Iscove’s Modified Dulbecco’s Medium (IMDM) from Sigma-Aldrich (St. Louis, MO, USA), fetal bovine serum (FBS) from Gibco (Grand Island, NY, USA), lipopolysaccharide (LPS) from Sigma Chemical Co. (St. Louis, MO, USA), recombinant Human TGF-β1 from Whiga (PeproTech Inc., USA), bicinchoninic acid (BCA) assay kits from Beyotime Biotechnology (Beijing, China), phycoerythrin (PE)-conjugated anti-CD11b, FITC-conjugated anti-Gr-1 and purified mouse Fc block (anti-CD16/CD32) from ThermoFisher Inc (Waltham, MA, USA),Enzyme-Linked Immunosorbent Assays (ELISA) kits for IL-17, TGF-β, IL-6, tumor necrosis factor-α (TNF-α) and IL-1β from eBioscience (Waltham, MA, USA). NF-κB p-p65, p-STAT3, E-cadherin, snail, vimentin, and β-actin antibodies were purchased from Abcam, Inc (Cambridge, MA, USA). Goat Anti-rabbit IgG/Alexa Fluor 488 and Goat Anti-mouse IgG/Alexa Fluor 546 were purchased from Whiga (PeproTech Inc, USA). Goat Anti-rabbit IgG/HRP antibody was purchased from ZhongShanJinQiao Biological Technology Co (Beijing, China). Hoechst 33342 was purchased from ThermoFisher Inc (MA, USA). JSH23 was purchased from TargetMol((MA, USA).
Cells and Animals
The human colorectal cell line SW480 was obtained from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China), and has been tested. All cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% FBS, 100 μg/mL streptomycin, and 100U/mL penicillin at 37°C in a humidified atmosphere with 5% CO2.
Female C57BL/6 mice (6–8 weeks old) were purchased from the laboratory animal center of Beijing Huafu Kang Biological Technology Co., Ltd. (Beijing, China). All animals were housed under controlled conditions (temperature 22 ± 1°C, humidity 40–60%, and a 12 h dark/light cycle) with free access to a standard laboratory diet and water. All animal care and experimental procedures were authorized by the Animal Care & Welfare Committee Institute of Materia Medica, CAMS&PUMC (Ethics inspection number: 00004611).
Development of CAC Model and Treatment Procedure
The procedures used for CAC animal models were induced by AOM and DSS. In this study, 60 mice were randomly divided into the following six groups: control group, model group (AOM/DSS), capecitabine group, low and high concentration group of HLJ2 (AOM/DSS+HLJ2 treatment), vehicle group (HLJ2 treatment without AOM/DSS).
Following adapted feeding for 7 days, mice were given an intraperitoneal injection of AOM (10 mg/kg). One week later, 3% DSS was added to the drinking water for 7 days followed by a 14-day period of tap water for recovery. This cycle was repeated for a total of three times. Capecitabine (250 mg/kg) group was suspended one week following two weeks of continuous administration. HLJ2 (25, 50 mg/kg) or the vehicle (50 mg/kg) group were administrated daily via gavage from the second week through the end of the ninth week.
Throughout the total experimental periods, the body weight was measured every week. At the end of the experiment, after the mice were anesthetized and sacrificed, spleen and colon tissues were processed as follows. After measuring the spleen weight, spleen cells were collected for flow cytometry analysis. Colon length was measured and then the colon was slit open longitudinally along the main axis and washed with phosphate buffer saline (PBS, pH7.4). The number of tumors in the colon was recorded, and the diameter of each tumor was measured using a sliding caliper. The tumor burden of each colon was then calculated by the square of the mean diameter multiplied by the number of lesions (adenoma and adenocarcinoma) evaluated per mouse. The distal colon tissues (1 cm) were then fixed in 4% paraformaldehyde buffer for further histopathological examination and immunohistochemical analysis. Others were flash-frozen in liquid nitrogen and maintained at −80°C for Western blot analysis.
Hematoxylin and Eosin (HE) Staining and Immunohistochemical (IHC) Evaluations
Colon tissues were fixed in 10% formalin, dehydrated using graded concentrations of ethanol, embedded in paraffin, and then sectioned (5 µm thick sections). Sections were then mounted on slides, cleared, and rehydrated. Tissue sections were stained with H&E as per the standard method. In the case of IHC studies, colon tissue was carried out according to standard protocols using antibodies at a 1:100 dilution.
ELISA for Inflammatory Cytokines
The concentrations of IL-1β, IL-6, TNF-α, IL-17, and TGF-β in colon tissues were measured using commercial Mouse ELISA Kits, respectively, according to the manufacturer’s protocols.
Flow Cytometric Analysis of MDSCs
After the spleen was removed from mice, the fat and fascia tissues were cut out and rinsed with physiological saline. The spleen was then placed on a 200 mesh screen and gently squeezed using a syringe core in order to allow individual cells to enter the solution via the mesh. Single spleen cell suspensions were stained using specific fluorescein-conjugated antibodies and the proportions of different cell populations were analyzed using an LSRII flow cytometer (BD Biosciences, San Jose, CA). For flow cytometric sorting, mouse splenocytes were stained with PE-conjugated anti-CD11b and FITC-conjugated anti-Gr-1 antibodies (Waltham, MA, USA), and PE-CD11b+/FITC-Gr-1+-MDSCs (Waltham, MA, USA) were isolated by cell sorting. All data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Immunofluorescence
Samples were permeabilized with 1mL Triton for 15 min, and washed in PBS 3 times. Next, samples were blocked in 1mL 5% BSA for 30 min. Blocking solution was then removed and 300 μL antibody (1:100, Cambridge, MA, USA) was added to the sample overnight at 4°C. The primary antibodies were then removed and the samples were washed 3 times with PBS. Secondary antibody was then added to the sample and incubated for 1 hour in the dark. After samples were then washed 3 times in PBS, Hochest 33342 was added and incubated in the dark for 15 min. The slides were then washed 3 times with PBS, and mounted in 50% glycerin. Slides were then covered and imaged using fluorescence microscopy (Olympus Inc., USA).
Western Blot Analysis
Cell lysates or supersonic lysates of colon tissues were processed for Western blot analysis as follows. First, lysates were centrifuged at 12,000 g for 10 min and quantified by BCA assay. Samples were then boiled at 100 °C for 5 min, and the supernatants were separated and immunoblotted. The membrane was blocked in Tris-buffered saline with 0.1% Tween-20 (TBST) solution containing 5% non-fat dried milk for 1 h, followed by an overnight incubation at 4 °C with the following antibodies: NF-κB p-p65 (1:2000), p-STAT3 (1:2000), E-cadherin (1:2000), snail (1:2000), vimentin (1:2000). Blots were then washed four times for 5 min each in TBST and incubated with secondary HRP-conjugated goat anti-rabbit or anti-mouse IgGs (1:5000) for 45 min at 37°C. The proteins of interest were visualized using enhanced chemiluminescence (ECL, Tanon Inc, Shanghai, China) and the protein bands were detected and analyzed using Image J software.
Statistics Analysis
Statistical analysis was carried out using GraphPad Prism 5. Data were typically expressed as the mean ± SEM. Differences between groups were analyzed using either unpaired two-tailed Student’s t-test or one-way ANOVA with Bonferroni correction. Significance parameters were set at *P < 0.05, **P < 0.01, compared with the indicated control group mice, #P < 0.05, ##P < 0.01, compared with the indicated AOM/DSS group mice.
Results
HLJ2 Improved Weight Loss, Colon Contracture, and Decreased Spleen Index in AOM/DSS Animal Model
HLJ2 effectively improved weight loss caused by CAC, and the contracture of colon length induced by AOM/DSS was also found to be improved by HLJ2 in a dose-dependent manner (Figure 1A and B). The increased spleen index induced by AOM/DSS was found to be reduced by HLJ2 supplementation in a dose-dependent manner (Figure 1C). It is important to note that HLJ2 did not appear to cause the similar toxic and side effects of weight loss as Capecitabine in the therapeutic process, especially at the 3w, 5w, 6w, 7w, 8w and 9w during the experiment cycle (supporting information: Table S1).
HLJ2 Ameliorated Tumor Formation and Inflammatory Damage in AOM/DSS-Induced CAC Mice
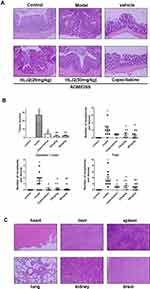
As depicted by HE staining, the majority of the lesions in the colon were found to be consistent with low-grade intraepithelial neoplasia or carcinoma in the AOM/DSS induced CAC mice group. Inflammation was also observed to exist in the entire mucosal epithelium and submucosal lamina propria in the AOM/DSS group tissue. These results demonstrated that the AOM/DSS animal model was successfully induced. Administration of HLJ2 (25, 50 mg/kg) and Capecitabine (250 mg/kg) was found to effectively improve the pathological damage and decrease the neoplasia (Figure 2A). HLJ2 (25, 50 mg/kg) significantly reduce tumor burden, size and number of colorectal neoplasms compared with AOM/DSS induced CAC model (Figure 2B).
HLJ2 Showed No Systemic Toxicity
Toxic side effects were evaluated in mice following long-term administration of HLJ2. First, the vehicle group did not exhibit any adverse effects on body weight in comparison with the control group (Figure 1A). In addition, there were no significant differences observed in the histological distribution in the vehicle group compared with the control group (Figure 2A). Abnormalities were not observed in other organs of the mice, including the heart, liver, spleen, lung, kidney, and brain (Figure 2C). All these data suggest that HLJ2 did not exhibit obvious systemic toxicity in mice at the conclusion of the experimental procedure.
HLJ2 Inhibited the Expression of NF-κB and p-STAT3 in Mice Treated with AOM/DSS
Uncontrolled inflammation is closely associated with tumor development and metastasis. Persistent inflammatory microenvironment can function to induce tumors by triggering genetic mutations.14 NF-κB is a key regulator of innate immune and inflammatory reactions and has been demonstrated to be a significant endogenous tumor promoter. Under the effect of carcinogenic factors, NF-κB pathway activation can result in genetic alterations and STAT phosphorylation.15
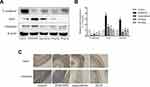
As depicted in Figure 3A, NF-κB p-p65 and p-STAT3 expression levels were obviously increased in the AOM/DSS-treated model group. This suggests that the NF-κB and STAT3 pathways may undergo activation in the CAC model. Compared with that of AOM/DSS-treated model group, HLJ2 was shown to reduce the expression of NF-κB p-p65 and p-STAT3Y5,7 induced by AOM/DSS. Immunohistochemical staining also demonstrated the same inhibition effect of HLJ2 on NF-κB and STAT3 (Figure 3C). These results demonstrate that HLJ2 may function to suppress AOM/DSS-mediated activation of NF-κB and STAT3 signaling.
HLJ2 Attenuated Proinflammatory Cytokines in Mice Treated with AOM/DSS
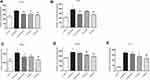
Inflammatory microenvironment which promotes CAC is generated by the signaling pathway which is mediated by TNF-α and IL-1β and also leads to other cytokine changes. Expression levels of the inflammatory cytokines, including IL-1β, IL-6, TNF-α, TGF-β, and IL-17 in mice treated with AOM/DSS were increased in comparison with the control group. HLJ2 can significantly reduce the secretion of IL-1β, IL-6, TNF-α, TGF-β, and IL-17 in a dose-dependent manner in comparison with the AOM/DSS induced group (Figure 4).
HLJ2 Decreased the Proportion of MDSCs in Spleen of Mice Treated with AOM/DSS
MDSCs plays a role in tumor immune escape, immune tolerance, immunosuppression, and other pathological processes. In addition, it promotes tumor occurrence and growth. The effect of HLJ2 on MDSC was analyzed using flow cytometric analysis of CD11b+Gr-1+ cells in the spleen (Figure 5). HLJ2 was observed to result in a decreased number of MDSCs in comparison with the model group. It should be noted that TGF-β1, the primary effector molecule of MDSCs, was observed increasing in the model group and decreased by HLJ2 treatment in a dose-dependent manner.
HLJ2 Inhibited EMT in Mice Treated with AOM/DSS
EMT plays a critical role in cancer metastasis and is also an important link in the transition from inflammation to cancer.16 In this study, EMT regulatory protein expression levels in the colon tissue were detected by Western blot analysis and immunohistochemical staining. As depicted in Figure 6A, a significant down-regulation of E-cadherin and an up-regulation of snail and vimentin were observed in AOM/DSS-induced CAC mice in comparison with the control group, indicating the occurrence of EMT in the AOM/DSS model group. HLJ2 treatment was found to dramatically increase E-cadherin expression and decrease snail and vimentin expression levels. Similarly, immunohistochemical staining further confirmed the effects of HLJ2 on snail and vimentin expression levels (Figure 6C). Therefore, these results suggest that HLJ2 can function to suppress EMT in the CAC mice model.
HLJ2 Inhibited the Expression of NF-κB and STAT3 in LPS-Induced SW480 Cell Line
NF-κB p-p65 and p-STAT3 expression levels in SW480 cells were analyzed following HLJ2 treatment and LPS stimulation (1µg/mL) for 24 h. As depicted in Figure 7, doses of 0.01µM, 0.1µM, and 1µM HLJ2 were observed to effectively reduce NF-κB p-p65 and p-STAT3 expression levels in a dose-dependent manner in comparison with the LPS-induced group. Therefore, these results suggest that HLJ2 could function to restrain the inflammatory reaction.
HLJ2 Inhibited the Expression of EMT Markers in TGF-β-Induced SW480 Cell Line
In order to gain further confirmation of the effect of HLJ2 on EMT during CAC process, we measured changes in expression levels of EMT markers in SW480 by Western blot and immunofluorescence staining. SW480 cells were stimulated with TGF-β (10ng/mL) for 48h in order to induce EMT. As depicted in Figure 8A, E-cadherin was up-regulated and snail, and vimentin expression levels were decreased by HLJ2 treatment in a concentration-dependent manner. In addition, immunofluorescence staining demonstrated that HLJ2 functioned effectively on EMT markers in SW480 cells (Figure 8B). JSH23, an NF-κB inhibitor could reverse TGF-β induced EMT. HLJ2 could not display its inhibition effect on EMT with JSH23 (Figure 8C). These results further confirmed that HLJ2 may inhibit EMT by the NF-κB pathway.
Discussion
The AOM/DSS-induced CAC mice model is a stable and specific model resembling the pathogenesis of human CAC with good organ selectivity.17 In this study, we used the AOM/DSS-induced CAC mouse model to study the efficacy of HLJ2 on CAC. Our results demonstrated that HLJ2 can effectively inhibit the pathological process of CAC (Figures 1 and 2). HLJ2 can also improve weight loss compared to the administration of Capecitabine. While alleviating CAC without weight loss, HLJ2 was found to have almost no physiological toxicity or side effects. On the basis of the effect on CAC, we further elucidated its mechanism of HLJ2 during inflammatory carcinogenesis programs.
Intestinal inflammatory reactions play a very critical role in the process of CAC. NF-κB signal has been demonstrated to regulate the conversion of inflammatory lesions into cancer. It is thought that NF-κB could enable various factors to maintain an inflammatory microenvironment in metastatic tumors, leading to the transition from the inflammatory response to the CAC phenotype.18 STAT3 is highly expressed in intestinal epithelial cells and has been demonstrated to increase the susceptibility of colonic epithelium to colitis. HLJ2 was demonstrated to have a good inhibition effect on the expression of NF-κB and STAT3 (Figures 3 and 7) in vivo and in vitro.
It has also been shown to increase CAC tumor incidence by NF-κB mediating IL-6, IL-1β and IL-17 secretion.19 A significant positive correlation was also observed between IL-17 and MDSC levels in gastrointestinal cancer patients. HLJ2 had good effect on IL-6, IL-1β and IL-17 secretion (Figures 4 and 5). So HLJ2 could effectively inhibit NF-κB and downstream inflammatory process during the inflammatory carcinogenesis programs of CAC.
EMT is a critical event in the inflammatory-cancer regulation network and can promote colon cancer invasion, metastasis, etc.20,21 The data in this study demonstrated that HLJ2 reduced TGF-β levels in the tissue supernatant of model mice (Figure 4). In addition, it was found to upregulate the expression levels of E-cadherin, while inhibiting snail and vimentin expression levels (Figure 6) in vivo. The effect of HLJ2 on EMT was further confirmed in vitro in TGF-β induced SW480 cells (Figure 8). These results demonstrated that HLJ2 could function to effectively inhibit the inflammation-cancer transformation through inhibition of TGF-β-induced EMT. To further determine the effect of HLJ2 on TGF-β induced EMT by inhibiting NF-κB, JSH23 was used in TGF-β induced SW480 cells. It was shown that EMT in TGF-β induced cells was reversed by JSH23 intervention. Meanwhile, the effect of HLJ2 on suppressing TGF-β induced EMT in the SW480 cells has not appeared. These findings manifested that the HLJ2 inhibiting TGF-β induced EMT might be by inhibiting NF-κB signaling. According to the data described above, HLJ2 was able to effectively relieve CAC through the inhibition of the inflammation-cancer chain including NF-κB and inflammatory factors, and then downstream TGF-β-induced EMT.
It is worthy mentioning that HLJ2 could improve the weight loss significantly compared to Capecitabine. Capecitabine, as a commonly used clinical drug, has many disadvantages of oral chemotherapy drugs, including high toxicity and side effects. In contrast to Capecitabine for CAC, HLJ2 possesses the advantages of high efficiency, a simple administration route, no side effects, low cost, etc. Therefore, we expect HLJ2 to make up for the vacancy in clinical treatments for colitis-associated cancer.
Of course, there are still many questions about anti-CAC mechanism of HLJ2. For example, in this study HLJ2 could decrease the percentage of MDSCs and the secretion of IL-17. MDSCs have been shown to function to promote the proliferation and metastasis of cancer cells through TGF-β-induced EMT.21–24 So in future, we would investigate the relation among MDSCs, IL-17 and EMT effected by HLJ2 in order to elucidate its anti-CAC mechanism more clearly.
Conclusions
In summary, HLJ2 was demonstrated to exert positive effects on the amelioration of CAC. A combination of both in vivo and in vitro data demonstrated that HLJ2 inhibited the conversion of inflammation to cancer through inhibition of NF-κB and STAT3 expression, decreasing TGF-β mediated EMT, ultimately alleviating carcinogenesis of CAC. Compared with Capecitabine for CAC, HLJ2 displayed no obvious toxic and side reaction. Therefore, we expect HLJ2 will be developed as a candidate drug for the treatment of CAC.
Ethics Statement
All animal experimental procedures were performed in accordance with the guidelines of the Animal Welfare and Research Ethics Committee of Peking Union Medical College.
Acknowledgments
We are grateful to the department of instrumental analysis, institute of Materia Medica, Chinese Academy of Medical Science and Peking Union Medical College, for the measurement of fluorescence microscopy. We thank for the help of all members, especially for the confocal microscopy technically guidance by Dr. Jinze Li (Chinese academy of medical science and Peking union medical college, Beijing 100050, China), the flow cytometry analysis by Ms Wei Cui (Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, China), the data collection by Yunfeng Song (the junior high school of Beijing Yucai school, Beijing 100050, China).
Funding
This work was financially supported by the fundamental Research Funds for the Central Universities (3332019073), the drug innovation major project (2018ZX09711001-002-002 and 2018ZX09711001-003-001), CAMS Innovation Fund for Medical Sciences (2016-12M-3-011 and 2017-12M-3-011) and the Opening Funds for State Key Laboratory of Bioactive Substances and Functions of Natural Medicines (GTZK201906). There is no conflict to declare regarding funding.
Disclosure
The authors declare no competing financial interests. The authors report no conflicts of interest for this work.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi:10.1002/ijc.25516
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi:10.1002/ijc.29210
3. Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;373:195.
4. Kanneganti M, Mino-Kenudson M, Mizoguchi E. Animal models of colitis-associated carcinogenesis. J Biomed Biotechnol. 2011;2011:342637.
5. Hotta S, Shimada Y, Nakano M, et al. Feasibility of restorative proctocolectomy in patients with ulcerative colitis-associated lower rectal cancer: a retrospective study. Asian J Surg. 2019;42(1):267–273. doi:10.1016/j.asjsur.2018.01.003
6. Lissner D, Siegmund B. Cancer surveillance in inflammatory bowel diseases. Dtsch Med Wochenschr. 2019;144(11):753–756.
7. Brody H. Colorectal cancer. Nature. 2015;521(7551):S1. doi:10.1038/521S1a
8. Shivakumar BM, Chakrabarty S, Rotti H, et al. Comparative analysis of copy number variations in ulcerative colitis associated and sporadic colorectal neoplasia. BMC Cancer. 2016;16:271. doi:10.1186/s12885-016-2303-4
9. Zundler S, Neurath MF. Integrating immunologic signaling networks: the JAK/STAT pathway in colitis and colitis-associated cancer. Vaccines (Basel). 2016;4.
10. Saitoh M. Epithelial-mesenchymal transition is regulated at post-transcriptional levels by transforming growth factor-β signaling during tumor progression. Cancer Sci. 2015;106:481–488. doi:10.1111/cas.12630
11. Kim TW, Shin JS, Chung KS, Lee YG, Baek NI, Lee KT. Anti-Inflammatory mechanisms of Koreanaside A, a lignan isolated from the flower of Forsythia koreana, against LPS-induced macrophage activation and DSS-induced colitis mice: the crucial role of AP-1, NF-κB, and JAK/STAT signaling. Cells. 2019;8(10):1163. doi:10.3390/cells8101163
12. Sevko A, Umansky V. Myeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: thick as thieves. J Cancer. 2013;4:3–11. doi:10.7150/jca.5047
13. Zhang H, Song G, Zhang Z, et al. Colitis is effectively ameliorated by (±)-8-acetonyl-dihydrocoptisine via the XBP1-NF-κB pathway. Front Pharmacol. 2017;8:619. doi:10.3389/fphar.2017.00619
14. Lin C, Zhang J. Inflammasomes in inflammation-induced cancer. Front Immunol. 2017;8:271. doi:10.3389/fimmu.2017.00271
15. Crucitti A, Corbi M, Tomaiuolo PM, et al. Laparoscopic surgery for colorectal cancer is not associated with an increase in the circulating levels of several inflammation-related factors. Cancer Biol Ther. 2015;16:671–677.
16. Snider AJ, Bialkowska AB, Ghaleb AM, Yang VW, Obeid LM, Hannun YA. Murine model for colitis-associated cancer of the colon. Methods Mol Biol. 2016;1438:245–254.
17. Patras L, Sesarman A, Licarete E, et al. Dual role of macrophages in the response of C26 colon carcinoma cells to 5-fluorouracil administration. Oncol Lett. 2016;12:1183–1191. doi:10.3892/ol.2016.4708
18. Lai CS, Yang G, Li S, et al. 3ʹ-Hydroxypterostilbene suppresses colitis-associated tumorigenesis by inhibition of IL-6/STAT3 signaling in mice. J Agric Food Chem. 2017;65(44):9655–9664. doi:10.1021/acs.jafc.7b03712
19. Bhangu A, Wood G, Mirnezami A, Darzi A, Tekkis P, Goldin R. Epithelial mesenchymal transition in colorectal cancer: seminal role in promoting disease progression and resistance to neoadjuvant therapy. Surg Oncol. 2012;21:316–323. doi:10.1016/j.suronc.2012.08.003
20. Fu Y, Liu X, Zhou N, et al. MicroRNA-200b stimulates tumour growth in TGFBR2-null colorectal cancers by negatively regulating p27/kip1. J Cell Physiol. 2014;229:772–782.
21. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–324.
22. Liao CP, Booker RC, Brosseau JP, et al. Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis. J Clin Invest. 2018;128(7):2848–2861. doi:10.1172/JCI99424
23. Dominguez C, David JM, Palena C. Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin Cancer Biol. 2017;47:177–184. doi:10.1016/j.semcancer.2017.08.002
24. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev Mol Cell Biol. 2019;20(2):69–84.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.